Abstract
The extracellular matrix (ECM) of the lung serves as both a scaffold for resident cells and a mechanical support for respiratory function. The ECM is deposited during development and undergoes continuous turnover and maintenance during organ growth and homeostasis. Cells of the mesenchyme, including the tissue resident fibroblast, take a leading role in depositing and organizing the matrix and do so in an anatomically distinct fashion, with differing composition, organization, and mechanical properties within the airways, vessels, and alveoli of the lung. Recent technological advancements have allowed the lung’s ECM biochemical composition and mechanical properties to be studied with improved resolution, thereby identifying novel disease-related changes in ECM characteristics. In parallel, efforts to study cells seeded on normal and disease-derived matrices have illustrated the powerful role the ECM can play in altering key functions of lung resident cells. The mechanical properties of the matrix have been identified as an important modifier of cell–matrix adhesions, with matrices of pathologic stiffness promoting profibrotic signaling and cell function. Ongoing work is identifying both mechanically activated pathways in mesenchymal cells and disease-related ECM molecules that biochemically regulate cell function. Uncovering the control systems by which cells respond to and regulate the matrix, and the failures in these systems that underlie aberrant repair, remains a major challenge. Progress in this area will be an essential element in efforts to engineer functional lung tissue for regenerative approaches and will be key to identifying new therapeutic strategies for lung diseases characterized by disturbed matrix architecture.
Keywords: fibroblast, fibrosis, extracellular matrix, lung
The extracellular matrix (ECM) provides a structural scaffold on which lung cells grow, differentiate, organize, and function. This scaffold is laid down and continuously remodeling during organogenesis in the developing organism, with the cells of the mesenchyme playing a leading role in matrix deposition and organization. Mesenchymal cell types, chief among them the resident fibroblast, are then tasked with maintaining the ECM scaffold throughout an organism’s life. To optimally fulfill its scaffold functions, the matrix varies in composition and organization across the different compartments and anatomical features of the lung. Thus, the cells of the mesenchyme must respond to relevant anatomically localized cues to deposit, maintain, and remodel the appropriate ECM that serves the lung’s functional needs.
In addition to its role as a structural scaffold, the matrix plays an essential mechanical role in support of lung function. Several disease conditions in the lung are associated with progressive ECM deposition or destruction and corresponding alterations in lung mechanics, including idiopathic pulmonary fibrosis (IPF), in which progressive fibrotic scarring is associated with decreases in lung compliance (1, 2), and emphysema, in which destruction of elastic fibers is associated with increases in compliance (3). Recent evidence indicates that alterations in the matrix may shift resident cell functions in ways that promote disease progression rather than homeostasis (4, 5). Thus, signaling cues in the matrix, whether biochemical or biomechanical, can become part of the processes that promote disease progression, helping to account for the relentless progressive nature of lung dysfunction in chronic diseases and underscoring the need to understand the pathologic cues present in the matrix.
Matrix and Mesenchyme in Lung Diseases
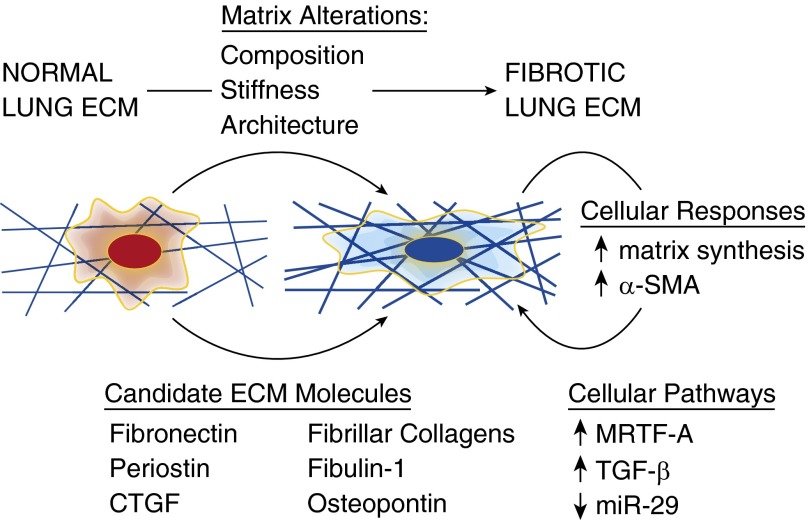
Strong evidence supporting a prominent role for ECM involvement in lung pathologies has emerged recently based on important technological and methodological advances. Recent application of proteomic approaches to the ECM has allowed the composition of the lung’s matrix to be dissected with unprecedented breadth and molecular resolution (4, 6). In parallel, detergent-based cellular extraction approaches have enabled study of the intact, but decellularized, lung matrix as a substrate for cell adhesion and function (4, 5, 7–9). Initial efforts have focused on matrices derived from the lungs of individuals with IPF and demonstrated broad alterations in matrix composition detected by proteomics (4). Decellularization of IPF-derived lungs, followed by seeding of naive fibroblasts onto the decellularized matrices, promoted cellular expression of the characteristic myofibroblast marker α-smooth muscle actin (Figure 1), a response that was absent when the same cells were seeded on control matrices derived from healthy lungs (4). Following this lead, recent work compared the transcriptional and translational programs expressed by healthy and IPF-derived lung fibroblasts when seeded on healthy or IPF-derived matrices. Remarkably, the dominant effect was matrix derived and not cell autonomous (5). Mechanistically, the authors identified a critical role for matrix-dependent suppression of miR-29 in engaging increased translation of matrix proteins when fibroblasts were seeded on IPF-derived matrices (5), elucidating a mechanism by which fibrotic matrix programs cells to produce greater amounts of matrix (Figure 1). Delineating the profibrotic cues that exist within pathological matrices will require intensive efforts, but several matrix or matrix-associated protein candidates have been identified that confer profibrotic signals and correlate with fibrotic pathologies (2), including fibulin-1 (10), osteopontin (11, 12), periostin (13, 14), connective tissue growth factor (15), and fibronectin (16, 17).
Figure 1.
Disease-related changes in the lung’s extracellular matrix (ECM) include changes in composition, mechanical properties such as matrix stiffness, and matrix architecture. Studies of naive fibroblasts seeded onto decellularized normal and disease-derived matrices indicate that important cellular responses are driven by the matrix, including the observation that a fibrotic lung ECM promotes expression of the myofibroblast marker α-smooth muscle actin (α-SMA) and a translational program leading to matrix synthesis. Although the molecular mechanisms by which the fibrotic ECM regulates mesenchymal cell function remain to be determined, mechanical signaling through myocardin-related transcription factor (MRTF)-A and transforming growth factor (TGF)-β mechano-signaling have both been demonstrated in simplified in vitro systems, as has an important role for fibrotic matrix in attenuation of miR-29 expression. In addition to mechanical signals provided by the fibrotic ECM, multiple matrix or matrix-associated candidate molecules have been identified that are associated with fibrosis in human subjects or animal models and may link the fibrotic ECM to alterations in mesenchymal cell function. CTGF, connective tissue growth factor.
Matrix- and Mechanotransduction in the Mesenchyme
Integrins, transmembrane receptors formed from heterodimers of α and β protein subunits, are the predominant receptors for ECM ligands (18). Although mammals express 18 α subunits and 8 β subunits, capable of forming 24 distinct integrin receptors, cells typically express a specific subset of functional integrin heterodimers, with specific cell types featuring a unique complement of integrin subunits and heterodimers. There is considerable overlap in integrin recognition of specific matrix molecules, and peptide sequences recognized by integrins, such as the tripeptide RGD sequence, are common to many different matrix molecules (18). Hence, there are inherent limitations in the capacity of integrins alone to discriminate among the large repertoire of matrix and matrix-associated ligands. This mismatch between ligands and receptors strongly suggests that additional cell surface proteins participate directly or indirectly in cell–matrix interactions and that additional contextual information must influence the signals transmitted by the ECM via integrin receptors.
Although matrix composition provides a primary means by which the matrix can communicate to resident cells, it is important to recognize that other aspects of the matrix can convey important information and regulate aspects of cellular function even in the presence of identical matrix composition. For instance, matrix ligand density, and how it is spatially displayed, can exert important effects. One important example relevant to fibroblast biology is the observation that α-smooth muscle actin–positive stress fiber formation depends on the ability of cells to form elongated, mature cell–matrix focal adhesions, which themselves depend on appropriate spatial display of ECM ligands supportive of these structures (19). Dimensionality clearly also plays an important role in defining cellular behaviors, and presentation of ECM ligands in three-dimensional space better mimics aspects of the interstitial matrix in which fibroblasts reside. Strikingly, cell migration through three-dimensional matrices can occur through multiple mechanisms depending on the density and biophysical properties of the matrix (20). In sparse matrices, fibroblast migration can be approximated as one-dimensional, as cell align and migrate along oriented ECM fibers (21). Recent work also demonstrates that mesenchymal cell invasion of matrices may respond to alterations in matrix biophysical properties, with preferential formation of invasive structures in areas of reduced matrix rigidity (22). Such matrix responsiveness could protect matrix barriers from invasion and allow cells to seek out paths of minimal resistance in trafficking to sites of matrix remodeling. These findings underscore the potential role that the mechanical properties of the matrix can play in modulating cellular responses to matrix signals, an area of investigation that has dramatically expanded in scope over the past decade.
Investigations of matrix mechanical properties in the lung have been aided by the use of atomic force microscopy to analyze micromechanical properties of lung tissue. Using such methods, it has been possible to compare the modulus of normal and fibrotic lung tissues, with pronounced matrix stiffening identified in human IPF-derived matrices (4) and similar changes observed in mouse models of lung fibrosis (23, 24). Fibroblasts grown on synthetic matrices spanning the range of matrix mechanical properties reported in these studies exhibit dramatic alterations in morphology, proliferation, migration, apoptosis, and matrix synthesis, indicative of the wide-ranging effects matrix stiffness alone exerts on fibroblast function (23). Hence, considerable effort has been expended to identify mechanisms by which matrix stiffness is transduced by fibroblasts. Although it is clear that integrin-mediated adhesions are critically involved, based on their altered size, frequency, and stability (19, 23, 25) across physiological variations in matrix stiffness, the mechanisms of mechanotransduction remain elusive. What is clear is that soft, compliant matrices do not support stable mature adhesions to form under traditional cell culture growth conditions, whereas increasingly rigid matrices inherently support the formation of stable, mature matrix adhesions. Elucidating the mechanochemical interactions that underlie this basic observation is fraught with complexity, in large part because integrins themselves have only small intracellular domains without inherent signaling capacity; instead, they rely on recruitment of additional proteins, many with inherent signaling and multiple protein–protein interacting domains, to form complexes containing 150 or more distinct proteins (26). Remarkably, simply altering the level of tension present in the actomyosin cytoskeleton, which itself controls the stability and size of focal adhesions, fundamentally alters the overall composition of these adhesion complexes (27, 28). Although not all elements of the adhesion complex are themselves mechanosensitive, compelling evidence now demonstrates that multiple key components of the adhesion complex can undergo conformational changes or unfolding to reveal cryptic binding sites that facilitate protein–protein interactions under permissive mechanical conditions (29). Thus, the mechanisms by which cells respond to alterations in matrix stiffness begin at the integrin interface with the ECM but fundamentally depend on mechanosensitive protein–protein interactions within the adhesion complex. Delineating key molecular players within this complex machinery is daunting but ultimately critical to understanding how the physical environment presented by the matrix shapes cellular responses.
Downstream of the adhesion complex, it is clear that increasing matrix stiffness promotes actin polymerization as a consequence of enhanced adhesion stability and cellular tractions, which themselves depend on stable cell–matrix adhesions and actin–myosin interactions in the cytoskeleton (30, 31). These observations have linked matrix stiffness to two critical signaling processes that appear to be pivotal in fibroblast activation: nuclear localization of myocardin-related transcription factor (MRTF) and cell force–mediated activation of transforming growth factor (TGF)-β. The MRTFs have been shown to be sequestered in the cytoplasm associated with G-actin and to respond to increases in actin polymerization by translocating to the nucleus. Consistent with this mechanism, it has been shown that fibroblast MRTF-A (also known as MKL1) accumulates in the nuclei of fibroblasts resident on stiff matrices but remains cytoplasmic on soft matrices (30). MRTF-A binds to another transcription factor, serum response factor, through which it interacts with the promoter region of the gene encoding α-smooth muscle actin. This pathway provides a molecular mechanism by which matrix mechanical properties can control expression of a key marker of the myofibroblast phenotype implicated in fibrotic pathologies (Figure 1). In support of the pathological relevance of this pathway, mice genetically deficient in MRTF-A are protected from bleomycin-induced lung fibrosis, and targeting of Rho kinase upstream of actin polymerization and MRTF-A effectively attenuates bleomycin-induced fibrosis in both preventive and therapeutic dosing regimens (32). Importantly, fibroblasts isolated from patients with IPF remain responsive to inactivation of this signaling pathway (32) and overall remain largely responsive to the matrix mechanical environment (33), suggesting that targeting the matrix mechanical environment or its downstream signaling pathways may be an effective strategy for interrupting profibrotic cellular activation.
Activation of TGF-β, which is secreted in a latent complex and tethered to the ECM via the latent TGF-β binding proteins, can be accomplished through integrin binding and cell-mediated force generation (34, 35). Matrix stiffness alters cellular capacity to generate tractions, the forces that cells transmit to the ECM (31). Not surprisingly, then, stiff matrices promote fibroblast ability to liberate active TGF-β from the matrix (36), implicating traction force–mediated TGF-β signaling in a positive feedback loop that amplifies profibrotic signaling. Interestingly, exogenous stimulation with TGF-β to enhance cellular expression of the contractile myofibroblast phenotype is only able to increase tractions on a stiff matrix (31). This finding suggests that tractions are mechanically limited on compliant matrices (25), as adhesions fail rather than transmit larger forces to the matrix in this mechanical context. Such a mechanism offers a putative protective brake on profibrotic signaling by limiting TGF-β activation under physiologically compliant mechanical conditions.
Very recent work brings the story full circle by demonstrating that matrix stiffness responses are also influenced by the specific composition of the matrix to which the cell is bound. Studies of cellular responses to matrix stiffness have thus far been overwhelmingly conducted on polyacrylamide hydrogels, which are themselves incapable of supporting cell adhesion; hence, they are coated with an ECM ligand, in almost all cases fibronectin or collagen. Strikingly, a recent study has found that the effects of a compliant matrix on limiting cell spreading and proliferation, which is observed on either collagen- or fibronectin-coated polyacrylamide, is eliminated by growing cells on a matrix of identical stiffness composed of a hyaluronan-based hydrogel conjugated with fibronectin (37). Although the exact mechanism is not understood, this study underscores how little we understand about how cells respond to combined biochemical and mechanical cues provided by the ECM. Interestingly, the cell spreading and proliferation on the compliant hyaluronan-based hydrogel are decoupled from cell tractions, which remain minimal (37) as they are on polyacrylamide gels. These results echo other recent observations that the functional responses to matrix stiffness, including proliferation and traction forces, can be decoupled and operate through independent mechanisms (25). These observations of matrix composition–dependent matrix stiffness responses, although limited, emphasize the potential complexity in cell–matrix responses and indicate that mechanical effects of matrix signaling can be highly dependent on the matrix composition.
Matrix and Mesenchyme in Development and Homeostasis
The growing evidence that matrix cues contribute to the pathogenesis of chronic lung disease underscore the importance of understanding the developmental origins of the lung ECM, how it is deposited during normal tissue morphogenesis, and how it is maintained and remodeled during organ growth and homeostasis. During lung development, the epithelium and mesenchyme engage in reciprocal interactions that are essential in guiding branching morphogenesis and appropriate cellular fate decisions (38). Hence, it is reasonable to speculate that the epithelium and mesenchyme cooperate in producing a matrix and soluble environment conducive for organ development and function and that the epithelium plays an instructive role in guiding mesenchymal matrix production. What is less well understood is whether distinct and stable mesenchymal cell types emerge in different anatomical regions of the lung or whether mesenchymal cell types remain responsive in a highly plastic fashion to their local environment. Fate mapping strategies are beginning to yield important insights into the developmental origin of mesenchymal cell subpopulations in the lung and revealing considerable complexity in both cell origin and plasticity (39, 40). Studies of fibroblasts isolated from adult lungs demonstrate durable phenotypic differences in cells cultured after isolation from proximal airways versus distal lung (41, 42), suggesting that functionally different mesenchymal cell subpopulations reside in different anatomical compartments within the lung. But our understanding of mesenchymal cell subpopulations and their origins and plasticity during disease and lung remodeling remains a major limitation in understanding the role of the mesenchyme in lung repair and remodeling.
Although the epithelium may play important roles in instructing mesenchymal cell function, it is equally important to recognize that the mesenchyme is not only tasked with matrix production but also contributes key morphogens and growth factors that support and instruct epithelial differentiation and expansion during development (38). Although much less is known about the support roles that mesenchymal cells play in the adult lung, recent work emphasizes potential roles for pericytes as vascular support cells (39, 40), whereas parenchymal lipofibroblasts are believed to serve as alveolar epithelial support cells (43–45). Although methods to identify and study these mesenchymal subpopulations in mouse models are rapidly emerging, more definitive markers of these (and other) mesenchymal cell subpopulations are needed to understand their localization in human lungs and their potential contributions to lung diseases. Recent observations from in vitro studies of lung progenitor cells suggest that the developmental role of mesenchyme in supporting lung morphogenesis may be recapitulated in support of lung epithelial progenitor function. Several groups have identified essential roles for mesenchymal cells, or their products, in efficient expansion of epithelial progenitor populations (43, 46–48), hinting at important but poorly understood roles for mesenchyme in establishing stem cell niches and supporting stem cell expansion and differentiation in response to lung injury. Whether chronic lung diseases that alter matrix composition and mechanics might disrupt this mesenchymal cell support function remains an open question, but one that could be essential to understanding failed reparative responses to lung injury and the divergence between normal wound healing and progressive lung scarring.
Finally, although substantial progress is being made in understanding mesenchymal cell origin and function in healthy and diseased lungs, we still understand relatively little about what signals control cells to maintain or alter the composition, architecture, and mechanical properties of the matrix. Are there, for instance, key regulators for depositing alveolar-, bronchial-, or vessel-associated matrix? Are these functions controlled by the surrounding epithelium, or are autonomous mesenchymal cell subpopulations responsible for the anatomical diversity of the ECM? And how does this control system fail in progressive matrix remodeling processes such as those found in IPF and emphysema? Moreover, although we have some basic understanding of how cells can control matrix mechanical properties by altering the density, composition, and cross-linking of the matrix, we have comparatively little knowledge of what signals regulate cellular control of this process (49) and what role specific matrix or matrix-associated proteins play in specifying matrix mechanical properties (14). Finally, further investigation is needed into the concept that fibrotic matrix deposition and corresponding changes in matrix mechanics are at least partially reversible biological processes (2, 50) amenable to therapeutic intervention (51–54). Uncovering the control systems that underlie these critical cell–matrix interactions represents a first step toward targeted manipulation of the ECM. Such information will be an essential element in efforts to engineer functional lung tissue for regenerative medicine approaches and will be a key step toward identifying new therapeutic strategies in lung diseases characterized by disturbed matrix architecture.
Footnotes
Supported by National Institutes of Health grant HL092961.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tschumperlin DJ, Jones JC, Senior RM. The fibrotic matrix in control: does the extracellular matrix drive progression of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2012;186:814–816. doi: 10.1164/rccm.201208-1561ED. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol. 2014;184:1643–1651. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suki B, Sato S, Parameswaran H, Szabari MV, Takahashi A, Bartolák-Suki E. Emphysema and mechanical stress-induced lung remodeling. Physiology (Bethesda) 2013;28:404–413. doi: 10.1152/physiol.00041.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11:M111 014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE. Future prospects for tissue engineered lung transplantation: decellularization and recellularization-based whole lung regeneration. Organogenesis. 2014;10:196–207. doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, Hoffman AM, Weiss DJ. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18:895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffar J, Unger S, Corte TJ, Keller M, Wolters PJ, Richeldi L, Cerri S, Prêle CM, Hansbro PM, Argraves WS, et al. Fibulin-1 predicts disease progression in patients with idiopathic pulmonary fibrosis. Chest. doi: 10.1378/chest.13-2688. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukui T, Ueha S, Abe J, Hashimoto S, Shichino S, Shimaoka T, Shand FH, Arakawa Y, Oshima K, Hattori M, et al. Qualitative rather than quantitative changes are hallmarks of fibroblasts in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2013;183:758–773. doi: 10.1016/j.ajpath.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, et al. COMET Investigators. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonniaud P, Martin G, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, White ES, Varga J. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci Transl Med. 2014;6:232ra250. doi: 10.1126/scitranslmed.3008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 19.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschumperlin DJ. Fibroblasts and the ground they walk on. Physiology (Bethesda) 2013;28:380–390. doi: 10.1152/physiol.00024.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu Z, Liu F, Tonkova EA, Lee SY, Tschumperlin DJ, Brenner MB. Soft matrix is a natural stimulator for cellular invasiveness. Mol Biol Cell. 2014;25:457–469. doi: 10.1091/mbc.E13-05-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol. 2013;229:25–35. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mih JD, Marinkovic A, Liu F, Sharif AS, Tschumperlin DJ. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J Cell Sci. 2012;125:5974–5983. doi: 10.1242/jcs.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo JC, Han X, Hsiao CT, Yates JR, III, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiller HB, Friedel CC, Boulegue C, Fässler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinković A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol. 2012;303:L169–L180. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marinković A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2013;48:422–430. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomini MM, Travis MA, Kudo M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin α(v)β(6)-dependent physical force. Exp Cell Res. 2012;318:716–722. doi: 10.1016/j.yexcr.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Raz-Ben Aroush D, Galie PA, Pogoda K, Bucki R, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35:71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotaru C, Schoonover KJ, Trudeau JB, Huynh ML, Zhou X, Hu H, Wenzel SE. Regional fibroblast heterogeneity in the lung: implications for remodeling. Am J Respir Crit Care Med. 2006;173:1208–1215. doi: 10.1164/rccm.200508-1218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Wu W, Hu H, Milosevic J, Konishi K, Kaminski N, Wenzel SE. Genomic differences distinguish the myofibroblast phenotype of distal lung fibroblasts from airway fibroblasts. Am J Respir Cell Mol Biol. 2011;45:1256–1262. doi: 10.1165/rcmb.2011-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 45.Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol. 2007;292:L608–L611. doi: 10.1152/ajplung.00379.2006. [DOI] [PubMed] [Google Scholar]
- 46.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung LY, Tian D, Brangwynne CP, Weitz DA, Tschumperlin DJ. A new microrheometric approach reveals individual and cooperative roles for TGF-beta1 and IL-1beta in fibroblast-mediated stiffening of collagen gels. FASEB J. 2007;21:2064–2073. doi: 10.1096/fj.06-7510com. [DOI] [PubMed] [Google Scholar]
- 50.Decaris ML, Gatmaitan M, FlorCruz S, Luo F, Li K, Holmes WE, Hellerstein MK, Turner SM, Emson CL. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol Cell Proteomics. 2014;13:1741–1752. doi: 10.1074/mcp.M113.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee TH, McKleroy W, Khalifeh-Soltani A, Sakuma S, Lazarev S, Riento K, Nishimura SL, Nichols BJ, Atabai K. Functional genomic screen identifies novel mediators of collagen uptake. Mol Biol Cell. 2014;25:583–593. doi: 10.1091/mbc.E13-07-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]