Abstract
Background:
Typically, fibrin sealants (FSs) and fibrin glues (FGs) are used to strengthen dural repairs during spinal surgery. In 2014, Epstein demonstrated that one FS/FG, Tisseel (Baxter International Inc., Westlake Village, CA, USA) equalized the average times to drain removal and length of stay (LOS) for patients with versus without excess bleeding (e.g. who did not receive Tisseel) undergoing multilevel laminectomies with 1-2 level noninstrumented fusions (LamF).[6]
Methods:
Here Tisseel was utilized to promote hemostasis for two populations; 39 patients undergoing average 4.4 level lumbar laminectomies with average 1.3 level noninstrumented fusions (LamF), and 48 patients undergoing average 4.0 level laminectomies alone (Lam). We compared the average operative time, estimated blood loss (EBL), postoperative drainage, LOS, and transfusion requirements for the LamF versus Lam groups.
Results:
The average operative times, EBL, postoperative drainage, LOS, and transfusion requirements were all greater for LamF versus Lam patients; operative times (4.1 vs. 3.0 h), average EBL (192.3 vs. 147.9 cc), drainage (e.g. day 1; 199.6 vs. 167.4 cc; day 2; 172.9 vs. 63.9 cc), average LOS (4.6 vs. 2.5 days), and transfusion requirements (11 LamF patients; 18 Units [U] RBC versus 2 Lam patients; 3 U RBC).
Conclusions:
Utilizing Tisseel to facilitate hemostasis in LamF versus Lam still resulted in greater operative times, EBL, postoperative average drainage, LOS, and transfusion requirements for patients undergoing the noninstrumented fusions. Although Tisseel decreases back bleeding within the spinal canal, it does not reduce blood loss from LamF decorticated transverse processes.
Keywords: Fibrin sealant, hemostasis, laminectomy alone, length of stay, lumbar surgery, multilevel laminectomy, noninstrumented fusion, stenosis, tisseel
INTRODUCTION
Typically, fibrin sealants (FSs) and fibrin glues (FGs) have been utilized to strengthen repairs of deliberate (e.g. intradural tumors) or traumatic cerebrospinal fluid (CSF) fistulas occurring during spinal surgery. In 2014, Epstein documented that one FS, Tiseeel (Baxter International Inc., Westlake Village, CA, USA), equalized the average times to drain removal and length of stay (LOS) for patients undergoing multilevel laminectomies and 1-2 level noninstrumented fusions (LamF) with versus without increased bleeding (the latter did not receive Tisseel).[6] Here we compared the average operative time, estimated blood loss (EBL), postoperative average drainage, LOS, and transfusion requirements for 39 patients undergoing LamF versus 48 patients undergoing multilevel laminectomies (Lam) alone.
MATERIALS AND METHODS
Thirty-nine patients undergoing multilevel laminectomies/noninstrumented fusions
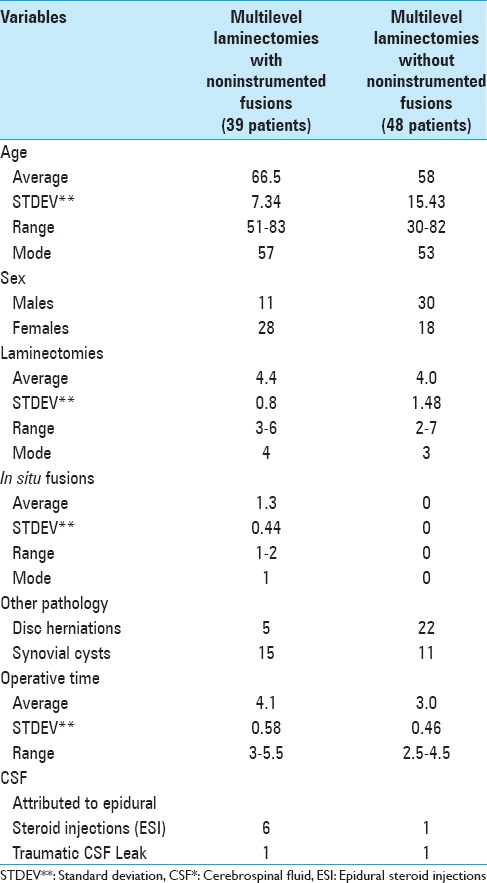
The 39 patients undergoing LamF averaged 66.5 years of age, and included more females than males (28 females, 11 males) [Table 1]. Patients underwent average 4.4 level laminectomies with average 1.3 level noninstrumented fusions. Only 5 patients had accompanying disc herniations, while 15 had synovial cysts. The average operative time, EBL, and average postoperative drainage, LOS, and transfusion requirements were evaluated for these patients.
Table 1.
Clinical data for patients undergoing multilevel laminectomy with versus without noninstrumented fusions using tisseel for hemostasis
Forty-eight patients undergoing multilevel laminectomies alone without fusions
The 48 patients undergoing Lam averaged 58 years of age, and included more males than females (30 males 18 females) [Table 1]. Patients underwent average 4.0 level Lam alone without fusions. A greater number of patients had accompanying disc herniations (22 patients), while fewer had synovial cysts (11 patients) when compared with the LamF group. For Lam patients, the average operative time, EBL, and postoperative average drainage, LOS, and transfusion requirements were also evaluated and compared with these data for LamF.
Use of tisseel to facilitate hemostasis
All patients in both series undergoing multilevel laminectomies with noninstrumented fusions (LamF) or with Lam alone received Tisseel intraoperatively to facilitate hemostasis in the spinal canal (e.g. from the epidural venous plexus or laminectomy bone back-bleeding). Additionally, Tisseel was utilized to facilitate treatment of CSF fistulas for LamF versus Lam patients; six LamF and one Lam patient exhibited CSF fistulas attributed to preoperative epidural steroid injections (ESI), while one patient in each group exhibited a traumatic intraoperative CSF fistula [Table 1].
RESULTS
Greater EBL, postoperative drainage, time to drain removal, and longer length of stay for lamF versus Lam alone patients
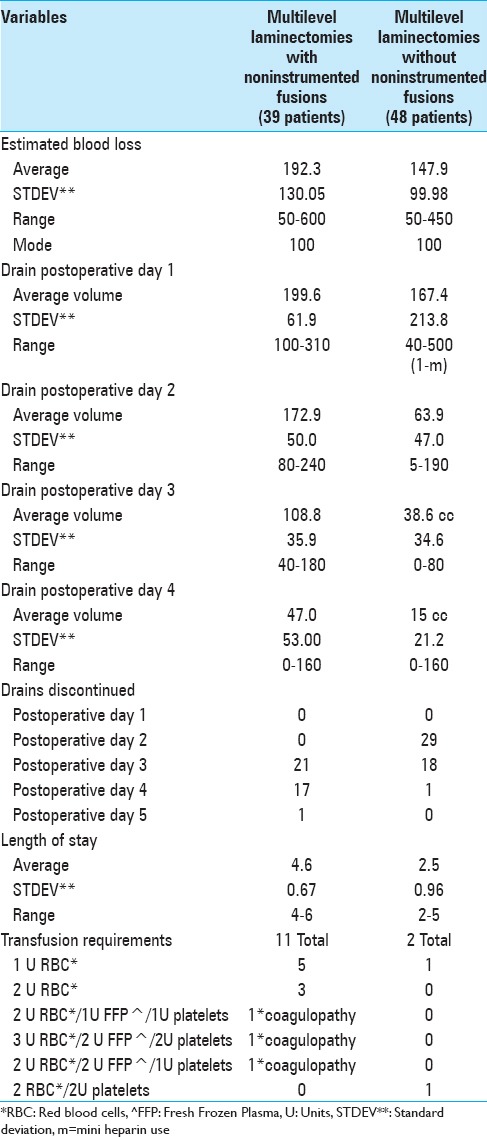
LamF patients exhibited greater EBL, postoperative drainage, longer times to drain removal, and LOS versus Lam alone patients. The intraoperative EBL was greater for LamF versus Lam patients (192.3 vs. 147.9 cc). The average amount of daily postoperative drainage was greater for LamF versus Lam patients particularly on postoperative days 1 and 2 when drains had not yet been removed in any patients; day 1: 199.6 versus 167.4 cc, and day 2; 172.9 versus 63.9 cc [Table 2]. For LamF patients, drains were mostly removed on postoperative days 3–4 (day 3; 21 drains, day 4; 17 drains), while for Lam patients, drains were mostly out by the second and third postoperative days (day 2; 29 drains, day 3; 18 drains). These data also correlated with the longer average LOS of 4.6 days for the LamF patients versus the average LOS of 2.5 days for the Lam alone patients.
Table 2.
Estimated blood loss, postoperative drainage, length of stay, and transfusion requirements following multilevel laminectomy with/without noninstrumented fusions using tisseel for hemostasis
Greater transfusion requirements for LamF versus Lam alone patients
LamF patients additionally exhibited greater transfusion requirements due to back-bleeding from decorticated transverse processes (TP) occurring during noninstrumented fusions versus Lam alone [Table 2]. Eleven of 39 LamF patients required 18 units (U) of packed red blood cells (RBC), 5 U of fresh frozen plasma (FFP), and 4 U of platelets (Plts) versus only 2 of 48 patients undergoing Lam alone requiring 3 U RBC and 2 U Plts (note: one patient with a coagulopathy required 2 U RBC/2 U Plts).
DISCUSSION
Frequency of dural tears
Traumatic dural tears (DT) occur during 1–17% of lumbar spinal surgical procedures, 1% for anterior cervical discectomy/fusion (ACDF), 1.7% for single-level anterior cervical corpectomy/fusion (ACF), and up to 12.5% for multilevel ACF involving resection of ossification of the posterior longitudinal ligament (OPLL).[2,4,9,10] Additionally, Epstein reported that for 33 patients undergoing LamF who received an average of 4.1 (range 2–12) preoperative lumbar ESI, 6 (18.2%) patients had DT documented intraoperatively that warranted surgical repair.[7]
Safety/efficacy of “fibrin sealants” and “fibrin glues” to repair DT
Epstein reviewed the relative safety/efficacy of supplementing/strengthening repairs of spinal DT utilizing two “FSs” or two “FG” [Table 1].[5] The FS included DuraSeal (Confluent Surgical Inc., Waltham, MA, USA) and BioGlue (Cryolife, Kennesaw, GA, USA), while the two FGs included Evicel (Johnson and Johnson Wound Management, Ethicon Inc., Somerville, NJ, USA) and Tisseel (Baxter International Inc., Westlake Village, CA, USA).
Paralytic complications with duraseal (FS)
Although the Food and Drug Administration (FDA) approved Duraseal (FS) for use in the brain and spine in 2009, Duraseal contributed to two cases of reported paralysis due to spinal injections. In 2009, Mulder et al. noted that DuraSeal, applied in the lumbar spine to facilitate DT repair following a laminotomy/diskectomy, migrated superiorly, became “swollen,” and contributed to a cauda equina syndrome 6 days postoperatively.[12] In 2010, Thavarajah et al. used DuraSeal to repair a traumatic anterior C5-C6 cervical diskectomy/fusion CSF fistula; postoperatively, the patient's quadriplegia was similarly due to “swollen” Duraseal, which was then surgically removed.[14]
Use of tisseel for hemostasis in cranial and anterior cervical surgery
Injecting Tisseel facilitated hemostasis both intracranially and in the anterior cervical spine. In 2007, Sekhar et al. safely achieved hemostasis by injecting Tisseel into the cranial epidural space (n = 200 patients), anterior cavernous sinus (n = 46 patients), and vertebral venous plexus (n = 20 patients); however, it resulted in venous occlusion/brain stem infarction when injected into the superior petrosal sinus.[13] In 2008, Yeom et al. showed that Tisseel (2.0 mL of aerosolized spray) reduced postoperative drainage and LOS following 30 multilevel ACDF (3 or more levels) versus 30 comparable controls.[19] Tisseel successfully reduced the total postoperative drainage (47 vs. 98 ml), time to drain removal (17 vs. 24 h), and average LOS (1.2 vs. 2.1 days).[19]
Sandwich versus conventional dural repair techniques utilizing fibrin sealant
Wang et al. compared the efficacy of utilizing the “sandwich” (interlocking suture/FS/FG glue/gelatin sponge/FS/FG glue) versus conventional (interlocking sutures/gelatin sponge) techniques for dural repair following removal of 54 spinal subdural tumors to prevent recurrent postoperative CSF leaks.[17] Sandwich repairs were more effective as they significantly reduced postoperative drainage while avoiding recurrent CSF fistulas.
Tisseel's safety/efficacy in reducing spinal surgical site infections
In 2012 and 2013, two authors noted that Tisseel impregnated with antibiotics reduced the risk of spinal infection.[3,16] In 2012, utilizing both an in-vitro model and a clinical series, Tofuku et al. demonstrated that antibiotic-impregnated FS (AFS) helped prevent instrumented surgical site infections (SSI).[16] When 5 of 10 infected nuts were exposed to vancomycin-impregnated FS, antimicrobial efficacy was documented using agar diffusion testing. Additionally, deep-instrumented SSI were reduced to 0% for 196 clinical procedures utilizing AFS versus a much higher 11 (5.8 %) of 188 patients undergoing instrumented spinal fusions without AFS. In 2013, Cashman et al. utilized in-vitro and in-vivo rat animal models to assess whether FS could effectively administer antibiotics to infected instrumented spinal fusions.[3] Both required mixing cefazolin, fusidic acid, or 5-fluorouracil with Vitagel (Orthovita, Malvern, Pennsylvania, USA) tissue sealant. When exposed to Staphylococcus aureus in vitro (e.g. zone of inhibition), and in vivo (e.g. a rat instrumented spinal fusion model), over a 2- to 4-day period, Vitagel/FS effectively delivered antibacterial activity.
FS and evicel; successful hemostatic agents in total joint surgery
In total joint surgery in 2009, Thoms and Marwin found that FS reduced the time to achieve intraoperative hemostasis, EBL, and the incidence of infections, while facilitating wound healing, and increasing postoperative range of motion.[15] Later, in 2014, Bou Monsef et al. found that the addition of Evicel (Johnson and Johnson Wound Management, Ethicon, Somerville, NJ) reduced blood loss, and therefore, the need for autologous preoperative blood donation (PABD) for anemic patients undergoing total knee replacement (TKR).[1]
Different FS/FG promote intraoperative lumbar spinal hemostasis
Different FS/FG have been utilized to promote intraoperative lumbar spinal hemostasis.[6,8] In 2014, Epstein applied Tisseel in 22 of 39 patients undergoing LamF who exhibited increased intraoperative bleeding; notably, the 17 LamF patients who did not demonstrate increased bleeding did not receive Tisseel. The addition of Tisseel in the LamF versus no Tisseel in the Lam patients equalized the time to drain removal for both groups (e.g. 3.41 days with vs. 3.38 days without Tisseel) along with the LOS (e.g. 5.86 days with vs. 5.82 days without), without increasing the infection rates or the average times to fusion (e.g. 5.9 vs. 5.5 months).[6] Also in 2014, Misusci et al. compared the short/long-term (e.g. 3 months, and 1 year) safety/efficacy of BioGlue versus Tisseel for DT repairs during noninstrumented lumbar fusions in 23 patients.[11] They found that all fistulas in both groups resolved without new neurological deficits or infections, and that patients demonstrated comparable outcomes (Visual Analog Scales, VAS). Additionally in 2014 Wu et al. evaluated 82 consecutive patients undergoing posterior lumbar fusion (PLF) or posterior lumbar interbody fusion (PLIF) who were randomized to receive absorbable gelatin sponge versus no sponge.[18] They found that those receiving the gelatin sponge versus no sponge showed reduced average drainage (173 ml vs. 392 ml), reduced perioperative blood transfusion requirements (34.15% vs. 58.5%), and reduced LOS (12.58 vs. 14.46 days) without adverse sequelae.
Tisseel in LamF patients undergoing noninstrumented postero-lateral fusions does not limit back bleeding from decorticated transverse processes
Despite the use of Tisseel to facilitate hemostasis in the LamF versus Lam groups, noninstrumented fusions with decorticated TP for uniquely contributed to greater operative times, intraoperative EBL, postoperative drainage, LOS, and transfusion requirements. Although LamF versus Lam patients underwent nearly comparable multilevel laminectomies (4.4 vs. 4.0 levels), the average 1.3 level noninstrumented fusions were responsible for these differences. LamF patients exhibited longer operative times (e.g. 1.1 h more), higher average EBL (44.4 cc), greater average daily drainage (day 1: 32.3 cc, day 2: 109 cc), longer times until drain removal (e.g. LamF days 3–4 [38 of 39 patients] vs. Lam days 2–3 [47 of 48 patients]), greater transfusion requirements (11 LamF patients requiring 18 U RBC vs. 2 Lam patients requiring 3U RBC), and longer LOS (average 4.6 vs. 2.5 days).
CONCLUSION
Tisseel, applied in the lumbar spinal canal locally limits drainage from epidural veins and largely stops back bleeding from the exposed edges of laminectomy defects. It cannot, however, limit back bleeding from TP decorticated during the performance of noninstrumented postero-lateral fusions. The intent of this study was to document for LamF versus Lam patients that the noninstrumented fusions contributed to the longer operative times, greater average EBL, higher volume of postoperative drainage, longer LOS, and higher transfusion requirements. These data may prove useful when deciding whether to perform LamF versus Lam alone especially in older patients with greater comorbidities who may not tolerate the greater risks enumerated above associated with the noninstrumented fusions.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/5/172/156561
REFERENCES
- 1.Bou Monsef J, Buckup J, Waldstein W, Cornell C, Boettner F. Fibrin sealants or cell saver eliminate the need for autologous blood donation in anemic patients undergoing primary total knee arthroplasty. Arch Orthop Trauma Surg. 2014;134:53–8. doi: 10.1007/s00402-013-1876-5. [DOI] [PubMed] [Google Scholar]
- 2.Cammisa FP, Jr, Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS. Incidental durotomy in spine surgery. Spine (Phila Pa 1976) 2000;25:2663–7. doi: 10.1097/00007632-200010150-00019. [DOI] [PubMed] [Google Scholar]
- 3.Cashman JD, Jackson JK, Mugabe C, Gilchrist S, Ball K, Tredwell S, et al. The use of tissue sealants to deliver antibiotics to an orthopaedic surgical site with a titanium implant. J Orthop Sci. 2013;18:165–74. doi: 10.1007/s00776-012-0325-6. [DOI] [PubMed] [Google Scholar]
- 4.Dafford EE, Anderson PA. Comparison of dural repair techniques. (00719-5).Spine J. 2013;pii(13):S1529–9430. doi: 10.1016/j.spinee.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Epstein NE. Dural repair with four spinal sealants: Focused review of the manufacturers’ inserts and the current literature. Spine J. 2010;10:1065–8. doi: 10.1016/j.spinee.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Epstein NE. Tisseel utilized as a hemostatic in spine surgery impacts time to drain removal and length of stay. Surg Neurol Int. 2014;5(Suppl 7):S354–61. doi: 10.4103/2152-7806.139668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein NE. Commentary: Unnecessary preoperative epidural steroid injections lead to cerebrospinal fluid leaks confirmed during spinal stenosis surgery. Surg Neurol Int. 2014;5(Suppl 7):S325–8. doi: 10.4103/2152-7806.139622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein NE. Hemostasis and other benefits of fibrin sealants/glues in spine surgery beyond cerebrospinal fluid leak repairs. Surg Neurol Int. 2014;5(Suppl 7):S304–14. doi: 10.4103/2152-7806.139615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannalah D, Lee J, Khan M, Donaldson WF, Kang JD. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am. 2008;90:1101–5. doi: 10.2106/JBJS.F.01114. [DOI] [PubMed] [Google Scholar]
- 10.Jankowitz BT, Atteberry DS, Gerszten PC, Karausky P, Cheng BC, Faught R, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. 2009;18:1169–74. doi: 10.1007/s00586-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miscusi M, Polli FM, Forcato S, Coman MA, Ricciardi L, Ramieri A, et al. The use of surgical sealants in the repair of dural tears during non-instrumented spinal surgery. Eur Spine J. 2014;23:1761–6. doi: 10.1007/s00586-013-3138-1. [DOI] [PubMed] [Google Scholar]
- 12.Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: Case report. Spine (Phila Pa 1976) 2009;34:E144–8. doi: 10.1097/BRS.0b013e31818d5427. [DOI] [PubMed] [Google Scholar]
- 13.Sekhar LN, Natarajan SK, Manning T, Bhagawati D. The use of fibrin glue to stop venous bleeding in the epidural space, vertebral venous plexus, and anterior cavernous sinus: Technical note. Neurosurgery. 2007;61(3 Suppl):E51. doi: 10.1227/01.neu.0000289711.95426.50. [DOI] [PubMed] [Google Scholar]
- 14.Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): A possible complication. Spine (Phila Pa 1976) 2010;35:E25–6. doi: 10.1097/BRS.0b013e3181b9fc45. [DOI] [PubMed] [Google Scholar]
- 15.Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17:727–36. doi: 10.5435/00124635-200912000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Tofuku K, Koga H, Yanase M, Komiya S. The use of antibiotic-impregnated fibrin sealant for the prevention of surgical site infection associated with spinal instrumentation. Eur Spine J. 2012;21:2027–33. doi: 10.1007/s00586-012-2435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HR, Cao SS, Jiang YQ, Li JN, Li XL, Fu YG, et al. A comparison between “sandwich” and conventional methods of repairing spinal dura rupture. Orthop Surg. 2012;4:233–40. doi: 10.1111/os.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Jin Y, Zhang J, Shao H, Yang D, Chen J. Hemostatic Techniques Following Multilevel PosteriorLumbar Spine Surgery: A Randomized Control Trial. J Spinal Disord Tech. 2014;27:442–6. doi: 10.1097/BSD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 19.Yeom JS, Buchowski JM, Shen HX, Liu G, Bunmaprasert T, Riew KD. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: A retrospective matched pair analysis. Spine (Phila Pa 1976) 2008;33:E543–7. doi: 10.1097/BRS.0b013e31817c6c9b. [DOI] [PubMed] [Google Scholar]


