Abstract
Objectives
Physician specialty is often positively associated with disease-specific outcomes and negatively associated with primary care outcomes for people with chronic conditions. People with HIV have increasing comorbidity arising from antiretroviral therapy (ART) related longevity, making HIV a useful condition to examine shared care models. We used a previously described, theoretically developed shared care framework to assess the impact of care delivery on the quality of care provided.
Design
Retrospective population-based observational study from 1 April 2009 to 31 March 2012.
Participants
13 480 patients with HIV and receiving publicly funded healthcare in Ontario were assigned to one of five patterns of care.
Outcome measures
Cancer screening, ART prescribing and healthcare utilisation across models using adjusted multivariable hierarchical logistic regression analyses.
Results
Models in which patients had an assigned family physician had higher odds of cancer screening than those in exclusively specialist care (colorectal cancer screening, exclusively primary care adjusted OR (AOR)=3.12, 95% CI (1.90 to 5.13), family physician-dominant co-management AOR=3.39, 95% CI (1.94 to 5.93), specialist-dominant co-management AOR=2.01, 95% CI (1.23 to 3.26)). The odds of having one emergency department visit did not differ among models, although the odds of hospitalisation and HIV-specific hospitalisation were lower among patients who saw exclusively family physicians (AOR=0.23, 95% CI (0.14 to 0.35) and AOR=0.15, 95% CI (0.12 to 0.21)). The odds of antiretroviral prescriptions were lower among models in which patients’ HIV care was provided predominantly by family physicians (exclusively primary care AOR=0.15, 95% CI (0.12 to 0.21), family physician-dominant co-management AOR=0.45, 95% CI (0.32 to 0.64)).
Conclusions
How care is provided had a potentially important influence on the quality of care delivered. Our key limitation is potential confounding due to the absence of HIV stage measures.
Keywords: Human Immunodeficiency Virus, PRIMARY CARE, chronic disease, comorbidity, health services delivery
Strengths and limitations of this study.
Population-based study capturing almost all people living with HIV and receiving care in Ontario, the Canadian province with the highest number of HIV-positive individuals.
Longitudinal and comprehensive healthcare utilisation.
Limited ability to capture HIV-specific morbidity may result in residual confounding arising from unmeasured disease stage indicators, such as CD4 cell count.
Cannot identify people with HIV not receiving care or unaware of their HIV-positive status.
Introduction
Patients with chronic disease often see more than one physician for their care. Those with single conditions who receive care from specialist physicians typically have improved disease-specific outcomes.1 The proportion of patients with chronic disease with single conditions and those who are treated exclusively by a specialist is low;2 3 however, these patients are less likely to have needs outside of these specialist's scope of practice met.3 4 Despite the belief that shared care by family physicians and specialists should lead to improved condition-specific and more general outcomes, evidence of this is lacking.5 Consensus remains, however, that sharing care may mitigate gaps from seeing either provider alone, and that a primary care foundation is required to effectively and economically balance specialist expertise.6–9
As people with HIV on combination antiretroviral therapy (ART) have lifespans that approach those of HIV-uninfected individuals,10 they are likely to acquire comorbidities related to ageing as well as from the effects of HIV and its treatment.11–13 As with other chronic conditions, there is evidence that HIV specialists and those providers with more HIV experience provide higher quality of care as measured by HIV-specific indicators.14–16 However, there is increasing recognition that some care needed for people with HIV falls outside the scope and comfort of many HIV specialists.17–21 The literature also demonstrates that patients with chronic conditions who do not have a regular family physician are more likely to have emergency department visits and hospital admissions.22 As such, it is essential that we study to what extent different patterns of care address the needs of this complex population.
Administrative data have been used to measure care for several populations in Ontario. For example, it has been used to study the relative extent to which patients with chronic conditions receive care from specialists and family physicians, as well as the quality of care provided.22 23 Building on a theoretical framework of the specialist–primary care physician interface,24 we have previously developed and characterised a typology of care for people with HIV based on actual patterns of care.25 We delineated the following care models: exclusively primary care, shared care with the family physician being the dominant HIV physician, shared care with the HIV specialist being the dominant HIV physician, exclusively specialist care and low engagement. We found that most HIV patients were linked to their usual family physician, and few saw exclusively HIV specialists.
Most of the existing literature exploring outcomes for specialist versus generalist care has used a dichotomous approach, ignoring the reality that many patients with chronic disease require care from more than one physician to meet their varied needs. Furthermore, the lack of standardisation of provider terminology in the HIV literature has complicated delineation of which physicians are currently providing care.26 For example, studies describe ‘HIV primary care’ regardless of the specialty of the physician. Our typology overcomes these issues by using a continuum of shared care provided by physician specialty. The main objective of this study is to assess the delivery of care as described by this typology, in particular, to examine differences in health services delivery and adherence to cancer screening among the main typology models. This evaluative approach allows for adjustment of factors known to affect the receipt of primary care services, including patient demographic and clinical characteristics, as well as the intensity of outpatient visits.
Methods
Data sources
We used the administrative databases at the Institute for Clinical Evaluative Sciences (ICES) for this cross-sectional study. These databases are made available to accredited researchers through a data sharing agreement with the Ontario Ministry of Health and Long Term Care. These individual-level data are linked using a coded identification number in accordance with the provincial Personal Health Information Protection Act. The databases used include the Registered Persons Database, which includes mortality and demographic data for all residents eligible for provincial healthcare, the Ontario Health Insurance Plan (OHIP) billing claims system, which contains 95% of physician services conducted in the province; the Discharge Abstract Database, which captures all provincial hospital discharge data; the National Ambulatory Care Reporting System, which contains information on visits to emergency departments; Citizen and Immigration Canada data, which contain information on individuals granted permanent residency in Canada; the Client Agency Program Enrolment registry, which tracks patient enrolment to individual family physicians; the ICES Physician Database, which comprises information from the OHIP Corporate Provider Database (CPDB), the Ontario Physician Human Resource Data Centre (OPHRDC) database and the OHIP database of physician billings regarding physician demographics, training and practice setting; and the Ontario Drug Benefits (ODB), claims database of prescriptions to individuals covered by the public system (those aged 65 years and older and those receiving social assistance (Ontario Works, Ontario Disability Support Program or the subsidised Trillium programme)).
Eligible population
We identified eligible individuals in Ontario from the Registered Persons Database. Using OHIP billing claims, we applied a previously validated algorithm to people 18 years of age and older and living in Ontario between 1 April 1992 and 31 March 2012.27 The algorithm has a sensitivity of 96.2% (95% CI 95.2% to 97.9%) and specificity of 99.6% (95% CI 99.1% to 99.8%) for identifying people with HIV and receiving care in Ontario. We excluded patients with an invalid or out-of-province residence on 1 July 2009 (n=277). As physicians in community health centres (CHCs) do not provide billing to OHIP, we excluded those patients within our cohort who were known to be receiving care in CHCs between 2008 and 2010 (n=17).28 Furthermore, we excluded patients who died during the 3-year study period (n=510) to avoid misclassifying their typology of care based on censored visit pattern data.
Assignment of patients to a typology category
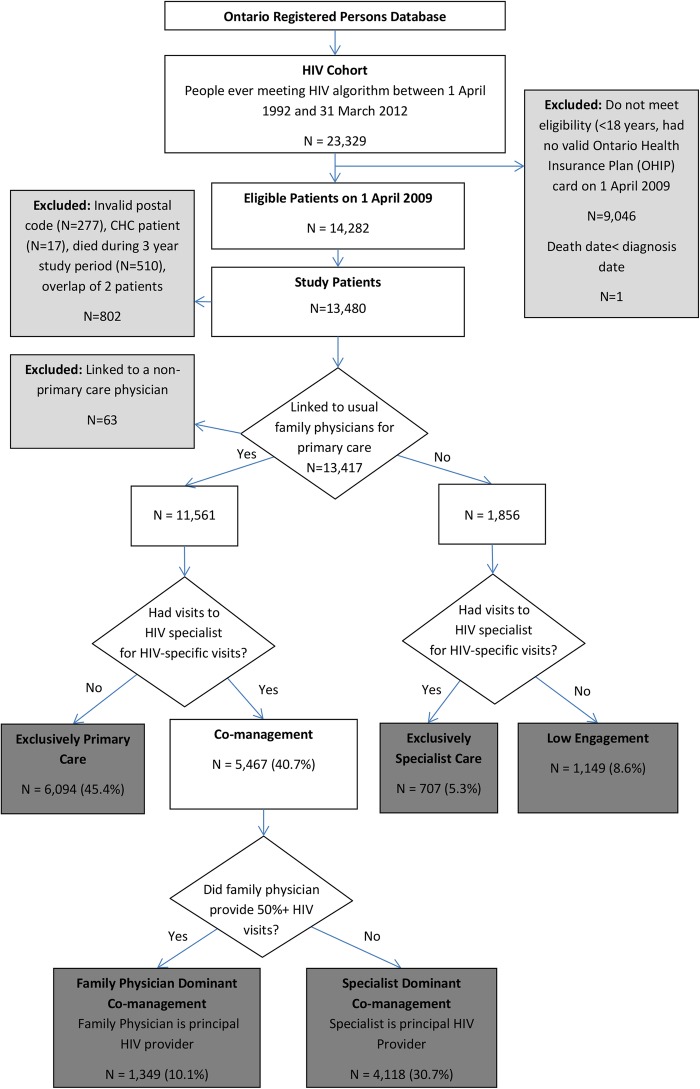
We used the OHIP database to identify all outpatient healthcare visits made by cohort patients between 1 April 2009 and 31 March 2012. We then used a previously reported and validated approach to assign patients to one of five typology models (table 1):25 exclusively primary care, family physician-dominant co-management, specialist-dominant co-management, exclusively specialist care and low engagement. Briefly, patients were assigned based on their outpatient healthcare visits, including the specialty of the physician seen (primary care or HIV specialist) and their billing codes submitted (HIV related or not) for the study period of 1 April 2009 to 31 March 2012 (figure 1). Patients were assigned to a usual provider of primary care if they were contractually rostered to a family physician, or if they had at least 50% of their primary care services provided by one individual family physician. In contrast to the USA, internal medicine specialists in Canada are primarily consultant physicians, and do not routinely provide ongoing primary care; thus, both infectious disease and internal medicine specialists are included as HIV specialists in our models.
Table 1.
Typology of specialist–primary care physician interface
| Primary care- dominant models | 1. Exclusively primary care—patient is assigned to a regular family physician who provides most care; no infectious disease or internal medicine physician provides any HIV care |
| 2. Family physician-dominant co-management—patient is assigned to a regular family physician who provides the majority (50% or more) of HIV-related care; specialist physician provides some HIV care | |
| Specialist- dominant models | 3. Specialist-dominant co-management—patient is assigned to a regular family physician, but specialist physician provides the majority (50% or more) of HIV-related care |
| 4. Specialist care only—patient is not assigned to a regular family physician; specialist physician provides all HIV-related care | |
| 5. Low engagement—patient is not assigned to a regular family physician and has no specialist physician providing HIV care |
Figure 1.
Flow diagram of study participants and typology assignments (CHC, community health centre).
Patient characteristics
Patient age, sex and postal code on 1 April 2009 were obtained from the Registered Persons Database (RPDB). We used postal codes at the neighbourhood level linked to 2006 Statistics Canada census data to assign income quintiles and rurality scores. Rurality was assigned categorically into major urban areas (score 0–9), non-major urban areas (10–39) and rural areas (40 or higher) according to the Rurality Index of Ontario.29
We used data from Citizenship and Immigration Canada to identify recent immigrants as well as immigrants from HIV-endemic regions of Africa and the Caribbean. This group represents a growing proportion of new and prevalent HIV infections in Canada, is often demographically different from other groups and has health outcomes that differ from other groups living with HIV.30–33
We used the ODB claims database to identify individuals who were eligible for public drug coverage.
The Johns Hopkins Adjusted Clinical Group System was used to ascertain comorbidity by assigning patients to up to 32 distinct Aggregated Diagnosis Groups (ADGs).34 We assigned patients to low (<5 ADGs), medium (6–9 ADGs) and high (≥10 ADGs) comorbidity categories.34 35 People with mental health conditions between 1 April 2007 and 1 April 2009 were broadly captured using an algorithm previously validated to identify people receiving mental health services in the primary care setting.36
We used OHIP billing claims to identify the number of outpatient visits patients made to the family physicians and HIV specialists to whom they were assigned during the study period.
Physician specialty
We obtained information about self-designated physician specialty for the fiscal year 2009 from the ICES Physician Database.
Outcome measures
Outcomes were selected using preselected indicators of health services delivery and technical quality of primary care using administrative data in the primary care setting.37 Quality of primary care outcomes (cancer screening) included: adherence to colorectal cancer screening (ascertained as one screening test (faecal occult blood or colonoscopy) over 2 years for eligible individuals aged 50–74 years), adherence to breast cancer screening (ascertained as one mammography test over 2 years for eligible women aged 50–69 years) and adherence to cervical cancer screening (ascertained as one cervical screening test over 2 years in eligible women aged 21–69 years). Health service utilisation outcomes were measured over the latter 18 months of the 3-year study period (1 October 2010–31 March 2012) and included: any emergency department visits, any low-acuity emergency department visits, any hospital admissions (excluding maternity and same day surgery) and any HIV-specific hospitalisations (defined as any hospitalisation for which HIV was the primary diagnosis). Finally, one HIV-specific outcome, the receipt of any ART prescription among those eligible for ODB (n=8302), was included.
Statistical analysis
Descriptive statistics were generated to compare patient demographic and clinical characteristics among the five typologies. Summary measures of the outcome measures (frequencies and proportions) were calculated for patients in each of the typologies.
We conducted multivariable hierarchical logistic regression analyses to examine the association between typology category and quality of care outcomes. We excluded patients in the low engagement category from this analysis, because their healthcare utilisation and outcome patterns differed significantly from the other models. Family physicians and HIV specialists were modelled as random effects, where possible, to account for clustering of patients by providers. Typology category, modelled as a four-level categorical variable, was entered as the main effect of interest. We estimated unadjusted and adjusted ORs for typology, together with 95% CIs. We adjusted for the following patient covariates: age, sex, neighbourhood income quintile, rurality, immigrant status, comorbidity, mental health comorbidity, as well as the number of outpatient visits. The method of pseudolikelihood estimation was used to estimate the models. If a model adjusting for the two sources of clustering failed to converge, we specified only the family physician as a random effect. All statistical analyses used the SAS procedure GENMOD using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 13 480 individuals were eligible for our study on 1 April 2009 and were assigned to typology categories as described in figure 1. The characteristics of HIV patients differed among the care models, as shown in table 2. Both specialist-dominant models (exclusively specialist care and specialist-dominant co-management) had the highest proportions of female patients, those in younger age categories, those living in the lowest income neighbourhoods and non-major urban settings, and non-Canadian born patients, in particular those originating from Africa and the Caribbean. The family physician-dominant co-management model had the highest proportion of patients in the highest comorbidity category.
Table 2.
Patient characteristics among typology models
| Patient characteristics | Exclusively primary care | Family physician- dominant co-management | Specialist-dominant co-management | Exclusively specialist care | Low engagement |
|---|---|---|---|---|---|
| N=6094 | N=1349 | N=4118 | N=707 | N=1149 | |
| Male sex | 5128 (84.1%) | 1194 (88.5%) | 3013 (73.2%) | 525 (74.3%) | 959 (83.5%) |
| Age category (years) | |||||
| 18–35 | 1064 (17.5%) | 169 (12.5%) | 753 (18.3%) | 161 (22.8%) | 166 (14.4%) |
| 36–49 | 3265 (53.6%) | 705 (52.3%) | 2136 (51.9%) | 387 (54.7%) | 617 (53.7%) |
| 50–65 | 1523 (25.0%) | 439 (32.5%) | 1092 (26.5%) | 149 (21.1%) | 317 (27.6%) |
| >65 | 242 (4.0%) | 36 (2.7%) | 137 (3.3%) | 10 (1.4%) | 49 (4.3%) |
| Neighbourhood income quintile | |||||
| Quintile 1 (lowest) | 1623 (26.6%) | 430 (31.9%) | 1538 (37.3%) | 288 (40.7%) | 373 (32.5%) |
| Quintile 2 | 1236 (20.3%) | 248 (18.4%) | 881 (21.4%) | 156 (22.1%) | 218 (19.0%) |
| Quintile 3 | 1025 (16.8%) | 220 (16.3%) | 638 (15.5%) | 92 (13.0%) | 206 (17.9%) |
| Quintile 4 | 1024 (16.8%) | 193 (14.3%) | 526 (12.8%) | 93 (13.2%) | 174 (15.1%) |
| Quintile 5 (highest) | 1129 (18.5%) | 238 (17.6%) | 510 (12.4%) | 75 (10.6%) | 159 (13.8%) |
| Missing | 57 (0.9%) | 20 (1.5%) | 25 (0.6%) | ≤5 | 19 (1.7%) |
| Rurality index | |||||
| Major urban | 5553 (91.1%) | 1263 (93.6%) | 3596 (87.3%) | 617 (87.3%) | 1030 (89.6%) |
| Non-major urban | 450 (7.4%) | 61 (4.5%) | 409 (9.9%) | 73 (10.3%) | 86 (7.5%) |
| Rural | 80 (1.3%) | 20 (1.5%) | 96 (2.3%) | 16 (2.3%) | 30 (2.6%) |
| Missing | 11 (0.2%) | ≤5 | 17 (0.4%) | ≤5 | ≤5 |
| Immigrant status | |||||
| Canadian born | 5145 (84.4%) | 1144 (84.8%) | 3009 (73.1%) | 505 (71.4%) | 968 (84.2%) |
| Immigrant from Africa or the Caribbean | 413 (6.8%) | 108 (8.0%) | 718 (17.4%) | 153 (21.6%) | 90 (7.8%) |
| Immigrant from Europe and Western Nations | 132 (2.2%) | 22 (1.6%) | 75 (1.8%) | 13 (1.8%) | 36 (3.1%) |
| Immigrant from other nations | 404 (6.6%) | 70 (5.2%) | 315 (7.6%) | 36 (5.1%) | |
| Mental health condition | 2384 (39.1%) | 675 (50.0%) | 1688 (41.0%) | 216 (30.6%) | 160 (13.9%) |
| Number of ADGs | |||||
| High | 1167 (19.1%) | 496 (36.8%) | 1121 (27.2%) | 145 (20.5%) | 76 (6.6%) |
| Medium | 1968 (32.3%) | 502 (37.2%) | 1547 (37.6%) | 166 (23.5%) | 66 (5.7%) |
| Low | 2959 (48.6%) | 351 (26.0%) | 1450 (35.2%) | 396 (56.0%) | 1007 (87.6%) |
| Number of visits to the usual family physician (mean, SD) | 13.6±13.4 | 19.6±15.1 | 8.6±15.9 | NA | NA |
| Number of visits to HIV specialists (mean, SD) | NA | 4.9±4.5 | 9.1±6.1 | 8.2±6.9 | NA |
ADGs, adjusted diagnosis groups; NA, not available.
Prevalence of outcomes varied by patients among typology models (table 3). Patients in exclusively specialist care had the lowest observed proportions of all cancer screening manoeuvres. Models with two providers (both family physician and HIV specialist) had the highest observed proportion of having an emergency department visit. The observed proportions of patients with any low-acuity emergency department visit, hospital admission and HIV-specific hospital admission were lowest among patients in exclusively primary care. The observed proportion of patients prescribed ART when eligible for public drug coverage was lowest among patients in exclusively primary care. Patients in low engagement primary care had uniformly poor quality of care on all measures, and were not compared with other care delivery models in further analyses.
Table 3.
Prevalence of quality indicators among typology models
| Prevalence of quality indicator (N reflects the number eligible for the outcome) | Exclusively primary care | Family physician-dominant co-management | Specialist-dominant co-management | Exclusively specialist care | Low engagement |
|---|---|---|---|---|---|
| N=6094 | N=1349 | N=4118 | N=707 | N=1149 | |
| Cancer screening outcomes | |||||
| Colorectal cancer screening (N=2829) | 478 (42.2%) | 125 (48.1%) | 306 (33.1%) | 24 (16.2%) | 12 (3.3%) |
| Cervical cancer screening (N=2323) | 421 (50.9%) | 66 (45.8%) | 464 (46.0%) | 57 (33.7%) | 33 (18.9%) |
| Mammography (N=591) | 139 (55.4%) | 26 (63.4%) | 112 (48.7%) | 10 (32.3%) | 4 (10.5%) |
| Health services delivery outcomes | |||||
| Any emergency department visits | 1815 (29.8%) | 483 (35.8%) | 1452 (35.3%) | 221 (31.3%) | 122 (10.6%) |
| Any low-acuity emergency department visits | 876 (14.4%) | 233 (17.3%) | 714 (17.3%) | 119 (16.8%) | 70 (6.1%) |
| Any hospital admissions | 470 (7.7%) | 226 (16.8%) | 479 (11.6%) | 82 (11.6%) | 33 (2.9%) |
| Any HIV-specific hospital admissions | 57 (0.9%) | 76 (5.6%) | 121 (2.9%) | 25 (3.5%) | ≤5 |
| Number of specialist types seen ≥2 | 3630 (59.6%) | 1024 (75.9%) | 2712 (65.9%) | 395 (55.9%) | 150 (13.1%) |
| HIV-specific outcome | |||||
| Any receipt of ART among ODB eligible patients(N=8302) | 2271 (66.1%) | 866 (85.7%) | 2683 (86.6%) | 467 (87.3%) | 72 (32.3%) |
ART, antiretroviral therapy; ODB, Ontario Drug Benefits.
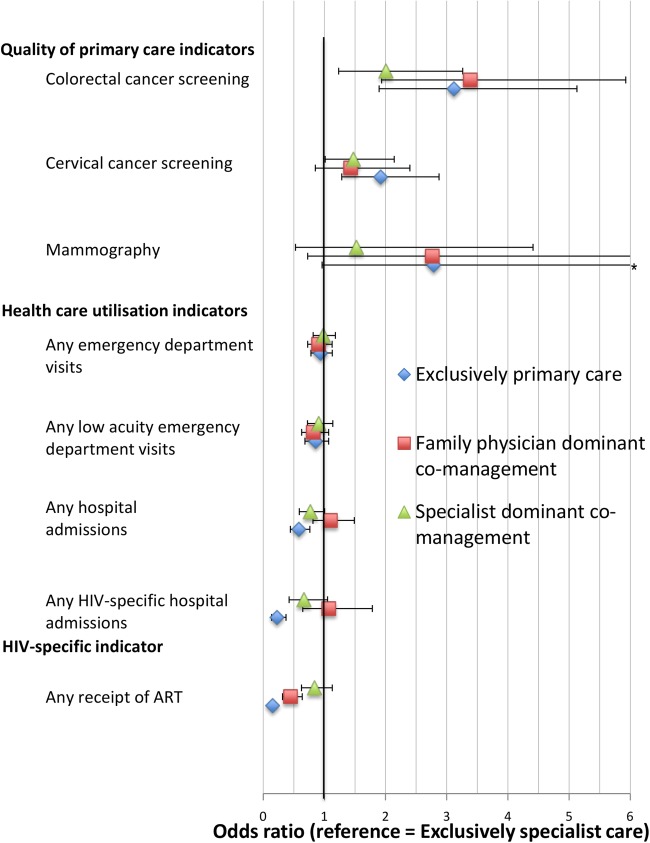
Figure 2 summarises the results of the hierarchical logistic regression analyses of all outcomes, adjusted for patient-level characteristics (the adjusted model results for all outcomes are shown in the online supplementary appendix). The adjusted ORs for each typology category versus the reference category (exclusively specialist care) are displayed, together with 95% CIs. Compared with those in exclusively specialist care, patients in all other typology categories had higher odds of adherence to cancer screening, although not all findings were statistically significant (colorectal cancer screening, exclusively primary care adjusted OR (AOR)=3.12, 95% CI (1.90 to 5.13), family physician-dominant co-management AOR=3.39, 95% CI (1.94 to 5.93), specialist-dominant co-management AOR=2.01, 95% CI (1.23 to 3.26)).
Figure 2.
Hierarchical logistic regression analyses of study outcomes by typology category (ORs (95% CIs)) (reference=exclusively specialist care). ART, antiretroviral therapy.
There were no significant differences in the odds of any emergency department visit and any low-triage emergency department, but the odds of any hospital admission (AOR=0.23, 95% CI (0.14 to 0.35)) and any HIV-specific hospital admission were significantly lower among patients in exclusively primary care (AOR=0.15, 95% CI (0.12 to 0.21)).
The odds of receiving ART were significantly lower in the exclusively primary care and family physician-dominant co-management (exclusively primary care AOR=0.15, 95% CI (0.12 to 0.21), family physician-dominant co-management AOR=0.45, 95% CI (0.32 to 0.64), specialist-dominant co-management AOR=0.84, 95% CI (0.63 to 1.13)).
Discussion
Healthcare should evolve to meet the needs of people living with HIV as they increasingly age and become a more diverse population. Quality care is influenced by many patient, provider and system factors. Of factors that influenced care in our study, we found by far the most important influence on care was how care is provided and shared between specialist and primary care physicians. We found that people with HIV with more family physician care received better cancer screening and had lower odds of hospital admissions. In fact, patients in exclusively specialist care had colorectal cancer screening rates that were half of those in primary care-dominant models. Those with more HIV specialist care received better disease-specific care (both diabetes care and, importantly, ART). Those identified as having low engagement in care, that is, no identified usual primary care provider and no HIV specialist, had very poor care. These findings highlight that there is no one current model of healthcare delivery for patients with HIV. However, it does suggest on a population level the need for specialist and primary care expertise to cover the broad range of care needs for this increasingly complex population.
People with HIV are known to have lower rates of disease screening than their non-HIV counterparts.21 38 Compared with specialty care, primary care leads to better health promotion and disease prevention, even in areas of high inequality.9 As such, we accurately anticipated that patients in models that include the usual family physician would be more likely to have recommended cancer screening manoeuvres, and that this would be most pronounced for models in which the family physician provided the majority of care. This is consistent with some18 but not all39 research comparing prevention interventions between generalists and specialists for HIV patients, and as such we were surprised by the magnitude of this difference. In our study, improved cancer screening most likely arises in part due to the continuity of care with a prevention-oriented provider40 41 who can adhere to prevention recommendations within their own clinical setting.20
We found that HIV patients in models in which HIV care is provided predominantly by family physicians were less likely to receive prescriptions for ART. It is possible that there are differences in the patient characteristics pertaining to HIV stage or ODB eligibility that are not captured within our data. However, despite recent work demonstrating similar ART prescribing between generalists and specialists,42 it is most likely that this finding supports previous extensive literature demonstrating that specialist care is closely linked to adequate antiretroviral treatment.14 16
People with chronic conditions who do not have a regular medical doctor have been shown to be more likely to have emergency department visits and hospital admissions.22 41 43 Surprisingly, we failed to observe significant differences in the frequency of emergency department visits (including low-acuity visits), although patients in exclusively specialist care were more likely to have had a hospital admission (including HIV-specific admissions). Inappropriate emergency department use among HIV patients has been previously attributed to both generalist care and to having multiple clinicians,44 45 but we saw no influence of these features on emergency department usage. People with HIV in Ontario have higher rates of hospitalisation than those in the general population, with rates higher among those of lower socioeconomic status.46 Thus, our findings may reflect changes in HIV care and treatment over time, or may be affected by unmeasured indicators of disadvantage, such as HIV disease stage,47 housing status or employment.48
While this typology provides one theoretically grounded and intuitive way to describe and measure how care is delivered, the delivery of primary care is complex. Thus, it is possible that the differences found (and not found) between typology models are influenced by organisational, practice and community factors that are not measured by an administrative definition of shared care.49 For example, the quality of HIV care has been shown to be improved through case management, multidisciplinary and group practices, extended hours, decision support and clinical information systems, and collocation of clinic activity.14 20 44 50 In addition, extensive work has shown that physician experience has a strong impact on HIV-specific outcomes,14–16 whereas our study focused on the impact of physician specialisation.
There are additional limitations to our work. While we used validated measures of comorbidity burden, there are measures of HIV-specific morbidity, such as CD4 counts and time since diagnosis, that are unmeasured in this study. Most importantly, increased HIV severity or advanced disease stage may have led to less focus on preventative care and resultant higher hospitalisations among those in exclusively specialist care.51 Second, we were unable to measure care delivered outside of the provincial health insurance plan, such as in CHCs (where about 1% of the Ontario population receives primary care28), and to those federally insured such as refugee claimants and some Aboriginal populations. Third, it is possible that a small proportion of patients switched physicians or models of care during the 3-year study period.52 Fourth, ART prescribing only represents the receipt of one prescription over 18 months and may not reflect actual medication adherence. Fifth, it is possible that residual confounding, such as by income or immigration, could have influenced these results. Finally, while our study provides insight into how patterns of care delivery influence general and disease-specific outcomes for people with HIV, it does not provide information regarding the quality of interaction between providers associated with improved patient outcomes53 or measures of patient satisfaction associated with different care models.54
This study found that a theoretically based typology of how care is delivered had a strong relationship with the quality of care provided for people with HIV, even after adjustment for important patient characteristics such as neighbourhood income, rurality and number of comorbidities. However, it does suggest on a population level the need for both specialist and primary care expertise to cover the broad range of care needs for this increasingly complex population. Further work is required to understand how best to integrate specialist and primary care, and to evaluate how this typology relates to actual coordination between providers and patient satisfaction with care.
Footnotes
Contributors: CEK, DGM, WH, MT and RHG contributed to the design of the work, and to the acquisition, analysis and interpretation of the data. JY contributed to the acquisition and analysis of the data. All the authors listed contributed to the drafting of the work and revisions. All authors gave their final approval of the final manuscript submitted.
Funding: This research was funded in part by the Ontario Ministry of Health and Long Term Care (MOHLTC). This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the MOHLTC. CEK holds a Canadian Institutes for Health Research (CIHR) Fellowship in the Area of Health Services/Population Health HIV/AIDS Research. RHG is supported as a Clinician Scientist in the Department of Family and Community Medicine at the University of Toronto. DGM holds a chair in Applied Public Health Sciences, CIHR/Public Health Agency of Canada.
Competing interests: None declared.
Ethics approval: This study was approved by the Ottawa Hospital and Sunnybrook Health Sciences Centre Research Ethics Boards.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Administrative data used for this study could be accessed because of comprehensive research agreements between Institute for Clinical Evaluative Sciences (ICES) and Ontario's Ministry of Health and Long-Term Care, and between ICES and Citizenship and Immigration Canada (CIC).
References
- 1.Smetana GW, Landon BE, Bindman AB et al. . A comparison of outcomes resulting from generalist vs specialist care for a single discrete medical condition. Arch Intern Med 2007;167:10–20. 10.1001/archinte.167.1.10 [DOI] [PubMed] [Google Scholar]
- 2.Jaakkimainen L, Schultz SE, Klein-Geltink J et al. . Ambulatory physician care for adults. In: Jaakkimainen L, Upshur R, Klein-Geltink J, Leong A, Maaten S, Schultz S, eds. Primary care in Ontario: ICES atlas. Toronto: Institute for Clinical Evaluative Sciences, 2006:53–76. [Google Scholar]
- 3.Katz A, Martens P, Chateau D et al. . Understanding the health system use of ambulatory care patients. Winnipeg, MB: Manitoba Centre for Health Policy, Department of Community Health Sciences, Faculty of Medicine, University of Manitoba; (Beaconsfield, Quebec: Canadian Electronic Library, 2013). [Google Scholar]
- 4.Lafata JE, Martin S, Morlock R et al. . Provider type and the receipt of general and diabetes-related preventive health services among patients with diabetes. Med Care 2001;39:491–9. 10.1097/00005650-200105000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Smith SM, Allwright S, O'Dowd T. Does sharing care across the primary-specialty interface improve outcomes in chronic disease? A systematic review. Am J Manag Care 2008;14:213–24. [PubMed] [Google Scholar]
- 6.Rothman AA, Wagner EH. Chronic illness management: what is the role of primary care? Ann Intern Med 2003;138:256–61. 10.7326/0003-4819-138-3-200302040-00034 [DOI] [PubMed] [Google Scholar]
- 7.Dahrouge S, Devlin RA, Hogg B et al. . The economic impact of improvements in primary healthcare performance. Ottawa: Canadian Health Services Research Foundation, 2012. [Google Scholar]
- 8.Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med 2009;7:293–9. 10.1370/afm.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83:457–502. 10.1111/j.1468-0009.2005.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada N, Jacobson LP, Cohen M et al. . Cause-specific mortality among HIV-infected individuals, by CD4+cell count at HAART initiation, compared with HIV-uninfected individuals. AIDS 2014;28:257–65. 10.1097/QAD.0000000000000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasse B, Ledergerber B, Furrer H et al. . Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011;53:1130–9. 10.1093/cid/cir626 [DOI] [PubMed] [Google Scholar]
- 12.Guaraldi G, Orlando G, Zona S et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–6. 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- 13.Goulet JL, Fultz SL, Rimland D et al. . Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007;45:1593–601. 10.1086/523577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handford CD, Tynan AM, Rackal JM et al. . Setting and organization of care for persons living with HIV/AIDS. Cochrane Database Syst Rev 2006;(3):CD004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handford CD, Rackal JM, Tynan AM et al. . The association of hospital, clinic and provider volume with HIV/AIDS care and mortality: systematic review and meta-analysis. AIDS Care 2012;24:267–82. 10.1080/09540121.2011.608419 [DOI] [PubMed] [Google Scholar]
- 16.Rackal JM, Tynan AM, Handford CD et al. . Provider training and experience for people living with HIV/AIDS. Cochrane Database Syst Rev 2011;15:CD003938 10.1002/14651858.CD003938.pub2 [DOI] [PubMed] [Google Scholar]
- 17.Fultz SL, Goulet JL, Weissman S et al. . Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clin Infect Dis 2005;41:738–43. 10.1086/432621 [DOI] [PubMed] [Google Scholar]
- 18.Leece P, Kendall C, Touchie C et al. . Cervical cancer screening among HIV-positive women. Retrospective cohort study from a tertiary care HIV clinic. Can Fam Physician 2010;56:e425–31. [PMC free article] [PubMed] [Google Scholar]
- 19.Duffus WA, Barragan M, Metsch L et al. . Effect of physician specialty on counseling practices and medical referral patterns among physicians caring for disadvantaged human immunodeficiency virus-infected populations. Clin Infect Dis 2003;36:1577–84. 10.1086/375070 [DOI] [PubMed] [Google Scholar]
- 20.Sheth AN, Moore RD, Gebo KA. Provision of general and HIV-specific health maintenance in middle aged and older patients in an urban HIV clinic. AIDS Patient Care STDS 2006;20:318–25. 10.1089/apc.2006.20.318 [DOI] [PubMed] [Google Scholar]
- 21.Reinhold JP, Moon M, Tenner CT et al. . Colorectal cancer screening in HIV-infected patients 50 years of age and older: missed opportunities for prevention. Am J Gastroenterol 2005;100:1805–12. 10.1111/j.1572-0241.2005.50038.x [DOI] [PubMed] [Google Scholar]
- 22.Glazier RH, Moineddin R, Agha MM et al. . The impact of not having a primary care physician among people with chronic conditions. ICES Investigative Report Toronto: Institute for Clinical Evaluative Sciences, 2008:1–30. [Google Scholar]
- 23.Muggah E, Graves E, Bennett C et al. . The impact of multiple chronic diseases on ambulatory care use; a population based study in Ontario, Canada. BMC Health Serv Res 2012;12:452 10.1186/1472-6963-12-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrest CB. A typology of specialists’ clinical roles. Arch Intern Med 2009;169:1062–8. 10.1001/archinternmed.2009.114 [DOI] [PubMed] [Google Scholar]
- 25.Kendall CE, Younger J, Manuel DG et al. . The derivation and validation of a typology of care for patients with chronic disease using administrative data. Submit September 2014.
- 26. Monitoring HIV care in the United States: indicators and data systems. Institute of Medicine, The National Academic Press, 2012. [PubMed]
- 27.Antoniou T, Zagorski B, Loutfy MR et al. . Validation of case-finding algorithms derived from administrative data for identifying adults living with human immunodeficiency virus infection. PLoS ONE 2011;6:e21748 10.1371/journal.pone.0021748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glazier R, Zagorski B, Rayner J. Comparison of primary care models in Ontario by demographics, case mix and emergency department use, 2008/09 to 2009/10. ICES Investigative Report Toronto, Ontario: Institute for Clinical Evaluative Sciences, 2012. [Google Scholar]
- 29.Kralj B. Measuring “rurality” for purposes of health care planning: an empirical measure for Ontario. Ont Med Rev 2000;67:33–52. [Google Scholar]
- 30.Public Health Agency of Canada. HIV/AIDS epi update: national HIV prevalence and incidence estimates in Canada for 2008. Surveillance and Risk Assessment Division, Centre for Communicable Diseases and Infection Control, 2010:1–7. [Google Scholar]
- 31.Rebeiro P, Althoff KN, Buchacz K et al. . Retention among North American HIV-infected persons in clinical care 2000–2008. J Acquir Immune Defic Syndr 2013;62:356–62. 10.1097/QAI.0b013e31827f578a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krentz HB, Gill MJ. Comparison of healthcare costs between local and immigrant HIV populations living in Southern Alberta, Canada. Health Policy 2011;103:124–9. 10.1016/j.healthpol.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 33.Dombrowski JC, Kent JB, Buskin SE et al. . Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS 2012;26:77–86. 10.1097/QAD.0b013e32834dcee9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johns Hopkins University. Johns Hopkins ACG Case-Mix Adjustment System. http://www.acg.jhsph.edu. [Google Scholar]
- 35.Huntley AL, Johnson R, Purdy S et al. . Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med 2012;10:134–41. 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steele LS, Glazier RH, Lin E et al. . Using administrative data to measure ambulatory mental health service provision in primary care. Med Care 2004;42:960–5. 10.1097/00005650-200410000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Jaakkimainen L, Klein-Geltink JE, Guttmann A et al. . Indicators of primary care based on administrative data. In: Jaakkimainen L, Klein-Geltink J, Leong A, Maaten S, Schultz S, Wang L, eds. Primary care in Ontario: ICES atlas. Toronto: Institute for Clinical Evaluative Sciences, 2006:207–38. [Google Scholar]
- 38.Rahangdale L, Sarnquist C, Yavari A et al. . Frequency of cervical cancer and breast cancer screening in HIV-infected women in a county-based HIV clinic in the Western United States. J Womens Health (Larchmt) 2010;19:709–12. 10.1089/jwh.2009.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koethe JR, Moore RD, Wagner KR. Physician specialization and women's primary care services in an urban HIV clinic. AIDS Patient Care STDS 2008;22:373–80. 10.1089/apc.2007.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med 2005;3:159–66. 10.1370/afm.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menec VH, Sirski M, Attawar D. Does continuity of care matter in a universally insured population? Health Serv Res 2005;40:389–400. 10.1111/j.1475-6773.2005.0p364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu C, Umanski G, Blank A et al. . HIV-infected patients and treatment outcomes: an equivalence study of community-located, primary care-based HIV treatment vs. hospital-based specialty care in the Bronx, New York. AIDS Care 2010;22:1522–9. 10.1080/09540121.2010.484456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontaine P, Flottemesch TJ, Solberg LI et al. . Is consistent primary care within a patient-centered medical home related to utilization patterns and costs? J Ambul Care Manage 2011;34:10–19. 10.1097/JAC.0b013e3181ff7019 [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez HP, Marsden PV, Landon BE et al. . The effect of care team composition on the quality of HIV care. Med Care Res Rev 2008;65:88–113. 10.1177/1077558707310258 [DOI] [PubMed] [Google Scholar]
- 45.Keitz SA, Box TL, Homan RK et al. . Primary care for patients infected with human immunodeficiency virus: a randomized controlled trial. J Gen Intern Med 2001;16:573–82. 10.1046/j.1525-1497.2001.016009573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antoniou T, Zagorski B, Loutfy MR et al. . Socio-economic- and sex-related disparities in rates of hospital admission among patients with HIV infection in Ontario: a population-based study. Open Med 2012;6:e146–54. [PMC free article] [PubMed] [Google Scholar]
- 47.Fleishman JA, Moore RD, Conviser R et al. . Associations between outpatient and inpatient service use among persons with HIV infection: a positive or negative relationship? Health Serv Res 2008;43(1 Pt 1):76–95. 10.1111/j.1475-6773.2007.00750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glazier RH, Tepper J, Agha MM et al. . Primary care in disadvantaged populations. In: Jaakkimainen L, Klein-Geltink JE, Leong A, Maaten S, Schultz SE, Wang LU, eds. Primary care in Ontario: ICES atlas. Institute for Clinical Evaluative Sciences, 2006. [Google Scholar]
- 49.Comino EJ, Davies GP, Krastev Y et al. . A systematic review of interventions to enhance access to best practice primary health care for chronic disease management, prevention and episodic care. BMC Health Serv Res 2012;12:415 10.1186/1472-6963-12-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasricha A, Deinstadt RT, Moher D et al. . Chronic care model decision support and clinical information systems interventions for people living with HIV: a systematic review. J Gen Intern Med 2013;28:127–35. 10.1007/s11606-012-2145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akgün KM, Gordon K, Pisani M et al. . Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected veterans. J Acquir Immune Defic Syndr 2013;62:52–9. 10.1097/QAI.0b013e318278f3fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez HP, Wilson IB, Landon BE et al. . Voluntary physician switching by human immunodeficiency virus-infected individuals: a national study of patient, physician, and organizational factors. Med Care 2007;45:189–98. 10.1097/01.mlr.0000250252.14148.7e [DOI] [PubMed] [Google Scholar]
- 53.Foy R, Hempel S, Rubenstein L et al. . Meta-analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med 2010;152:247–58. 10.7326/0003-4819-152-4-201002160-00010 [DOI] [PubMed] [Google Scholar]
- 54.Page J, Weber R, Somaini B et al. . Quality of generalist vs. specialty care for people with HIV on antiretroviral treatment: a prospective cohort study. HIV Med 2003;4:276–86. 10.1046/j.1468-1293.2003.00157.x [DOI] [PubMed] [Google Scholar]