Abstract
Background:
Typical aneurysmal bone cysts (ABCs) are osteolytic, multicystic lesions with parietal sclerosis and blood-filled cysts. In rare instances, the cystic components may be completely absent. Such solid variants in ABC (s-ABC) exhibit a solid architecture; making the clinical, radiological, and histological differentiation from other solid bone tumors like osteosarcoma (especially giant cell rich osteosarcoma) and giant cell tumor, a difficult task.
Case Report:
We report the case of a 45–year-old male presenting with a giant solid cervical spine lesion. Histopathology revealed solid variant of ABC, even though the radiological and fine needle aspiration cytology studies pointed toward a giant cell tumor.
Conclusion:
We aim to discuss the clinical, radiological, and histological findings of solid ABC (a rare benign entity) vis-à-vis the common neoplastic entities of osteosarcoma and giant cell tumor. The histopathological nuisances in making the diagnosis of s-ABC are put forth, along with its impact on management of such giant bony spinal lesions.
Keywords: Bony tumors of the cervical spine, pathology of solid variant of aneurysmal bone cyst, solid variant of aneurysmal bone cyst, spinal aneurysmal bone cysts
INTRODUCTION
The conventional aneurysmal bone cyst (ABC) is a nonneoplastic bony lesion, characterized by cavernous spaces with fibrous walls, intermixed with bone and giant cells.[9] Rarely, ABCs may be solid, with predominant fibroblastic proliferation, giant cells, and areas of heterotopic calcification; and hence, may be easily confused with spindle cell neoplasms like giant cell rich osteosarcoma and giant cell tumor (GCT).[7,9] Very few cases of solid variant of ABC (s-ABC) involving the vertebral column have been reported.[1,4,6,8,9] We report a case of s-ABC presenting as a giant cervical spine lesion and present the clinical, radiological and histological findings of this rare benign entity vis-à-vis the common neoplastic entities of osteosarcoma and GCT, and discuss its diagnostic implications.
CASE REPORT
Clinical presentation
A 45-year-old male presented with a gradually progressive, painless, firm, lobulated swelling over the left side of neck, of one year duration; without any radiculopathy or myelopathy.
Investigations
Computed tomography (CT) of neck showed a large expansile lytic lesion with epicentre in the left pedicle and body of C4 vertebrae, extending into the lamina and spinous process [Figure 1a–c]. The lesion had large prevertebral and paravertebral soft tissue components causing anterior displacement of the carotid vessels and partial encasement of the V2 segment of the left vertebral artery [Figure 1c]. On magnetic resonance imaging (MRI), the lesion was iso- to hypo-intense on T1W images and heterogenously iso- to hyper-intense on T2W images; with intense, relatively homogenous, postcontrast enhancement [Figure 1d–f]. There was an intraspinal epidural component displacing the spinal cord postero-laterally to the right. With the working diagnosis of a primary bone tumor (GCT/osteosarcoma), fine needle aspiration (FNA) was attempted. FNA cytology showed numerous osteoclast-like multinucleated giant cells with stromal fragments dispersed with blood and hemosiderin laden macrophages suggesting GCT.
Figure 1.
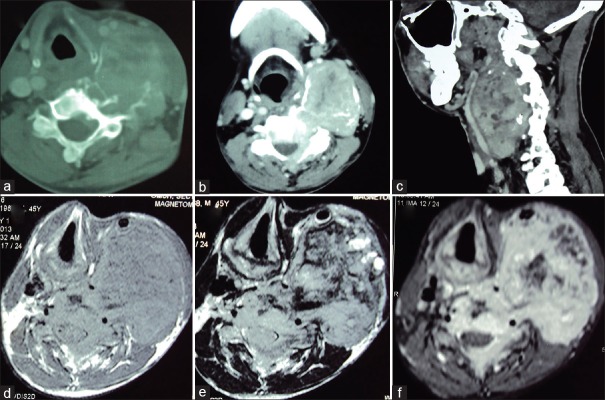
Computed tomography axial bone window (a) and soft-tissue mediastinal window (b) sections show an expansile, osteolytic lesion arising from the left lateral arch elements of C4 vertebra having an enhancing soft tissue component. The cranio-caudal extent and displacement of vascular and soft tissue structures is appreciated on sagittal CT reconstruction (c) image. Axial T1-weighted precontrast (d), T2-weighted (e) and T1-weighted postcontrast (f) images better demonstrate the extent of the soft tissue component
Treatment
Through anterolateral approach, C4-C5 corpectomy and radical excision of the tumor along with excision of left side pedicle of C4 and C5 was performed by the senior author (PS). Grossly, tumor was solid, fibrous, and highly vascular. Spine was stabilized with C3 to C6 anterior cervical plating with interposed iliac bone graft. Postoperatively, the patient developed transient hoarseness of voice, which resolved over time.
Histopathology
Histology revealed marked fibroblastic proliferation and scattered giant cells, with presence of focal osteoid production, which was not associated with malignant cells [Figure 2a]. A few blood-filled spaces, rimmed by giant cells and hemosiderin laden macrophages, were also seen [Figure 2b].
Figure 2.
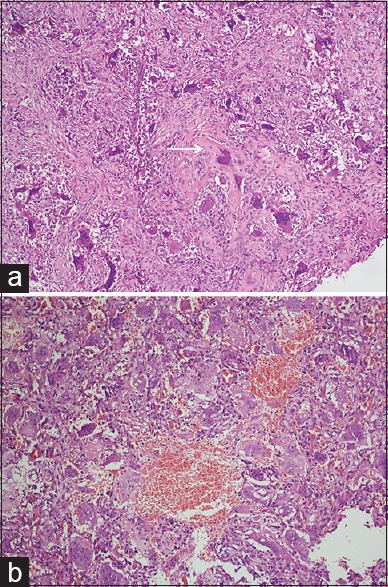
(a) Photomicrograph showing scattered multinucleated osteoclastic giant cells and focal osteoid production (arrow) in a background of fibroblastic proliferation (H and E ×10). (b) A few dilated blood filled spaces are seen lined by multinucleated osteoclastic giant cells (H and E ×10). The absence of atypia is key to diagnosis of s-ABC
Follow-up
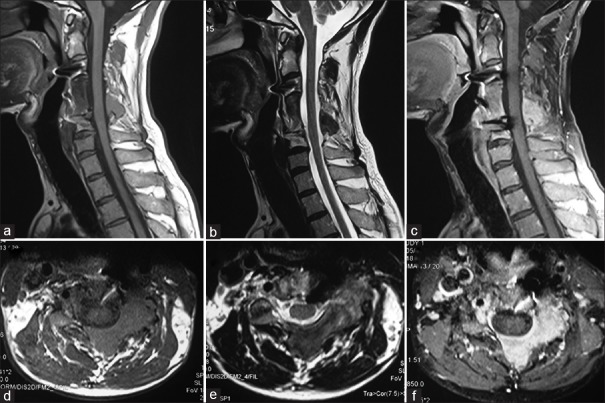
Postoperative Positron Emission Tomography (PET)-CT scan showed small, solitary, residual FDG (Fluorodeoxyglucose) avid lesion over the posterior elements of C4 and C5 vertebrae. Follow-up MRI at 10 months showed residual lesion centered at the left side lamina and spinous process of C4 and C5 vertebrae with no significant associated soft tissue component or recurrence [Figure 3a–f]. Regular follow-up of the patient is planned, till there is any recurrence or patient becomes symptomatic for the small residual lesion.
Figure 3.
Follow-up MR imaging (at 10 months postsurgery): Sagittal T1-weighted (a), T2-weighted (b) and T1-weighted postcontrast (c) images depict spinal fixation with decompressed spinal cord. The T1-weighted (d), T2-weighted (e) and T1-weighted postcontrast (f) axial sections confirm the residual lesion along the left lateral arch of the vertebra with associated small soft tissue component
DISCUSSION
ABCs are expansile, benign, tumor-like lesions, commonly affecting long bones around the knee and occurring rarely in the vertebral column.[9] Typical ABCs are osteolytic lesions, composed of vesicular structural lacunae with para-osseous tumor expansion, with well-defined adjacent normal bone.[6] On gross examination, they are multicystic lesions with some parietal sclerosis and blood-filled cysts. Histology shows multiple, cystoid structures without endothelial lining, along with fibromyxoid stroma embedded with osteoclastic giant cells.
In rare cases, the cystic components may be completely absent, and the tumor exhibits a solid architecture; making it impossible to differentiate from other solid bone tumors like osteosarcoma, GCT, etc.[7] Sanerkin et al., in 1983, first described the ‘solid variant’ of ABC, which differs from the characteristic histological picture seen in the classical ABC.[7] Solid ABC, consists of a fibroblastic lesion with scattered osteoclastic, osteoblastic, and fibromyxoid elements, without a predominant component of cavernous channels, and such solid, bony, ‘giant cell’-rich lesions can easily be mistaken for GCTs or ‘giant cell’ rich osteosarcoma.[4]
Clinical considerations
Solid variants of ABC are rare lesions, accounting for 3.4–7.5% of all ABCs, and most commonly affect long bones like femur and tibia.[1,4,9] The spine is very rarely affected, with only 15 cases reported till date.[4,9] Solid ABCs have been known to occur exclusively in the pediatric age group, with a predilection for the female sex.[4] Half of the reported cases have occurred in the thoracic spine.[4] Solid ABCs, similar to the conventional ABC, usually originate from the posterior elements of the vertebrae and involvement of the vertebral body is rare.[9] Our patient was an adult male, with the lesion arising from the body and pedicle of the fourth cervical vertebra.
Radiological considerations
Routine radiography and CT shows an expansile, osteolytic lesion and both conventional and solid variant ABCs appear similar on it.[4,9] On MRI, these lesions are T1 hypointense and T2 hyperintense, except that instead of septations, solid variants appear as solid lesions with homogenous contrast enhancement. MRI aids in differentiating the two morphological extremes of ABCs.[9] However, s-ABC closely resembles other solid primary bony tumors; and differentiating these solid lesions based solely on radiology is a daunting task, as seen in our case. Soft pointers toward the same are a very well-demarcated lesion, slow growing nature, and presence of intralesional ‘fluid–fluid’ levels.
Histopathological considerations
Among the differentials, giant-cell rich osteosarcoma, GCT, and s-ABC may show overlapping histological features. GCT shows many multinucleated, osteoclastic giant cells, admixed with round to slightly oval stromal cells showing similar nuclear character.[3] The giant cells are uniformly distributed throughout the lesion and the mononuclear stromal cells show relatively monomorphic nuclei. The term ‘giant cell-rich osteosarcoma’ is reserved for those osteosarcomas that contain abundant osteoclast-like giant cells distributed throughout the tumor.[2,5] At low-power view, these lesions show multinucleated giant cells simulating a GCT; but on high-power view, cytologic anaplasia of the stromal cells and malignant osteoid production can usually be identified.[5] The tumor cells show nuclear pleomorphism and are usually mitotically active.[2] The giant cells are seen scattered within the lesion. S-ABC is characterized by extensive fibroblastic proliferation along with scattered osteoclastic giant cells. There may be focal osteoid production, but it is not associated with obvious malignant cells.[9] The absence of cytologic atypia in s-ABC should prevent its being mistaken for conventional osteosarcoma.[1] Small areas of blood-filled spaces lined by multinucleated giant cells may or may not be seen. In our case, there was marked fibroblastic proliferation and scattered giant cells. Focal osteoid production was present, but not associated with malignant cells. A few blood-filled spaces rimmed by giant cells and hemosiderin laden macrophages were also seen. Features thus favored a diagnosis of solid variant of ABC over GCT or giant-cell rich osteosarcoma.
Treatment strategy
For giant, solid, bony lesions involving the cervical spine (like the one described above), maximally safe resection followed by spinal stabilization should be the goal. The primary reason for this strategy is the fact that even after complete radiological evaluation, the pathology may vary within a wide spectrum, from benign (like s-ABC) to malignant (like giant-cell rich osteosarcoma). Hence, maximum benefit should be offered to the patient at first surgery. Adjuvant radiotherapy is not prescribed in view of benign nature of the lesion; and incidences of postradiation sarcoma and postradiation myelopathy. Long-term follow-up (up to 6 years) is available in literature, after treatment with resection or radiation; and it shows good prognosis, with no recurrence of lesions.[4,7,9] The differentials like GCT and giant-cell rich osteosarcoma are highly malignant lesions requiring adjuvant therapies like radiation and chemotherapy.
Our patient has shown a good recovery after surgery and the follow-up MRI at 10-month follow-up shows adequate spinal cord decompression, without any increase in size of small residual posterior lesion.
CONCLUSION
Misdiagnosis of spinal s-ABC with solid bony tumors like GCT and giant-cell rich osteosarcoma is common. Hence, s-ABC should always be considered as a differential diagnosis in solid bony spinal lesions. Radiologically, these lesions appear similar; thus, histology needs to be carefully reviewed to differentiate between them. Though the initial treatment strategy in the form of maximally safe radical resection is the same for all three, the long-term outcome and recurrence rate is definitely better with s-ABC.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/5/182/156570
Contributor Information
Amey R. Savardekar, Email: ameysavardekar@gmail.com.
Deviprasad Patra, Email: devosy4ever@gmail.com.
Debajyoti Chatterjee, Email: devchat1984@gmail.com.
Chirag K. Ahuja, Email: chiragkahuja@gmail.com.
Pravin Salunke, Email: drpravinsalunke@yahoo.co.uk.
REFERENCES
- 1.Bertoni F, Bacchini P, Capanna R, Ruggieri P, Biagini R, Ferruzzi A, et al. Solid variant of aneurysmal bone cyst. Cancer. 1993;71:729–34. doi: 10.1002/1097-0142(19930201)71:3<729::aid-cncr2820710313>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Feng D, Yang X, Liu T, Xiao J, Wu Z, Huang Q, et al. Osteosarcoma of the spine: Surgical treatment and outcomes. World J Surg Oncol. 2013;11:89. doi: 10.1186/1477-7819-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junming M, Cheng Y, Dong C, Jianru X, Xinghai Y, Quan H, et al. Giant cell tumor of the cervical spine: A series of 22 cases and outcomes. Spine (Phila Pa 1976) 2008;33:280–8. doi: 10.1097/BRS.0b013e318162454f. [DOI] [PubMed] [Google Scholar]
- 4.Karampalis C, Lenthall R, Boszczyk B. Solid variant of aneurysmal bone cyst on the cervical spine of a child: Case report, differential diagnosis and treatment rationale. Eur Spine J. 2013;22:523–31. doi: 10.1007/s00586-012-2548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinra P, Valdamani S, Singh V, Dutta V. Diaphyseal giant cell-rich osteosarcoma: Unusual histological variant in an unusual site. Indian J Pathol Microbiol. 2012;55:600–2. doi: 10.4103/0377-4929.107848. [DOI] [PubMed] [Google Scholar]
- 6.Pennekamp W, Peters S, Schinkel C, Kuhnen C, Nicolas V, Muhr G, Frangen TM. Aneurysmal bone cyst of the cervical spine (2008:7b) Eur Radiol. 2008;18:2356–60. doi: 10.1007/s00330-008-0944-7. [DOI] [PubMed] [Google Scholar]
- 7.Sanerkin NG, Mott MG, Roylance J. An unusual intraosseous lesion with fibroblastic, osteoclastic, osteoblastic, aneurysmal and fibromyxoid elements: “Solid” variant of aneurysmal bone cyst. Cancer. 1983;51:2278–86. doi: 10.1002/1097-0142(19830615)51:12<2278::aid-cncr2820511219>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Sato K, Sugiura H, Yamamura S, Takahashi M, Nagasaka T, Fukatsu T. Solid variant of an aneurysmal bone cyst (giant cell reparative granuloma) of the 3 rd lumbar vertebra. Nagoya J Med Sci. 1996;59:159–65. [PubMed] [Google Scholar]
- 9.Suzuki M, Satoh T, Nishida J, Kato S, Toba T, Honda T, et al. Solid variant of aneurysmal bone cyst of the cervical spine. Spine (Phila Pa 1976) 2004;29:E376–81. doi: 10.1097/01.brs.0000137053.08152.a6. [DOI] [PubMed] [Google Scholar]