Abstract
Objective
The authors have recently been using a surgical technique of minimally invasive direct lateral interbody fusion (DLIF) for correcting of coronal imbalance. The purpose of this study was to evaluate the surgical outcome and complication of DLIF.
Methods
We undertook retrospective analysis of a consecutive series of 8 DLIF procedures in Degenerative lumbar spine disease since May 2011. Four patients underwent DLIF only, and the others underwent combined DLIF and posterior fixation. Data on intra- and postoperative complications were collected. The pre- and postoperative X-rays were reviewed. We investigated coronal deformity, Cobb's angle, and apical vertebral translation (AVT). The mean follow-up period was months with a range of 2 to 8 months.
Results
A mean preoperative coronal Cobb's angle was 21.8° (range 11.5-32.4°). Following after DLIF, the mean Cobb's angle was decreased to 13.0° (range 2.9-21.5°). Following additional posterior screw fixation, mean Cobb's angle was further decreased to 7.4° (range 2.9-13.2°). A mean preoperative AVT was 2.0 cm(range 0.6-3.5 cm), and improved to 1.4 cm(range 0.3-2.4 cm) and 0.8 cm(range 0.2-1.8 cm) postoperatively (DLIF and, posterior fixation respectively). One patient (12.5%) showed cage migration during follow-up period. Two patients (25%) developed motor weakness, and 4 patients (50%) experienced postoperative thigh paresthesias or dysesthesias. During follow up period, motor weakness had resolved in 1 patient. Sensory symptoms were improved in all patients at the last follow-up.
Conclusion
Degenerative lumbar disease can be effectively corrected by DLIF with acceptable complications.
Keywords: Degenerative lumbar disease, Coronal imbalance, DLIF, Lumbosacral plexus, Postoperative complication
INTRODUCTION
Recently surgical trends are toward minimally invasive operations, which decreases blood loss and post-operative pain and enables patients to return to their ordinary life after short hospital stays4,7,14,20,28). The first laparoscopic lumbar discectomy was introduced in 1991 by Obenchain17). Following that, new surgical methods such as transforaminal lumbar interbody fusion (TLIF) and direct lateral interbody fusion (DLIF) were introduced for degenerative lumbar spine disease.
DLIF, also called as Extreme Lateral Interbody Fusion (XLIF)19), is a method of maximizing the advantages of anterior lumbar interbody fusion (ALIF) and improving accessibility to anterior spinal body that facilitates minimum invasive treatment by transpsoas approach. This technique has been used to treat degenerative lumbar disease including degenerative scoliosis1,4,6,13). The authors have recently been using a surgical technique of minimally invasive direct lateral interbody fusion (DLIF) for correcting coronal imbalance. The purpose of this study was to evaluate surgical outcome and complications of DLIF for Degenerative lumbar spine disease.
MATERIALS AND METHODS
1. Patient population
This study included a consecutive series of 8 patients (mean age 65.8, range 51-76, M:F=2:7) who underwent DLIF in our institute between May, 2011 and Feb, 2012. Among a total of 31 DLIF cases, 8 patients underwent DLIF for Degenerative lumbar spine disease. Four patients underwent DLIF only, and the others underwent combined DLIF and posterior fixation(Table 1). Mean follow up period was 3 months
Table 1. Summary of demographic profiles.
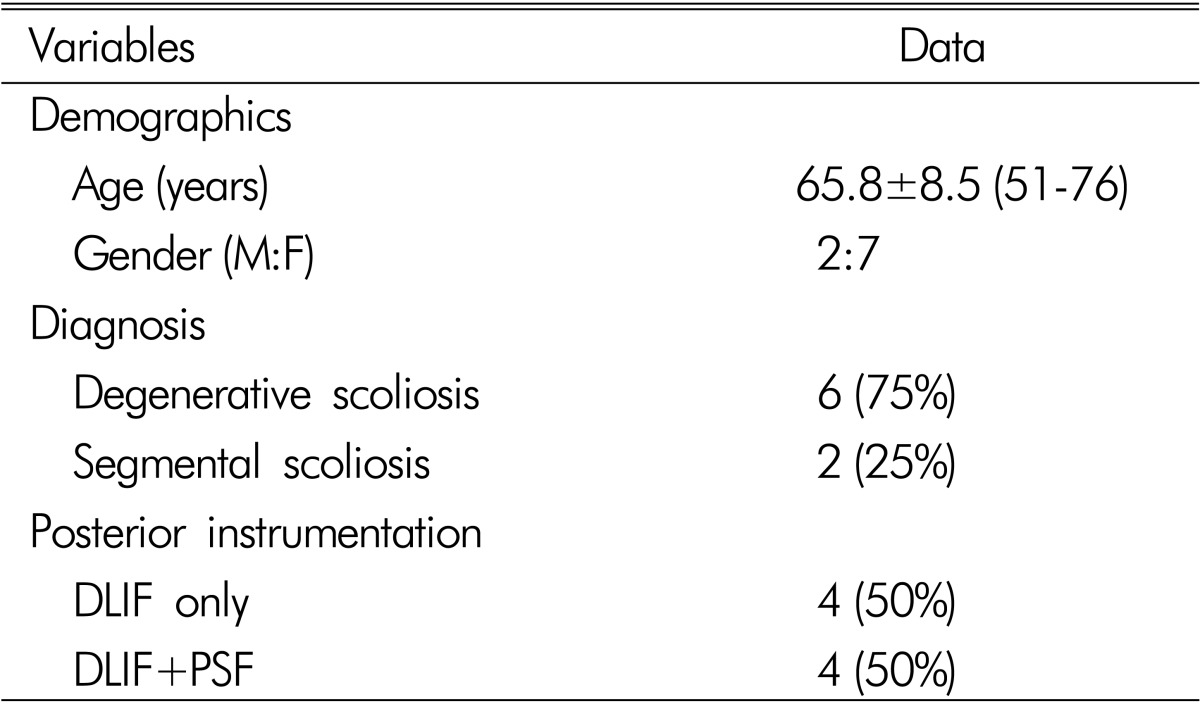
DLIF; Direct Lateral Interbody Fusion
PSF; Pedicle Screw Fixation
2. Surgical procedures
The patient is placed on a surgical table in a true 90° lateral decubitus position and taped in this position. C-arm guided cross table anterior-posterior (AP) image help to confirm the true 90° position. Surgical table and patient are flexed in such a way as to increase the distance between the iliac crest and the rib cage, especially at L4-L5. It is helpful to place a pillow or roll under the contralateral flank19). EMG electrodes are placed for intra-operative monitoring as previous reports. Intra-operative EMG monitoring system(NIM-Spine, Medtronic Sofamor Danek, TN, USA) is installed to estimate close proximity of the lumbosacral plexus to the advancing tubular dilator3,5,9,27). A 4-cm-sized oblique incision is made and retroperitoneal finger dissection is performed. Through this small incision, atraumatic tissue dilators and an expandable retractor are inserted, which can be the working portal19). We check for normal EMG and then conduct discectomy. During discectomy, we check that it is released to the opposite annulus. After sufficient discectomy, interbody fusion is conducted using DLIF cage. In most cases, we make 1 incision for multilevel DLIF but for some patients another incision is made.
We use Clydesdale cages (Medtronic Sofamor Danek, TN, USA) for DLIF, with heights from 8 to 12mm, lengths from 45 to 55mm and a total lordosis angle of 0°, 6°. All DLIF grafts were packed with β-tricalcium phosphate (ChronOS□ strip, Synthes, PA, USA) and demineralized bone matrix (DBX□ putty, Synthes, PA, USA). Since DLIF cannot be conducted to L5/S1 which is covered by sacral crest, for the group who were receiving pedicle screw fixation, PLIF was followed by pedicle screw fixation to this lesion.
3. Radiologic evaluation
L-spine series X-ray was examined to compare the 8 patients' pre and post operative status and coronal Cobb's angle and apical vertebral translation (AVT) were compared to check the improvement of degenerative lumbar scoliosis. Cobb's angle is an index to determine the severity of sagittal or coronal deformity by measuring the angle made by 2 perpendicular lines from the extended lines of upper and lower endplate with the severest scoliosis26).
AVT is an indicator of determining the degree of scoliosis by measuring the distant from the center sacral vertebral line (CSVL) to the midpoint of the apical vertebral body of the curve 26).
4. Data analysis
Measurements were collected and analyzed by using software (Microsoft Excel, Microsoft Corp., WA, USA). A p<0.05 was regarded as statistical significance.
RESULTS
1. Demographics
In our experience, 31 cases of DLIF were conducted since May, 2011 and 8 cases among them were done to the patients with coronal imbalance. 4 patients underwent DLIF procedures and the other 4 underwent additional pedicle screw fixation (PSF) by posterior approach after transpsoas DLIF.
2. Radiologic outcomes
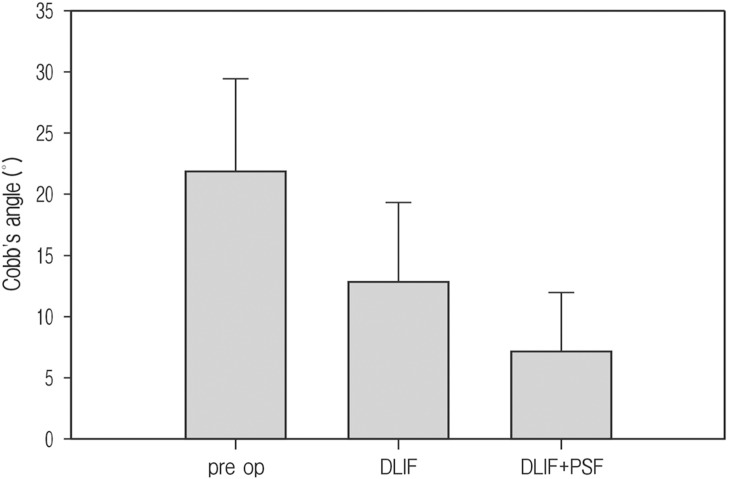
The pre-operative average Cobb's angle of all patients is 21.8° (range 11.5-32.4°) and the average of 4 patients who operated additional PSF is 24.1°, which shows a little more serious scoliosis than that of the entire. After surgical management for scoliosis, all 8 patients who were received DLIF got better and their average Cobb's angle improved to 13° (range 2.9-21.5°). The 4 patients who were received additional PSF after DLIF, saw their average final Cobb's angle much improved to 7.4° (range 2.9-13.2°)(Fig. 1).
Fig. 1. Correction of Cobb's angle; pre op mean Cobb's angle was 21.8° that improved to 13° after DLIF, and more improved to 7.3° after additional pedicle screw fixation.
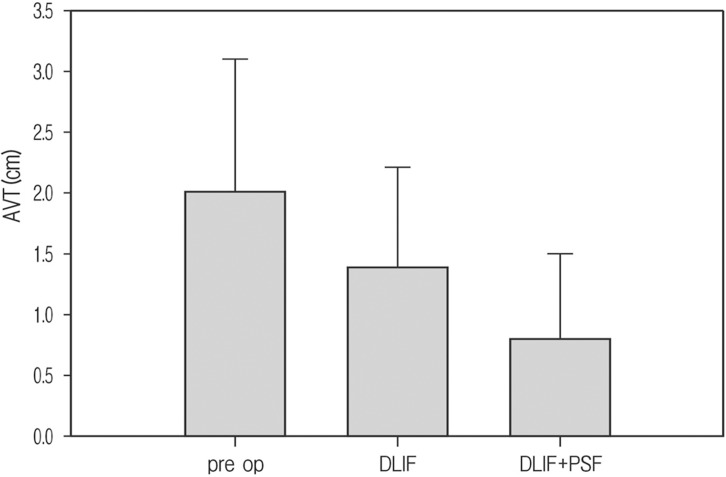
As with Cobb's angle, AVT also showed marked improvement after operation. Total mean AVT was 2.0 cm(range 0.6-3.5 cm) before surgery, but in which improved to 1.4 cm (range 0.3-2.4 cm) after DLIF only and the additional PSF group was much improved to 0.8 cm(range 0.2-1.8 cm) (Fig. 2).
Fig. 2. Correction of Apical Vertebral Translation (AVT); pre op mean AVT was 2.0 cm that improved to 1.4 cm after DLIF, and more improved to 0.8 cm after additional pedicle screw fixation.
Of course, it's expected that the additional PSF group has much improvement of Cobb's angle and AVT, but the standalone DLIF group shows sufficient correction of coronal imbalance. And standalone DLIF group's correction rate of Cobb's angle was stiffer than the additional PSF group's rate.
3. Complications
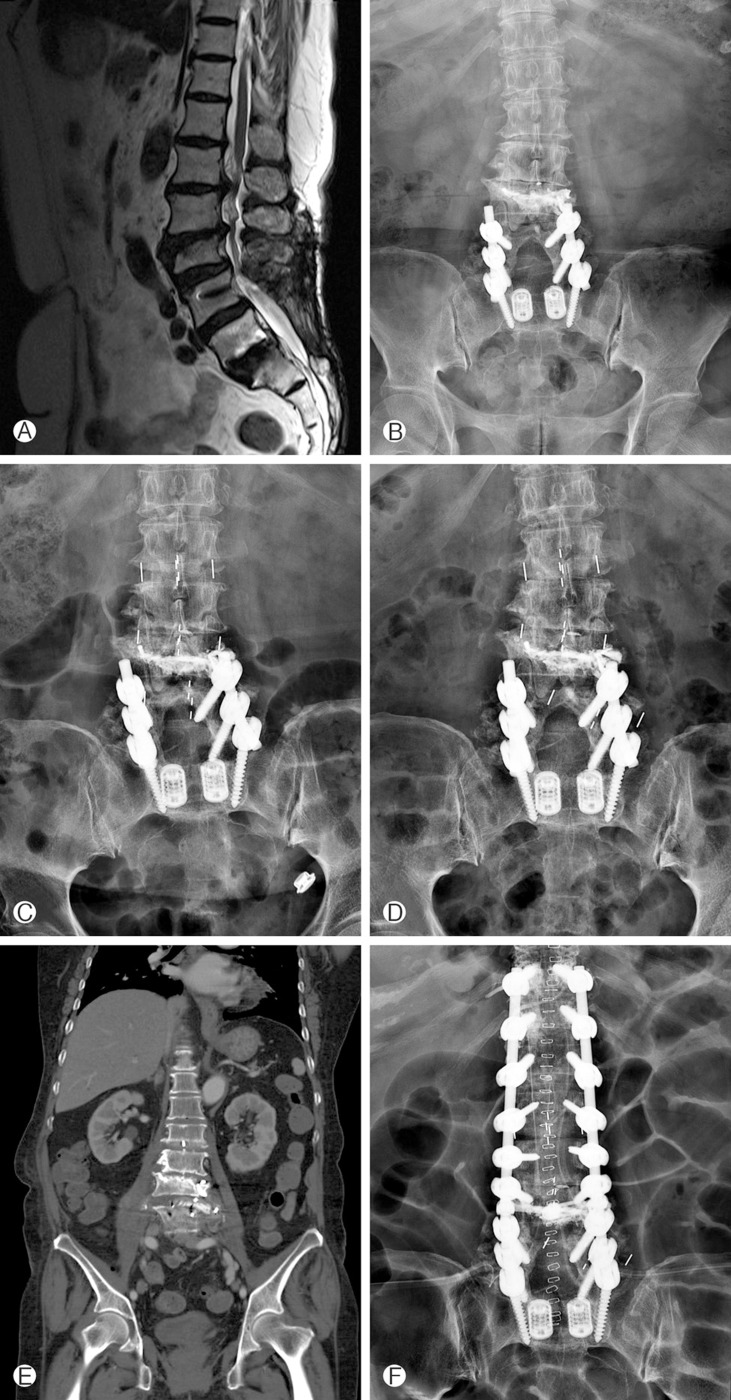
Some patients had cage-related complications. Two cases of cage subsidence were detected in standalone DLIF group, and one case of cage migration was detected. Cage migration patients who received additional expansive posterior pedicle screw fixation for prevent spinal instability (Fig. 3). This patient was included additional PSF group in this study.
Fig. 3. Case 4 patient is 74-year-old female; she has been operated PLIF L5-S1 and pedicle screw fixation on L4-5-S1 in 2009. And L3 percutaneous vertebroplasty was done. (A, B) She visited our hospital due to topping off and segmental scoliosis L1-2-3-4. (C) Performed DLIF surgery. (D) After 1 month follow up L-spine X-ray and (E) abdominal CT coronal image was shown L3-4 DLIF cage migration. (F) Finally, We did expansive pedicle screw fixation for prevent spinal instability (PLIF; Posterior Lumbar Interbody Fusion, DLIF; Direct Lateral Interbody Fusion).
Also, lumbosacral plexus related complications were detected. Two patients (25%) developed motor weakness, and 4 patients (50%) experienced postoperative thigh paresthesias or dysesthesias. But, motor weakness had recovered in few weeks for 1 patient. Sensory symptoms were improved in all patients at the last follow-up.
No cases had serious complications and no severe morbidity and mortality resulted from Degenerative lumbar spine disease.
DISCUSSION
The lateral, transpsoas approach was first introduced in 2001 by Pimenta21). It was developed into XLIF in 2006 by Ozgur19) and presented in Korea in May 2011 under the name of DLIF. DLIF approaches to vertebral body directly and can be conducted without any help from an extra-surgeon who performs an anterior abdominal approach like ALIF1,10,19,24). It also has the advantage of enabling a surgical approach without any risk to great vessels2) or retrograde ejaculation22) which are potential problems in anterior approach1,10,19). The fusion surface area is enhanced by using a much larger and higher cage than in historical PLIF or TLIF, thus improving the reduction rate in degenerative spine disease8,18,23). In addition, it is effective in reducing peri-operative bleeding and operation time with minimal invasive technique19). It has also, less postoperative pain, leading to early recovery and faster return to ordinary life16). DLIF technique is very effective and has many advantages.
Minimally invasive scoliosis correction is a very challenging to a spinal deformity surgeon. But traditional open extensive scoliosis correction was hard work and very dangerous surgery. For the patient's safety and appropriated surgical correction of deformity, minimally invasive deformity correction technique was needed. DLIF provide for bilateral annulus release and after disectomy, placement of a large interbody spacer that allows correction of coronal deformity. This reason can make the people believe DLIF is valuable tool for scoliosis correction. Anand et al report correction of Cobb's angle form 18.93° to 6.19° through minimal invasive multilevel percutaneous screw fixation1). Some studies compare combined transpsoas approach and traditional posterior approach for scoliosis treatment. Tormenti et al report radiographic outcome as the Cobb's angle and AVT were significantly improved in patients who underwent a combined transpsoas and posterior approach26). In this study, we retrospectively reviewed radiograph to contrast standalone DLIF and DLIF with open posterior PSF for scoliosis correction. According to our data, standalone DLIF improved Cobb's angle by 40.3%(from 21.8 to 13.0) and AVT by 30%(from 2.0 cm to 1.4 cm).
Despite of this effective correction, standalone DLIF has some complication, such as, subsidence of cage and, cage migration. Marchi et al reported standalone DLIF subsidence rate was 17.3%15). Recently, minimally invasive percutaneous pedicle screw fixation systems were developed well. Long-level percutenous screw fixation is available now. So the combination of DLIF and percutaneous screw fixation is a good tool for degenerative scoliosis correction. This combination can prevent cage related complication such as cage subsidence and migration.
Another major DLIF complication is lumbosacral plexus injury. In our experience, transient thigh sensory change (25%) and psoas muscle weakness (12.5%) rate were similar to previous literature reports11,12,25). The key to solving this problem is less damage to the psoas muscle during finger transpsoas disse ction and retracted dilator insertion. And close observation of intra-operative EMG monitoring was needed.
As for our result, standalone DLIF gets enough reduction of spinal alignment and is a good treatment tool for segmental scoliosis15). To prevent cage related complications, a combination of DLIF and additional reinforcement surgery is the best choice for minimal invasive scoliosis correction.
Our study is limited by small numbers and lack of sufficient experiences. We need longer follow up data.
CONCLUSION
Degenerative lumbar spine disease with coronal imbalance can be effectively corrected by DLIF with acceptable complication rates.
Table 2. Summary of surgical profiles (Cobb's angle/AVT) mean±SD.
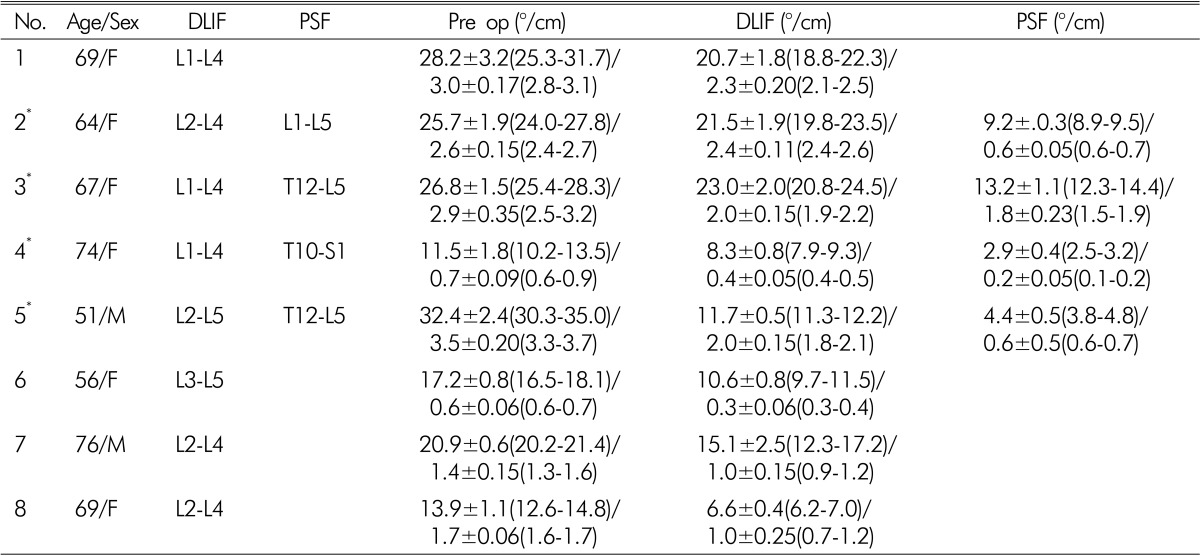
*DLIF+PSF
DLIF; Direct Lateral Interbody Fusion, PSF; Pedicle Screw Fixation, AVT; Apical Vertebral Translation
References
- 1.Anand N, Baron EM, Thaiyananthan G, Khalsa K, Goldstein TB. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21(7):459–467. doi: 10.1097/BSD.0b013e318167b06b. [DOI] [PubMed] [Google Scholar]
- 2.Baker JK, Reardon PR, Reardon MJ, Heggeness MH. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976) 1993;18(15):2227–2230. doi: 10.1097/00007632-199311000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Banagan K, Gelb D, Poelstra K, Ludwig S. Anatomic mapping of lumbar nerve roots during a direct lateral transpsoas approach to the spine: acadaveric study. Spine (Phila Pa 1976) 2011;36(11):E687–E691. doi: 10.1097/BRS.0b013e3181ec5911. [DOI] [PubMed] [Google Scholar]
- 4.Benglis DM, Elhammady MS, Levi AD, Vanni S. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery. 2008;63(3 Suppl):191–196. doi: 10.1227/01.NEU.0000325487.49020.91. [DOI] [PubMed] [Google Scholar]
- 5.Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10(2):139–144. doi: 10.3171/2008.10.SPI08479. [DOI] [PubMed] [Google Scholar]
- 6.Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28(3):E8. doi: 10.3171/2010.1.FOCUS09282. [DOI] [PubMed] [Google Scholar]
- 7.Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg. 2007;15(6):321–329. doi: 10.5435/00124635-200706000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Elowitz EH, Yanni DS, Chwajol M, Starke RM, Perin NI. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg. 2011;54(5-6):201–206. doi: 10.1055/s-0031-1286334. [DOI] [PubMed] [Google Scholar]
- 9.Galloway GM. Direct lateral transpsoas approach to interbody fusion-may be risky after all. J Clin Neurophysiol. 2011;28(6):605–606. doi: 10.1097/WNP.0b013e31823db011.. [DOI] [PubMed] [Google Scholar]
- 10.Gumbs AA, Shah RV, Yue JJ, Sumpio B. The open anterior paramedian retroperitoneal approach for spine procedures. Arch Surg. 2005;140(4):339–343. doi: 10.1001/archsurg.140.4.339. [DOI] [PubMed] [Google Scholar]
- 11.Houten JK, Alexandre LC, Nasser R, Wollowick AL. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine. 2011;15(3):280–284. doi: 10.3171/2011.4.SPINE1127. [DOI] [PubMed] [Google Scholar]
- 12.Karikari IO, Nimjee SM, Hardin CA, Hughes BD, Hodges TR, Mehta AI, et al. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: Initial clinical experience and early outcomes. J Spinal Disord Tech. 2011;24(6):368–375. doi: 10.1097/BSD.0b013e3181ffefd2. [DOI] [PubMed] [Google Scholar]
- 13.Knight RQ, Schwaegler P, Hanscom D, Roh J. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech. 2009;22(1):34–37. doi: 10.1097/BSD.0b013e3181679b8a. [DOI] [PubMed] [Google Scholar]
- 14.Lee YG, Cha JH, Park JS. Clinical outcome of minimally invasive tubular retractor assisted microscopic discectomy in far lateral lumbar disc herniation. Korean J Spine. 2010;7:155–160. [Google Scholar]
- 15.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346. doi: 10.1100/2012/456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci. 2012;19(6):829–835. doi: 10.1016/j.jocn.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Obenchain TG. Laparoscopic lumbar discectomy: case report. J Laparoendosc Surg. 1991;1(3):145–149. doi: 10.1089/lps.1991.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35(26 Suppl):S331–S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 19.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (xlif): anovel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976) 2007;32(5):537–543. doi: 10.1097/01.brs.0000256473.49791.f4. [DOI] [PubMed] [Google Scholar]
- 21.Pimenta L. Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery: VIII Brazilian Spine Society Meeting; Belo Horizonte, Minas Gerais, Brazil. 2001. [Google Scholar]
- 22.Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(1 Suppl):60–64. doi: 10.3171/spi.1999.91.1.0060. [DOI] [PubMed] [Google Scholar]
- 23.Richards JC, Majumdar S, Lindsey DP, Beaupre GS, Yerby SA. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine (Phila Pa 1976) 2005;30(7):744–749. doi: 10.1097/01.brs.0000157483.28505.e3. [DOI] [PubMed] [Google Scholar]
- 24.Riedel CJ. Open anterior lumbar interbody fusion. Clin Neurosurg. 2000;47:534–540. [PubMed] [Google Scholar]
- 25.Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech. 2010;23(6):393–397. doi: 10.1097/BSD.0b013e3181b31729. [DOI] [PubMed] [Google Scholar]
- 26.Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28(3):E7. doi: 10.3171/2010.1.FOCUS09263. [DOI] [PubMed] [Google Scholar]
- 27.Uribe JS, Vale FL, Dakwar E. Electromyographic monitoring and its anatomical implications in minimally invasive spine surgery. Spine (Phila Pa 1976) 2010;35(26 Suppl):S368–S374. doi: 10.1097/BRS.0b013e3182027976. [DOI] [PubMed] [Google Scholar]
- 28.Wang MY, Anderson DG, Poelstra KA, Ludwig SC. Minimally invasive posterior fixation. Neurosurgery. 2008;63(3 Suppl):197–203. doi: 10.1227/01.NEU.0000320434.83458.10. [DOI] [PubMed] [Google Scholar]