Abstract
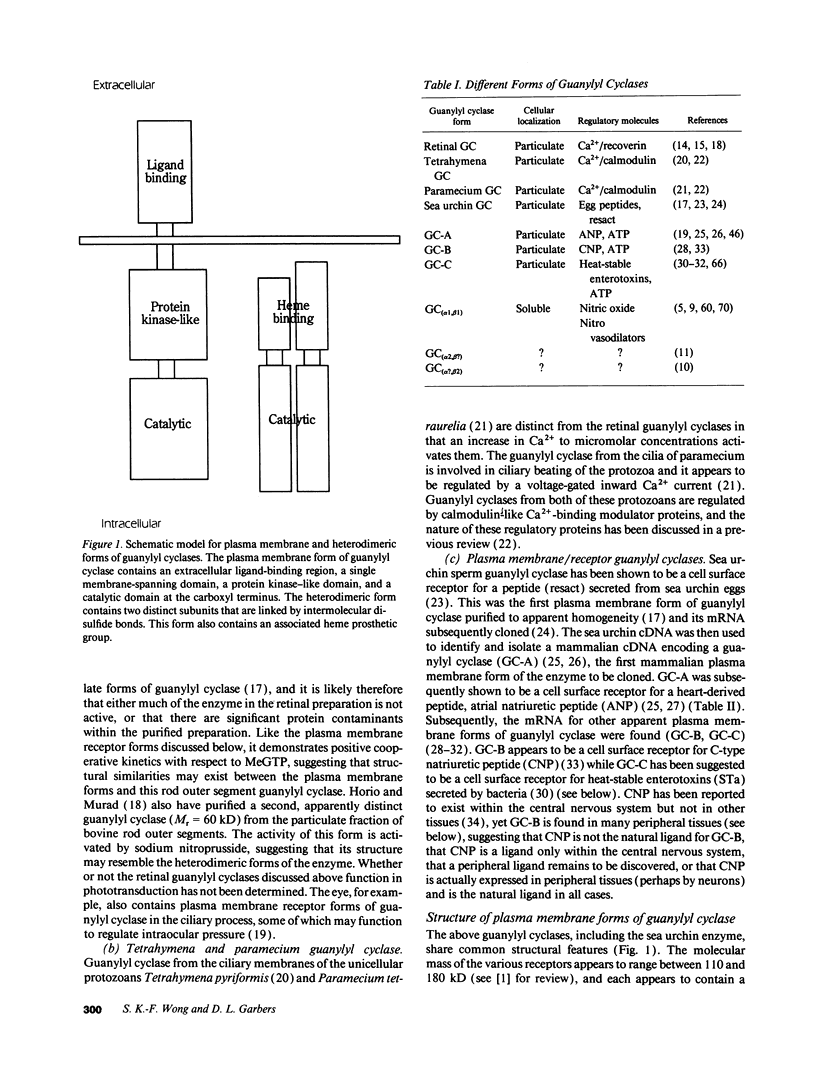
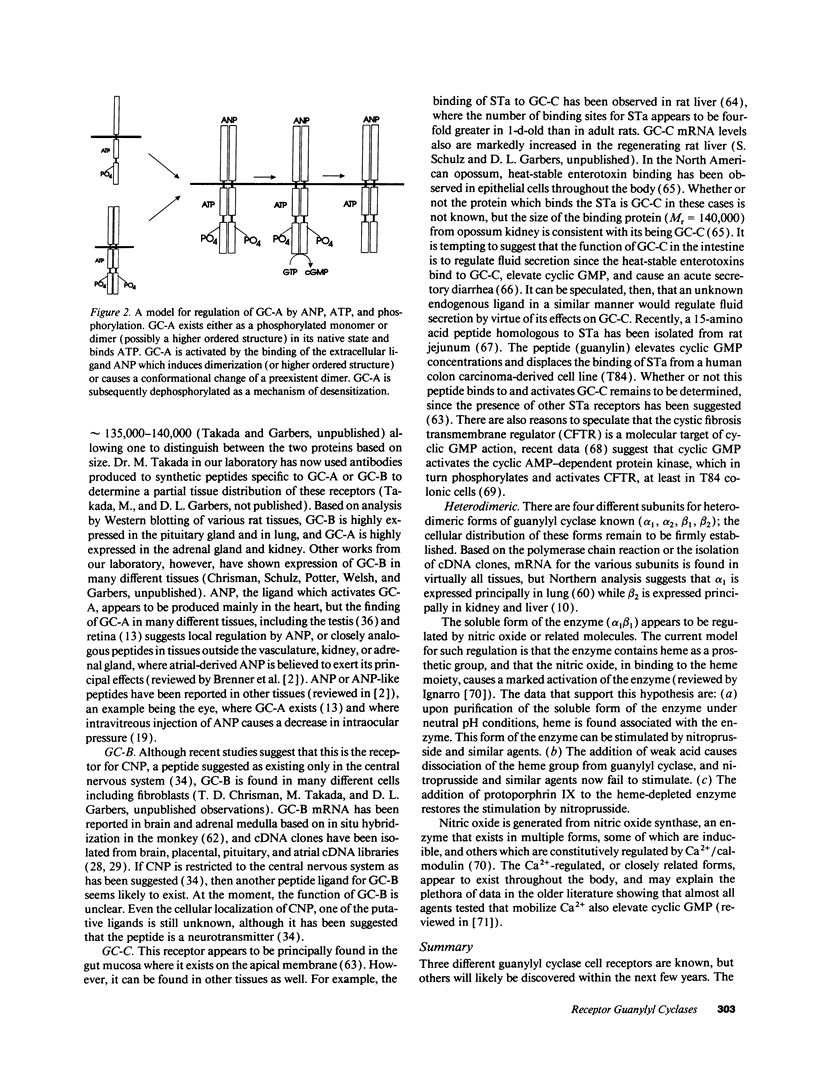
Three different guanylyl cyclase cell receptors are known, but others will likely be discovered within the next few years. The general function of these receptors appear to relate to the regulation of fluid volume or fluid movement. New receptors, or possibly the currently known receptors, therefore, may be discovered in areas of the body where fluid volume regulation is important. Such fluids whose volume or composition might be regulated by guanylyl cyclase receptors include synovial fluid, uterine/oviductal luminal fluid, follicular fluid, aqueous humor, cerebral spinal fluid, seminiferous tubule luminal fluid, epididymal luminal fluid, seminal plasma, and airway luminal fluid. The function of the heterodimeric forms of guanylyl cyclase appear to relate to a primary regulation of nitric oxide (or similar molecules) concentrations, which are in turn regulated by a Ca2+/calmodulin-sensitive nitric oxide synthase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura J. J., Minamino N., Kangawa K., Matsuo H. Isolation and identification of C-type natriuretic peptide in chicken brain. Biochem Biophys Res Commun. 1991 Jan 15;174(1):142–148. doi: 10.1016/0006-291x(91)90497-u. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Tubb D. J., Garbers D. L. Receptor-mediated activation of spermatozoan guanylate cyclase. J Biol Chem. 1986 Nov 15;261(32):14859–14862. [PubMed] [Google Scholar]
- Beuve A., Boesten B., Crasnier M., Danchin A., O'Gara F. Rhizobium meliloti adenylate cyclase is related to eucaryotic adenylate and guanylate cyclases. J Bacteriol. 1990 May;172(5):2614–2621. doi: 10.1128/jb.172.5.2614-2621.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990 Jul;70(3):665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Jiang B., Douglas J. G. Caged ATP potentiates guanylate cyclase activity stimulated by atrial natriuretic factor in rat lung membranes. Eur J Pharmacol. 1990 Jul 31;189(1):111–114. doi: 10.1016/0922-4106(90)90237-r. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Jiang B., Douglas J. G. Calcium reveals different mechanisms of guanylate cyclase activation by atrial natriuretic factor and ATP in rat lung membranes. Biochim Biophys Acta. 1991 Jun 7;1093(1):42–46. doi: 10.1016/0167-4889(91)90136-l. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Kohse K. P., Chang B., Hirata M., Jiang B., Douglas J. E., Murad F. Characterization of ATP-stimulated guanylate cyclase activation in rat lung membranes. Biochim Biophys Acta. 1990 Apr 9;1052(1):159–165. doi: 10.1016/0167-4889(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Chang M. S., Lowe D. G., Lewis M., Hellmiss R., Chen E., Goeddel D. V. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989 Sep 7;341(6237):68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. Signal transduction by guanylyl cyclases. Annu Rev Biochem. 1991;60:553–575. doi: 10.1146/annurev.bi.60.070191.003005. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989 Sep 22;245(4924):1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Singh S., Garbers D. L. Adenine nucleotides are required for activation of rat atrial natriuretic peptide receptor/guanylyl cyclase expressed in a baculovirus system. J Biol Chem. 1991 Mar 5;266(7):4088–4093. [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978 Dec 10;253(23):8433–8443. [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991 Feb 22;251(4996):915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sharma R. K. Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7882–7886. doi: 10.1073/pnas.88.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Porter J. G., Arfsten A. E., Miller J., Schilling J. W., Scarborough R. M., Lewicki J. A., Schenk D. B. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988 Jul 5;263(19):9395–9401. [PubMed] [Google Scholar]
- Gazzano H., Wu H. I., Waldman S. A. Activation of particulate guanylate cyclase by Escherichia coli heat-stable enterotoxin is regulated by adenine nucleotides. Infect Immun. 1991 Apr;59(4):1552–1557. doi: 10.1128/iai.59.4.1552-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981 Sep 14;132(1):71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Harteneck C., Wedel B., Koesling D., Malkewitz J., Böhme E., Schultz G. Molecular cloning and expression of a new alpha-subunit of soluble guanylyl cyclase. Interchangeability of the alpha-subunits of the enzyme. FEBS Lett. 1991 Nov 4;292(1-2):217–222. doi: 10.1016/0014-5793(91)80871-y. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Yamazaki A. Polymorphism in purified guanylate cyclase from vertebrate rod photoreceptors. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4746–4750. doi: 10.1073/pnas.88.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio Y., Murad F. Purification of guanylyl cyclase from rod outer segments. Biochim Biophys Acta. 1991 Dec 3;1133(1):81–88. doi: 10.1016/0167-4889(91)90244-r. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Heme-dependent activation of soluble guanylate cyclase by nitric oxide: regulation of enzyme activity by porphyrins and metalloporphyrins. Semin Hematol. 1989 Jan;26(1):63–76. [PubMed] [Google Scholar]
- Ivens K., Gazzano H., O'Hanley P., Waldman S. A. Heterogeneity of intestinal receptors for Escherichia coli heat-stable enterotoxin. Infect Immun. 1990 Jun;58(6):1817–1820. doi: 10.1128/iai.58.6.1817-1820.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Colbran J. L., Francis S. H., Corbin J. D. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem. 1992 Jan 15;267(2):1015–1019. [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Nagao S., Umeki S., Nozawa Y., Yazawa M., Yagi K. Ca2+-dependent modulator proteins from Tetrahymena pyriformis, sea anemone, and scallop and guanylate cyclase activation. J Biol Chem. 1981 Jan 10;256(1):19–22. [PubMed] [Google Scholar]
- Kamisaki Y., Saheki S., Nakane M., Palmieri J. A., Kuno T., Chang B. Y., Waldman S. A., Murad F. Soluble guanylate cyclase from rat lung exists as a heterodimer. J Biol Chem. 1986 Jun 5;261(16):7236–7241. [PubMed] [Google Scholar]
- Koch K. W. Purification and identification of photoreceptor guanylate cyclase. J Biol Chem. 1991 May 5;266(13):8634–8637. [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., Goeddel D. V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991 Apr 5;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- Korenfeld M. S., Becker B. Atrial natriuretic peptides. Effects on intraocular pressure, cGMP, and aqueous flow. Invest Ophthalmol Vis Sci. 1989 Nov;30(11):2385–2392. [PubMed] [Google Scholar]
- Krause W. J., Freeman R. H., Fort L. R. Autoradiographic demonstration of specific binding sites for E. coli enterotoxin in various epithelia of the North American opossum. Cell Tissue Res. 1990 May;260(2):387–394. doi: 10.1007/BF00318641. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Kuno T., Andresen J. W., Kamisaki Y., Waldman S. A., Chang L. Y., Saheki S., Leitman D. C., Nakane M., Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986 May 5;261(13):5817–5823. [PubMed] [Google Scholar]
- Kurose H., Inagami T., Ui M. Participation of adenosine 5'-triphosphate in the activation of membrane-bound guanylate cyclase by the atrial natriuretic factor. FEBS Lett. 1987 Jul 27;219(2):375–379. doi: 10.1016/0014-5793(87)80256-9. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Fletcher R. T., Chader G. J., Krishna G. Expression of guanylate cyclase-A mRNA in the rat retina: detection using polymerase chain reaction. Biochem Biophys Res Commun. 1992 Jan 31;182(2):851–857. doi: 10.1016/0006-291x(92)91810-d. [DOI] [PubMed] [Google Scholar]
- Larose L., McNicoll N., Ong H., De Léan A. Allosteric modulation by ATP of the bovine adrenal natriuretic factor R1 receptor functions. Biochemistry. 1991 Sep 17;30(37):8990–8995. doi: 10.1021/bi00101a012. [DOI] [PubMed] [Google Scholar]
- Lax I., Burgess W. H., Bellot F., Ullrich A., Schlessinger J., Givol D. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol. 1988 Apr;8(4):1831–1834. doi: 10.1128/mcb.8.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Chang M. S., Hellmiss R., Chen E., Singh S., Garbers D. L., Goeddel D. V. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989 May;8(5):1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane M., Arai K., Saheki S., Kuno T., Buechler W., Murad F. Molecular cloning and expression of cDNAs coding for soluble guanylate cyclase from rat lung. J Biol Chem. 1990 Oct 5;265(28):16841–16845. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pandey K. N., Singh S. Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J Biol Chem. 1990 Jul 25;265(21):12342–12348. [PubMed] [Google Scholar]
- Radany E. W., Gerzer R., Garbers D. L. Purification and characterization of particulate guanylate cyclase from sea urchin spermatozoa. J Biol Chem. 1983 Jul 10;258(13):8346–8351. [PubMed] [Google Scholar]
- Ramarao C. S., Garbers D. L. Purification and properties of the phosphorylated form of guanylate cyclase. J Biol Chem. 1988 Jan 25;263(3):1524–1529. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Schulz S., Chinkers M., Garbers D. L. The guanylate cyclase/receptor family of proteins. FASEB J. 1989 Jul;3(9):2026–2035. doi: 10.1096/fasebj.3.9.2568301. [DOI] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Shimomura H., Dangott L. J., Garbers D. L. Covalent coupling of a resact analogue to guanylate cyclase. J Biol Chem. 1986 Nov 25;261(33):15778–15782. [PubMed] [Google Scholar]
- Singh S., Lowe D. G., Thorpe D. S., Rodriguez H., Kuang W. J., Dangott L. J., Chinkers M., Goeddel D. V., Garbers D. L. Membrane guanylate cyclase is a cell-surface receptor with homology to protein kinases. Nature. 1988 Aug 25;334(6184):708–712. doi: 10.1038/334708a0. [DOI] [PubMed] [Google Scholar]
- Singh S., Singh G., Heim J. M., Gerzer R. Isolation and expression of a guanylate cyclase-coupled heat stable enterotoxin receptor cDNA from a human colonic cell line. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1455–1463. doi: 10.1016/0006-291x(91)91736-v. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Takayanagi R., Inagami T., Snajdar R. M., Imada T., Tamura M., Misono K. S. Two distinct forms of receptors for atrial natriuretic factor in bovine adrenocortical cells. Purification, ligand binding, and peptide mapping. J Biol Chem. 1987 Sep 5;262(25):12104–12113. [PubMed] [Google Scholar]
- Tang W. J., Krupinski J., Gilman A. G. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem. 1991 May 5;266(13):8595–8603. [PubMed] [Google Scholar]
- Thorpe D. S., Morkin E. The carboxyl region contains the catalytic domain of the membrane form of guanylate cyclase. J Biol Chem. 1990 Sep 5;265(25):14717–14720. [PubMed] [Google Scholar]
- Thorpe D. S., Niu S., Morkin E. Overexpression of dimeric guanylyl cyclase cores of an atrial natriuretic peptide receptor. Biochem Biophys Res Commun. 1991 Oct 31;180(2):538–544. doi: 10.1016/s0006-291x(05)81098-8. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Augustine A., Goeddel D. V., Lowe D. G. Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol Cell Biol. 1991 Jul;11(7):3454–3462. doi: 10.1128/mcb.11.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen P. S., Garbers D. L. Guanylyl cyclase-linked receptors. Annu Rev Neurosci. 1992;15:193–225. doi: 10.1146/annurev.ne.15.030192.001205. [DOI] [PubMed] [Google Scholar]
- Yuen P. S., Potter L. R., Garbers D. L. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry. 1990 Dec 11;29(49):10872–10878. doi: 10.1021/bi00501a002. [DOI] [PubMed] [Google Scholar]
- de Bold A. J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985 Nov 15;230(4727):767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Camerato T. R., Goeddel D. V. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991 Sep 25;266(27):17912–17918. [PubMed] [Google Scholar]


