Abstract
Objectives. We evaluated the effectiveness of offering adjunctive financial incentives for abstinence (contingency management [CM]) within a safety net hospital smoking cessation program.
Methods. We randomized participants (n = 146) from a Dallas County, Texas, Tobacco Cessation Clinic from 2011 to 2013 to usual care (UC; cessation program; n = 71) or CM (UC + 4 weeks of financial incentives; n = 75), and followed from 1 week before the quit date through 4 weeks after the quit date. A subset (n = 128) was asked to attend a visit 12 weeks after the scheduled quit date.
Results. Participants were primarily Black (62.3%) or White (28.1%) and female (57.5%). Most participants were uninsured (52.1%) and had an annual household income of less than $12 000 (55.5%). Abstinence rates were significantly higher for those assigned to CM than UC at all visits following the quit date (all Ps < .05). Point prevalence abstinence rates in the CM and UC groups were 49.3% versus 25.4% at 4 weeks after the quit date and 32.8% versus 14.1% at 12 weeks after the quit date. CM participants earned an average of $63.40 ($150 possible) for abstinence during the first 4 weeks after the scheduled quit date.
Conclusions. Offering small financial incentives for abstinence might be an effective means to improve abstinence rates among socioeconomically disadvantaged individuals participating in smoking cessation treatment.
Tobacco use is the leading cause of preventable death in the United States.1 Although the prevalence of smoking has declined to 18.1% among US adults, 27.9% of those living below the poverty threshold continue to smoke.2 Numerous studies have shown that socioeconomic disadvantage is associated with a reduced likelihood of smoking cessation,3–9 despite comparable numbers of quit attempts by individuals with higher socioeconomic status.10 Furthermore, abstinence rates among socioeconomically disadvantaged smokers participating in smoking cessation interventions are alarmingly low (e.g., point prevalence abstinence rates of 7%–13% and continuous abstinence rates of 2%–4% at 6-month follow-up).8,9,11–13 Factors, including exposure to stress or adversity (e.g., neighborhood problems, income instability), limited psychosocial resources (e.g., social support), greater nicotine dependence, greater negative affect, and poor adherence to smoking cessation treatments, may contribute to dismal smoking cessation outcomes and poor general health in socioeconomically disadvantaged populations.14–16
Notably, contingency management (CM), or the tangible reinforcement of abstinence and other related outcomes, is one approach that has been effective for the promotion of abstinence among individuals participating in treatment of substance abuse or dependence.17,18 The CM approach is also effective for promoting smoking abstinence in a variety of populations.19–31 Notably, Etter32 is evaluating the use of financial incentives for low-income smokers as part of an ongoing Internet-based smoking cessation program in Switzerland. However, the CM approach for smoking cessation has yet to be evaluated in mainstream clinic settings, such as safety net hospitals that serve economically disadvantaged smokers who are motivated to quit smoking. Recent survey research suggests that financial incentives for smoking cessation may be particularly appealing among individuals of low socioeconomic status.33 Thus, our purpose in this study was to test the effectiveness of offering small financial incentives to encourage short-term abstinence among economically disadvantaged smokers who enrolled in a tobacco cessation program at a safety net hospital.
METHODS
We recruited participants during their orientation visit to the Tobacco Cessation Clinic at the Dallas County, Texas, safety net hospital between August 2011 and April 2013. All patients newly enrolled in the tobacco cessation program heard a brief verbal description of the study from the study staff. We offered individuals the opportunity to be screened for eligibility to participate while they were waiting to meet with the clinic physician (or other prescribing provider) later that day. We obtained informed consent from all individuals before screening for eligibility. Individuals were eligible to participate in the study if they
demonstrated higher than a sixth grade English literacy level,
were willing to quit smoking 7 days after their first visit,
were aged 18 years or older,
had an expired carbon monoxide (CO) level of 8 parts per million or greater,
were smoking 5 or more cigarettes per day, and
were able to attend all study visits.
Measures
Demographic characteristics information.
We measured demographic and socioeconomic characteristics, including race/ethnicity, gender, age, marital or partner status, years of smoking, educational attainment, insurance status, employment status, and household income.
Literacy.
The Rapid Estimate of Adult Literacy in Medicine is an interviewer-administered checklist in which individuals are asked to read and pronounce 66 common medical terms.34,35 Individuals who pronounced 45 or more words correctly were considered to have greater than a sixth grade reading level.
Tobacco Use and Smoking Abstinence
We assessed tobacco use characteristics, including years of smoking, daily smoking rate, expired CO level (parts per million), and time to first cigarette upon waking in the morning. We calculated the Heaviness of Smoking Index based on the daily smoking rate and time to first cigarette at the baseline measurement.36
According to the Society of Research on Nicotine and Tobacco Subcommittee on Biochemical Verification,37 CO levels of 8 to 10 parts per million or greater suggest recent cigarette smoking with a sensitivity and specificity of approximately 90%. Thus, we defined abstinence on the quit date as a self-report of smoking abstinence since the previous evening at 10 p.m. (approximately 12 hours after quitting) in combination with an expired CO level of 10 parts per million or less. We defined 7-day point prevalence abstinence at weeks 1 to 4, and 12 weeks after the quit date as a self-report of abstinence from smoking over the past 7 days in combination with an expired CO level of less than 8 parts per million. We measured 30-day point prevalence abstinence at 12 weeks after the quit date, which we defined as self-reported abstinence over the past 30 days in combination with an expired CO level of less than 8 parts per million. Important advantages of point prevalence abstinence included the ability to consider individuals abstinent following delays in cessation or a return to abstinence after an initial lapse (for a review and discussion, see Velicer et al.38 and Hughes et al.39). In our study, point prevalence abstinence was a particularly important outcome because financial incentives might have encouraged participants to return to abstinence following a lapse.
In cases where abstinence status could not be determined because of missing data, participants were considered nonabstinent. Complete smoking status data (i.e., self-reported smoking with or without biochemical verification or self-reported abstinence with biochemical verification) were available for 97.9% of participants on the scheduled quit date, 100% at 1 week after the quit date, 89.7% at 2 weeks after the quit date, 71.9% at 3 weeks after the quit date, 86.3% at 4 weeks after the quit date, and 74.2% at 12 weeks after the quit date. The proportion with incomplete smoking status data did not differ between treatment groups at any visit, although attendance was lower overall at 3 and 12 weeks after the quit date (i.e., more participants were considered relapsed because of nonattendance at these visits).
Participants were considered continuously abstinent at 4 and 12 weeks after the scheduled quit date if they reported that they had been abstinent since the quit date assessment (with a 12-hour grace period) and demonstrated expired CO levels of less than 8 parts per million at all attended visits. Participants who self-reported abstinence since the quit date, but had missing smoking status data (because of nonattendence) at some time points were considered continuously abstinent if they missed no more than 2 consecutive assessments and the data at the surrounding time points indicated abstinence. Note that 2 additional continuous abstinence variables were created that either allowed missing data at only 1 time point or did not allow any missing data. These variables were also evaluated as outcomes to confirm the robustness of findings related to continuous abstinence.
Procedure
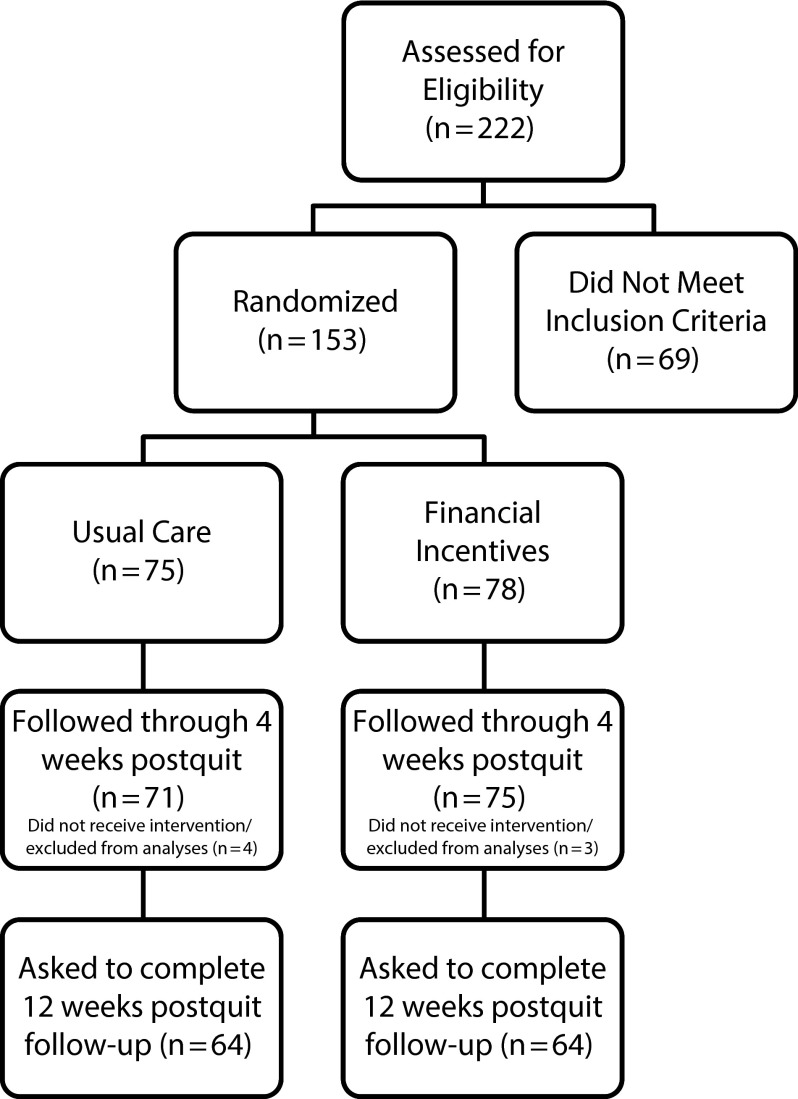
A total of 222 individuals were assessed for study eligibility, and 69 did not meet inclusion criteria (Figure 1). Participants were excluded for the following reasons: expired CO level less than 8 parts per million (n = 29); less than a seventh grade reading level (n = 27); smoked fewer than 5 cigarettes per day or not currently smoking (n = 14); unable to read, speak, and understand English (n = 2); and uninterested or other (n = 5). Participants might have been excluded for more than 1 reason. Excluded individuals did not differ from study participants on gender, race/ethnicity, or age. However, those excluded from the study had significantly lower CO (10.62 vs 17.86 ppm; P < .001) and literacy levels (Rapid Estimate of Adult Literacy scores of 45.22 vs 60.99; P < .001) than study participants, and they smoked fewer cigarettes per day (before the quit date; 14.60 vs 17.49; P = .056), although the latter finding did not reach statistical significance.
FIGURE 1—
Participant flow diagram: Financial Incentives for Abstinence in Smoking Cessation Treatment; Dallas County, TX; 2011–2013.
Eligible participants (n = 153) were randomly assigned to usual care (UC) for smoking cessation (n = 75) or to UC plus financial incentives for smoking abstinence (CM; n = 78). However, 7 participants did not return after the baseline visit (i.e., did not participate in the intervention) and were therefore excluded from all analyses, leaving a final study sample of 146 participants (71 UC participants, 75 CM participants). Participants were followed weekly from 1 week before their scheduled quit date through 4 weeks after the quit date. After the first 18 participants were enrolled, a 12-week follow-up assessment was added to the protocol. Thus, a subsample of 128 participants was asked to complete the 12-week follow-up assessment (64 UC participants, 64 CM participants).
Usual care.
The safety net hospital smoking cessation program offered all the recommended components of an intensive tobacco treatment intervention.40 Individuals interested in quitting smoking were referred (typically by treatment providers) to the tobacco cessation program. Individuals attended 1 initial clinic orientation and educational session provided by a respiratory therapist, followed by weekly group support sessions facilitated by social workers. Participants were seen individually by a physician or other prescribing provider on a weekly or as needed basis to receive pharmacotherapy and individual follow-up.
Contingency management.
Participants assigned to CM received all components of UC as described previously. In addition, participants had the opportunity to earn weekly incentives in the form of gift cards, if they (1) self-reported abstinence during the past approximately 12 hours on the quit day (i.e., abstinence since 10 p.m. the previous evening), or self-reported abstinence during the past 7 days at each weekly visit from 1 week through 4 weeks after the quit date; and (2) provided an expired CO sample consistent with abstinence (i.e., CO ≤ 10 ppm on the quit date, CO < 8 ppm at weeks 1–4 after the quit date). Participants earned a $20 gift card to a popular retail chain in exchange for biochemically confirmed abstinence on the quit date. An escalating schedule was employed, such that the amount of the incentives increased by $5 with each weekly successive abstinent visit through 4 weeks after the quit date (up to $150 total). Participants who were nonabstinent at any visit were eligible to earn incentives for abstinence at the next visit, although the amount was reset to $20.
Assessments.
Psychosocial assessments were completed at 1 week before the scheduled quit date (baseline), on the quit date, and at 1 and 4 weeks after the quit date, for which all participants were compensated with a $30 gift card at each assessment. Smoking status was assessed weekly from 1 week before quitting through 4 weeks after quitting. For a subset of participants (n = 128), smoking status was assessed at 12 weeks after the quit date, and participants were compensated for this assessment with a $30 gift card.
Statistical Analyses
We used SPSS version 20 (IBM, Armonk, NY) to generate descriptive statistics about the study sample. We conducted analysis of variance and χ2 analysis to determine whether there were differences in baseline characteristics between the intervention groups. We conducted unadjusted and adjusted logistic regression analyses to characterize the influence of CM relative to UC on point prevalence (quit date, and 1, 2, 3, 4, and 12 weeks after the quit date) and continuous abstinence (4 and 12 weeks after the quit date). Covariates in the adjusted analyses included pharmacological treatment, race/ethnicity, gender, age, years of education, and the number of cigarettes smoked per day before the quit date. We evaluated interaction effects by conducting unadjusted and adjusted analyses to determine whether the effect of intervention type on cessation outcomes over time varied by age, gender, race/ethnicity, education, cigarettes per day, pharmacological treatment, and week after quitting (time).
We used STATA version 13.0 (StataCorp, College Station, TX) to conduct unadjusted and adjusted mixed effects logistic regression analyses to evaluate the overall effect of treatment on 7-day point prevalence abstinence over time (1–4 weeks after the quit date, n = 146; 1–4 and 12 weeks after the quit date, n = 128).All repeated measures analyses included treatment week (time) in the model in addition to previously noted covariates.
RESULTS
Participants (n = 146) were primarily Black, and more than half reported that they were uninsured, unemployed, and had a household income of less than $12 000 per year. No significant differences were found between the treatment groups on demographic, socioeconomic, or smoking characteristics (Table 1). Participants attended an average of 57.3% of the weekly support group sessions offered from 1 week before the quit date through 4 weeks after the quit date, and the percentage of sessions attended did not differ significantly by treatment group.
TABLE 1—
Participant Characteristics: Financial Incentives for Abstinence in Smoking Cessation Treatment; Dallas County, TX; 2011–2013
| Characteristics | Usual Care (n = 71), % or Mean (SD) | Usual Care + Financial Incentives (n = 75), % or Mean (SD) | All Participants (n = 146), % or Mean (SD) |
| Demographic | |||
| Age, y | 52.6 (7.4) | 51.7 (7.3) | 52.2 (7.3) |
| Gender (female) | 63.4 | 52.0 | 57.5 |
| Race/ethnicity, % | |||
| Black/African American | 57.7 | 66.7 | 62.3 |
| White/Caucasian | 31.0 | 25.3 | 28.1 |
| Latino/Hispanic | 4.2 | 6.7 | 5.5 |
| Multirace/other | 7.0 | 1.3 | 4.1 |
| Partner status (married/living with significant other) | 31.0 | 32.0 | 31.5 |
| Socioeconomic | |||
| Education, y | 12.1 (1.8) | 12.0 (2.2) | 12.0 (2.0) |
| Annual household income (< $12 000) | 52.1 | 58.7 | 55.5 |
| Employment status (not employed) | 81.7 | 89.3 | 85.6 |
| Insurance status | |||
| Uninsured | 52.1 | 52.0 | 52.1 |
| Medicaid/Medicare | 39.4 | 40.0 | 39.7 |
| Private/job/combination | 8.5 | 8.0 | 8.2 |
| Smoking | |||
| Cigarettes smoked/d (before quit date) | 17.0 (7.7) | 18.0 (9.7) | 17.5 (8.8) |
| Years of smoking | 31.0 (9.6) | 31.9 (9.2) | 31.4 (9.4) |
| CO, ppm (baseline) | 17.5 (6.7) | 18.2 (8.6) | 17.9 (7.7) |
| Smoke ≤ 5 minutes of waking | 42.3 | 53.3 | 47.9 |
| Heaviness of Smoking Index | 3.1 (1.2) | 3.4 (1.3) | 3.3 (1.3) |
| Pharmacological treatment | |||
| Nicotine replacement therapy | 50.7 | 49.3 | 50.0 |
| Varenicline | 32.4 | 37.4 | 34.9 |
| Bupropion | 11.3 | 8.0 | 9.6 |
| Combination/missing | 5.6 | 5.3 | 5.5 |
Note. CO = carbon monoxide. No significant differences between treatment groups were found on any variable included in the table.
Pharmacological Treatment
Participants were most frequently prescribed nicotine replacement therapy (NRT; patch or gum, 50%; n = 73), followed by varenicline (34.9%; n = 51), and bupropion (9.6%; n = 14). The remaining 5.5% of participants were prescribed a combination of NRT and bupropion (n = 7), or their data were missing (n = 1). The χ2 analysis indicated that the intervention groups did not differ in the distribution of cessation medications prescribed. The proportion of participants prescribed each type of pharmacological treatment is presented by intervention group in Table 1. Abstinence rates at 4 and 12 weeks after quitting did not differ significantly by medication type. Seven-day point prevalence abstinence rates for those prescribed varenicline, NRT, and bupropion were 43.1%, 37.0%, and 35.7% at 4 weeks after quitting and 27.5%, 17.8%, and 21.4% at 12 weeks after quitting, respectively.
Smoking Cessation
Point prevalence abstinence.
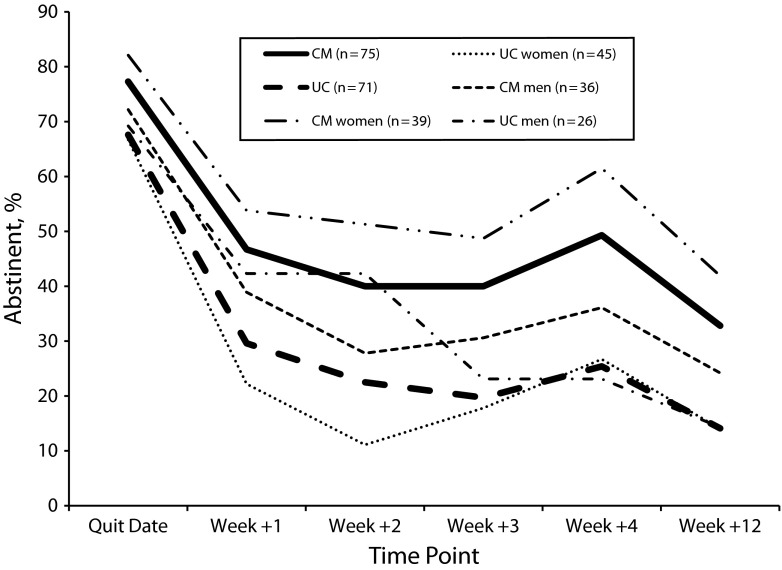
Unadjusted and adjusted logistic regression analysis indicated that CM participants were significantly more likely to achieve 7-day point prevalence abstinence than UC participants at 1 week after the quit date (46.7% [n = 35] vs 29.6% [n = 21] abstinent), 2 weeks after the quit date (40.0% [n = 30] vs 22.5% [n = 16] abstinent), 3 weeks after the quit date (40.0% [n = 30] vs 19.7% [n = 14] abstinent), and 4 weeks after the quit date (49.3% [n = 37] vs 25.4% [n = 18] abstinent; all Ps < 0.05). However, there were no between groups differences in point prevalence abstinence (approximately 12 hours) on the quit date. In the subsample (n = 128) that was offered the opportunity to complete the follow-up visit at 12 weeks after the quit date, CM participants were significantly more likely to achieve 7-day point prevalence abstinence than UC participants (32.8% [n = 21] vs 14.1% [n = 9] abstinent). CM participants were also more likely to achieve 30-day point prevalence abstinence at 12 weeks after the quit date (28.1% [n = 18] vs 10.9% [n = 7] abstinent). The odds of achieving point prevalence abstinence among those assigned to CM relative to UC are presented in Table 2. Point prevalence abstinence rates by intervention group are depicted in Figure 2.
TABLE 2—
Odds of Abstinence Among Those Who Received Financial Incentives Relative to Usual Care: Financial Incentives for Abstinence in Smoking Cessation Treatment; Dallas County, TX; 2011–2013
| 4 Weeks Postquit (n = 146) |
12 Weeks Postquit (n = 128) |
|||
| Values | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| 7-d point prevalence abstinence | 2.87** (1.42, 5.77) | 3.40*** (1.61, 7.16) | 2.98* (1.24, 7.16) | 3.76** (1.45, 9.70) |
| 30-d point prevalence abstinence | . . . | . . . | 3.19* (1.22, 8.29) | 4.26** (1.50, 12.05) |
| Repeated point prevalence abstinencea | 5.40*** (1.92, 15.14) | 5.36*** (1.93, 14.90) | 5.16** (1.75, 15.21) | 5.61** (1.88, 16.69) |
| Continuous abstinence | 2.34 (0.98, 5.59) | 2.59* (1.04, 6.42) | 3.01* (1.003, 9.01) | 3.61* (1.13, 11.49) |
Note. Values in the table reflect the odds of achieving abstinence for those assigned to the adjunctive financial incentives intervention relative to usual care. Pharmacological treatment, race, gender, age, years of education, and before quitting cigarettes smoked per day were included in the adjusted models.
Repeated measures analyses included 7-day point prevalence abstinence at 1, 2, 3, 4, and 12 weeks after the quit date as the outcomes. Treatment week (time) was additionally included in both the adjusted and unadjusted analyses.
*P < .05; **P < .01; ***P = .001.
FIGURE 2—
Biochemically verified point prevalence abstinence by treatment group and gender: Financial Incentives for Abstinence in Smoking Cessation Treatment; Dallas County, TX; 2011–2013.
Note. CM = contingency management; UC = usual care. A subsample of 128 participants was asked to complete the follow-up visit 12 weeks after the quit date. Values reflect 7-day point prevalence abstinence at all visits except for the quit date, which reflects approximately 12 hours of abstinence. Intervention group differences in abstinence rates were significant at all visits except for the quit date in both unadjusted and adjusted analyses (all Ps < .05).
Repeated point prevalence abstinence.
Mixed-effects logistic regression analysis indicated that CM participants were significantly more likely to achieve 7-day point prevalence abstinence over time (1–4 weeks after the quit date and 1–12 weeks after the quit date) than those assigned to UC (Table 2).
Continuous abstinence.
Logistic regression analysis indicated that CM participants were more likely to achieve continuous abstinence at 4 weeks after the quit date than UC participants (25.3% [n = 19] vs 12.7% [n = 9] abstinent). In the subsample (n = 128) that was offered the opportunity to complete the follow-up visit at 12 weeks after the quit date, CM participants were also significantly more likely to achieve continuous abstinence at 12 weeks after the quit date than UC participants (20.3% [n = 13] vs 7.8% [n = 5] abstinent). CM participants were more likely to achieve continuous abstinence at 4 and 12 weeks after the quit date, even when more conservative definitions of continuous abstinence were used (see descriptions in the Measures section; all Ps < .05; detailed results available upon request). The odds of achieving continuous abstinence in CM relative to UC are presented in Table 2.
Treatment Interactions
Age, race/ethnicity, education, cigarettes per day, pharmacological treatment, and weeks following the scheduled quit date (time) did not interact with the treatment group to predict repeated point prevalence abstinence. However, the treatment group interacted significantly with gender to predict 7-day point prevalence abstinence through 4 weeks after the quit date (n = 146) in unadjusted (P = .006) and adjusted analysis (P = .01). Similarly, the treatment group interacted significantly with gender to predict 7-day point prevalence abstinence through 12 weeks after the quit date (n = 128) in unadjusted (P = .028) and adjusted analysis (P = .03). Specifically, women assigned to the CM group had the highest abstinence rates over the first 4 and 12 weeks after the quit date, whereas women assigned to UC had the lowest abstinence rates. Conversely, abstinence rates were more similar among men in the CM and UC groups (Figure 2).
The average amount of incentives earned for abstinence among CM participants between the quit date and 4 weeks after the quit date was $63.40 (SD = $51.67) of a possible $150.
DISCUSSION
We demonstrated the short-term effectiveness of offering small financial incentives for biochemically verified abstinence as an adjunct to an existing smoking cessation program provided at an urban safety net hospital. Adjunctive financial incentives doubled abstinence rates relative to UC in an extremely socioeconomically disadvantaged and primarily Black sample. The positive effect of financial incentives on abstinence rates remained 8 weeks after the incentives were discontinued, and findings indicated that financial incentives might work particularly well for improving abstinence rates among socioeconomically disadvantaged women. Thus, small financial incentives for smoking cessation might be an affordable, practical, and effective means of increasing cessation rates and reducing tobacco-related disease in the safety net hospital setting.
Numerous studies showed that offering financial incentives for smoking cessation was an effective strategy for promoting smoking abstinence.19–31 We added to previous research by demonstrating that providing adjunctive short-term financial incentives is also an effective approach to smoking cessation in the safety net hospital setting. Furthermore, the CM approach used in our study was effective at increasing abstinence rates in an extremely disadvantaged population who would otherwise be less likely to quit smoking (for a review, see Hiscock et al.14). Lower rates of abstinence in UC were consistent with findings from other cessation studies in socioeconomically disadvantaged populations.8,9,11–13,31 Notably, the amount of incentives offered in the our study was low ($20–$40 per visit; $150 possible over a 4-week period) relative to the amounts offered in most other CM interventions for smoking cessation. Plausibly, using smaller incentives was feasible in this population because participants were highly motivated to quit smoking, and most had very little income, possibly increasing the subjective value and desirability of incentives.
Gender moderated the relationship between intervention group and point prevalence abstinence over the course of the study. Women assigned to the CM intervention were more likely to achieve abstinence over time than women assigned to the UC group. Women assigned to CM had the highest abstinence rates over time, whereas women assigned to UC had the lowest abstinence rates. Conversely, the abstinence rates among men were more similar between intervention groups. This finding was intriguing, because women were frequently shown to have poorer cessation outcomes than men.41–43 Study findings suggested that the CM approach might work particularly well among socioeconomically disadvantaged women. Additional research is needed to confirm gender-related findings.
The CM approach used in this study could be a cost-effective way to reduce health care expenditures. The total cost of incentives was $63.40 per participant, which might be affordable and sustainable in many safety net hospital tobacco clinic settings. Biochemical verification of abstinence via expired CO measurement is quick and inexpensive, and is already commonly used in cessation clinics. An estimated 200 new patients per year are treated in the clinic described in this study. If all were offered the opportunity to participate in this financial incentives program, the cost would amount to $12 680 annually. This relatively small expense could conceivably be covered in the hospital budget for smoking cessation, because this money would likely be saved through reduced tobacco-related health care expenditures. In 2010, the average cost associated with the initial year of lung cancer treatment ranged from $60 533 to $73 062.44 Thus, the prevention of a single case of lung cancer would cover the cost of at least 5 years of this financial incentives program without even considering the potential savings related to other tobacco-related health problems. Additional research is needed to quantify the cost-effectiveness of incentives for smoking cessation. Insurance companies might consider covering financial incentives for abstinence based on favorable cost-benefit analyses.
Researchers suggested that daily monitoring of smoking status might be required to accurately verify smoking status within CM interventions.45 However, daily monitoring is impractical for hospital programs and patients. At 4 weeks after the quit date, we found that only 3% of study participants (n = 4) provided a self-report of abstinence in combination with an expired CO level that suggested nonabstinence. It appears that most people were honest about their smoking status or were unaware of the limitations of measuring expired CO. Moreover, although the half-life of CO is up to 8 hours depending on a variety of factors,37 studies showed that expired CO is a valid indicator of smoking status and cessation outcomes, and compares favorably with cotinine and other biochemical measures with longer detection windows.38,46,47 Unfortunately, smoking status could not be validated with cotinine measurement in our study because half of study participants were prescribed NRT. Notably, the CM approach in our study was associated with higher point prevalence abstinence rates even when a more conservative CO level of less than 4 parts per million was used as verification of self-reported abstinence (results available upon request). As such, we believe that self-reports of abstinence combined with CO levels suggestive of recent abstinence provided a reasonably accurate, immediate, and practical measure of abstinence that is already available in many clinic settings.
Study Limitations
Our study had strengths and limitations. We were the first to examine the effectiveness of using small, weekly financial incentives to promote smoking abstinence as part of a safety net hospital smoking cessation program. In addition, the intervention was evaluated within a primarily Black and extremely socioeconomically disadvantaged population that had a reduced likelihood of smoking cessation. A major limitation of this study was the lack of long-term follow-up, although early abstinence and more time abstinent following a quit attempt were associated with a greater likelihood of achieving long-term smoking abstinence.48,49 Thus, the CM approach utilized in our study holds promise for promoting longer term abstinence in socioeconomically disadvantaged populations.
Conclusions
Future research should focus on characterizing the long-term influence of financial incentives on cessation, and refining the CM approach for use within socioeconomically disadvantaged populations. Research is needed to determine the optimal value of incentives and length of time that incentives should be offered. Other types of incentives should also be explored, such as lotteries in which abstinent participants are represented in drawings for prizes rather than receiving an individual payment.50,51 Finally, future research should focus on evaluating and implementing the CM approach for smoking cessation among socioeconomically disadvantaged patients in other real-world settings.
In summary, we demonstrated that offering adjunctive financial incentives approximately doubled short-term abstinence rates relative to UC among safety net hospital patients motivated to quit smoking. The positive effect of the incentives on cessation outcomes remained at 12 weeks after the quit date, which was 8 weeks after the incentives were discontinued. Thus, including small financial incentives as part of standard smoking cessation programs might help to improve cessation rates among socioeconomically disadvantaged smokers, and particularly among women, in the safety net hospital setting. In turn, increased rates of smoking cessation might reduce tobacco-related disease, tobacco-related hospital expenditures, and socioeconomic and racial/ethnic disparities in health.
Acknowledgments
Funding for this research was provided by the University of Texas Health Science Center, School of Public Health. Data analysis and article preparation were additionally supported by American Cancer Society grants MRSGT-10-104-01-CPHPS (to D. E. K) and MRSGT-12-114-01-CPPB (to M. S. B).
Human Participant Protection
This research was approved by the institutional review boards of the University of Texas Southwestern Medical Center (STU 042011-096) and the University of Texas Health Science Center (HSC-SPH-11-0269). Informed consent was obtained from all participants.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Agaku IT, King BA, Dube SR.Centers for Disease Control and Prevention (CDC). Current cigarette smoking among adults — United States, 2005–2012 MMWR Morb Mortal Wkly Rep 201463229–34. [PMC free article] [PubMed] [Google Scholar]
- 3.Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: results from a prospective study of an Australian national sample. Addiction. 2006;101(1):121–127. doi: 10.1111/j.1360-0443.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 4.Fernández E, Schiaffino A, Borrell C et al. Social class, education, and smoking cessation: long-term follow-up of patients treated at a smoking cessation unit. Nicotine Tob Res. 2006;8(1):29–36. doi: 10.1080/14622200500264432. [DOI] [PubMed] [Google Scholar]
- 5.Wetter DW, Cofta-Gunn L, Irvin JE et al. What accounts for the association of education and smoking cessation? Prev Med. 2005;40(4):452–460. doi: 10.1016/j.ypmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Businelle MS, Kendzor DE, Reitzel LR et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29(3):262–273. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagan P, Shavers VL, Lawrence D, Gibson JT, O’Connell ME. Employment characteristics and socioeconomic factors associated with disparities in smoking abstinence and former smoking among US workers. J Health Care Poor Underserved. 2007;18(4 suppl):52–72. doi: 10.1353/hpu.2007.0119. [DOI] [PubMed] [Google Scholar]
- 8.Kendzor DE, Businelle MS, Costello TJ et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health. 2010;100(4):702–706. doi: 10.2105/AJPH.2009.172676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendzor DE, Reitzel LR, Mazas CA et al. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Soc Sci Med. 2012;74(9):1394–1401. doi: 10.1016/j.socscimed.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotz D, West R. Explaining the social gradient in smoking cessation: it’s not in the trying, but in the succeeding. Tob Control. 2009;18(1):43–46. doi: 10.1136/tc.2008.025981. [DOI] [PubMed] [Google Scholar]
- 11.Froelicher ES, Doolan D, Yerger VB, McGruder CO, Malone RE. Combining community participatory research with a randomized clinical trial: the Protecting the Hood Against Tobacco (PHAT) smoking cessation study. Heart Lung. 2010;39(1):50–63. doi: 10.1016/j.hrtlng.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Okuyemi KS, James AS, Mayo MS et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav. 2007;34(1):43–54. doi: 10.1177/1090198106288046. [DOI] [PubMed] [Google Scholar]
- 13.Hahn EJ, Rayens MK, Chirila C, Riker CA, Paul TP, Warnick TA. Effectiveness of a quit and win contest with a low-income population. Prev Med. 2004;39(3):543–550. doi: 10.1016/j.ypmed.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248(1):107–123. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? Ann N Y Acad Sci. 2010;1186:146–173. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 16.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 18.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 19.Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2010;18(1):37–50. doi: 10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher based contingent reinforcement of smoking abstinence among methadone maintained patients: a pilot study. J Appl Behav Anal. 2008;41(4):527–538. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal. 2008;41(4):617–622. doi: 10.1901/jaba.2008.41-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: comparing 2 different cognitive behavioral treatments. Addict Disord Their Treat. 2010;9(2):64–74. [Google Scholar]
- 23.Tevyaw TOL, Colby SM, Tidey JW et al. Contingency management and motivational enhancement: a randomized clinical trial for college student smokers. Nicotine Tob Res. 2009;11(6):739–749. doi: 10.1093/ntr/ntp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correia CJ, Benson TA. The use of contingency management to reduce cigarette smoking among college students. Exp Clin Psychopharmacol. 2006;14(2):171–179. doi: 10.1037/1064-1297.14.2.171. [DOI] [PubMed] [Google Scholar]
- 25.Heil SH, Higgins ST, Bernstein IM et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins ST, Bernstein IM, Washio Y et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105(11):2023–2030. doi: 10.1111/j.1360-0443.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55(suppl):S33–S40. doi: 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpp KG, Levy AG, Asch DA et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev. 2006;15(1):12–18. doi: 10.1158/1055-9965.EPI-05-0314. [DOI] [PubMed] [Google Scholar]
- 29.Volpp KG, Troxel AB, Pauly MV et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 30.Stoops WW, Dallery J, Fields NM et al. An Internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug Alcohol Depend. 2009;105(1-2):56–62. doi: 10.1016/j.drugalcdep.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Businelle MS, Kendzor DE, Kesh A et al. Small financial incentives increase smoking cessation in homeless smokers: a pilot study. Addict Behav. 2014;39(3):717–720. doi: 10.1016/j.addbeh.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Etter JF. Financial incentives for smoking cessation in low-income smokers: study protocol for a randomized controlled trial. Trials. 2012;13:88. doi: 10.1186/1745-6215-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonevski B, Bryant J, Lynagh M, Paul C. Money as motivation to quit: a survey of a non-random Australian sample of socially disadvantaged smokers’ views of the acceptability of cash incentives. Prev Med. 2012;55(2):122–126. doi: 10.1016/j.ypmed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Davis TC, Long SW, Jackson RH et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 35.Murphy PW, Davis TC, Long SW, Jackson RH, Decker BC. Rapid estimate of adult literacy in medicine (REALM): a quick reading test for patients. J Read. 1993;37(2):124–130. [Google Scholar]
- 36.Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the heaviness of smoking index and its two components: findings from the International Tobacco Control Four Country Study. Nicotine Tob Res. 2010;12(suppl):S45–S50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 38.Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- 39.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 40.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ. Rockville, MD: US Department of Health and Human Services; 2008. Treating tobacco use and dependence: 2008 update. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed October 17, 2014. [Google Scholar]
- 41.Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 42.Piper ME, Cook JW, Schlam TR et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997;22(4):521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 44.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Prev Med. 2012;55(suppl):S24–32. doi: 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berlin I, Radzius A, Moolchan ET, Henningfield JE. Correlates of expired air carbon monoxide: effect of ethnicity and relationship with saliva cotinine and nicotine. Nicotine Tob Res. 2001;3(4):325–331. doi: 10.1080/14622200110050400. [DOI] [PubMed] [Google Scholar]
- 47.Fritz M, Wallner R, Grohs U, Kemmler G, Saria A, Zernig G. Comparable sensitivities of urine cotinine and breath carbon monoxide at follow-up time points of three months or more in a smoking cessation trial. Pharmacology. 2010;85(4):234–240. doi: 10.1159/000280435. [DOI] [PubMed] [Google Scholar]
- 48.Gilpin EA, Pierce JP, Farkas AJ. Duration of smoking abstinence and success in quitting. J Natl Cancer Inst. 1997;89(8):572–576. doi: 10.1093/jnci/89.8.572. [DOI] [PubMed] [Google Scholar]
- 49.Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- 50.Petry NM, Peirce JM, Stitzer ML et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- 51.Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: contingency management treatment of substance abusers in community settings. J Consult Clin Psychol. 2005;73(6):1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]