Abstract
Objectives. We demonstrated the effectiveness of an accelerated hepatitis B vaccination schedule in drug users.
Methods. We compared the long-term effectiveness of accelerated (0–1–2 months) and standard (0–1–6 months) hepatitis B vaccination schedules in preventing hepatitis B virus (HBV) infections and anti-hepatitis B (anti-HBs) antibody loss during 2-year follow-up in 707 drug users (HIV and HBV negative at enrollment and completed 3 vaccine doses) from February 2004 to October 2009.
Results. Drug users in the accelerated schedule group had significantly lower HBV infection rates, but had a similar rate of anti-HBs antibody loss compared with the standard schedule group over 2 years of follow-up. No chronic HBV infections were observed. Hepatitis C positivity at enrollment and age younger than 40 years were independent risk factors for HBV infection and antibody loss, respectively.
Conclusions. An accelerated vaccination schedule was more preferable than a standard vaccination schedule in preventing HBV infections in drug users. To overcome the disadvantages of a standard vaccination schedule, an accelerated vaccination schedule should be considered in drug users with low adherence. Our study should be repeated in different cohorts to validate our findings and establish the role of an accelerated schedule in hepatitis B vaccination guidelines for drug users.
One of the most common blood-borne pathogens, hepatitis B virus (HBV), has been estimated to infect approximately 2 billion people worldwide, including 350 million who live with chronic infection.1 From these chronic infections, approximately 15% to 40% will develop cirrhosis, liver failure, and hepatocellular carcinoma, which can lead to enormous medical expenses and loss of life.2 These staggering numbers are reflected in the US national HBV statistics as well, with a reported prevalence of 704 000 chronic HBV infections and 4.6% exposure in the general noninstitutionalized population during 1999 to 2008.3,4 Because of its asymptomatic progression and high infectious potential (50–100 times compared with HIV), HBV has a greater potential to spread in the population, especially in high-risk groups such as drug users.5
Hepatitis B vaccine has been proved to be highly immunogenic and effective in prevention of HBV infection in infants and healthy adults since its introduction in 1984. However, despite the availability of a highly efficacious vaccine, hepatitis B still remains highly prevalent in drug users. Impairment of inhibition regarding high-risk sexual behavior, sharing needles, shooting galleries, drug–sex exchanges, and the level of infection within the locality play an important role in increasing the risk of acquiring these infections in injecting and noninjecting drug users (IDUs and NIDUs).6–8 Surprisingly, hepatitis-related awareness is low among HIV knowledgeable drug users.9 A very high prevalence of HBV (64%) has been observed among IDUs compared with the general population.10 With approximately 51 000 new cases of HBV infections per year, 16% are estimated to be IDUs, and unvaccinated IDUs have an incidence density ranging from 10 to 31 infections per 100 person-years.11 Furthermore, an increasing trend of drug users who adopt noninjected routes of heroin administration has been observed in the United States and other countries since late 1980s.12 NIDUs may consist of former injectors who may have already been infected with HIV/HCV or never injectors who may become exposed to HBV infection through unprotected sex through high-risk sex partners.13 NIDUs may serve as a potential sexual transmission bridge between high prevalence IDUs to the low prevalence general population.14 Although studies examining HBV infection in NIDUs are generally lacking, a study conducted in adult noninjecting heroin users in New York City (1996–2001) reported that 24% of never injectors and 49% of former injectors were infected with HBV.13 Therefore, both IDUs and NIDUs are among the prioritized target population for immunization in the United States.
Low acceptance and adherence to the standard vaccination schedule (0, 1, 6 months) is one of the primary concerns in this unstable population. Drug users are a hard to reach and mobile population who often lack access to health care. They have multiple social, psychological, and medical needs that lead to frequent change of residence, imprisonment, or admission to a therapeutic community.15,16 Other barriers to vaccine compliance include competing needs, poor relationships with health care providers, and lack of information or education.17,18 In our recent hepatitis B vaccine intervention trial among not-in-treatment drug users, we identified that participants on an accelerated schedule (0, 1, 2 months) were significantly more likely to receive 3 doses of vaccine than those on the standard schedule (76% vs 66%, respectively; P < .05).19 Moreover, these participants also had a greater anti-hepatitis B (anti-HBs; antibody to hepatitis B surface antigen) seroconversion compared with the standard schedule group at 6 months (70% vs 46%; P < .001).20 With an earlier immune response and better adherence, the accelerated schedule seems more advantageous. Despite these encouraging results, the long-term effectiveness of this accelerated vaccination schedule remains unexamined. Because an anti-HBs level of more than 10 milli-international units per milliliter is needed to offer seroprotection against HBV, and because drug users develop a suboptimal immune response following hepatitis B vaccination (58%–77%),21 it is imperative to examine the levels of antibody protection offered by an accelerated schedule beyond 12 months.22
Thus, we aimed to compare the long-term effectiveness of an accelerated vaccination schedule with a standard vaccination schedule in preventing HBV infections in cohort of 707 drug users, who were free of HIV and HBV infection at enrollment and had completed 3 doses of vaccination during our HBV vaccine intervention trial. Our secondary aim was to identify the risk factors associated with anti-HBs antibody loss and HBV infection, respectively.
METHODS
We conducted a large HBV vaccine intervention trial from February 2004 to October 2009 in 2 highly endemic drug-using neighborhoods of Houston, Texas. Inclusion criteria for participants were aged 18 years or older, current illicit drug use in the 48 hours before enrollment, and negative screening tests for antibodies to HIV (anti-HIV), hepatitis B surface antigen (HBsAg), and anti-HBs.19 Of 1260 not-in-treatment current drug users enrolled and cluster randomized into the accelerated or standard HBV vaccination schedule, a total of 941 drug users completed all 3 vaccine doses.19 Of these, 234 drug users were excluded because they had immunity from natural hepatitis infection (presence of anti-HBs or hepatitis B core antibody [anti-HBc]; n = 222), active infection (presence of HBsAg with anti-HBc; n = 0), or vaccination before enrollment in the trial (presence of anti-HBs only; n = 9).20 The remaining 707 drug users were susceptible to new HBV infection at enrollment (negative tests for HBsAg, anti-HBc, and anti-HBs titer < 10 mIU/mL) and had completed all 3 HBV vaccine doses for their schedule group; thus, we included them for analysis in our study. All data collection procedures and laboratory methods were approved by the Committee for the Protection of Human Subjects at our institution.
Data Collection and Laboratory Methods
After a signed informed consent, participants provided data on their demographic, drug use, social, and behavioral characteristics. We collected this information through verbally administered questionnaires that were also recorded electronically via computer-assisted personal interviews (CAPI, QDS, Bethesda, MD). We collected variables such as age, gender, race/ethnicity, education level, marital status, living arrangements, employment, history of drug use patterns, and sexual habits. We recorded drug use patterns such as current drug use, past drug use, age at drug initiation and length of drug use, 30-day use frequency, and the pattern at the time of heaviest use. We collected these details for drugs such as crack cocaine, powder cocaine, methamphetamine, marijuana, “fry,” heroin, speedball, codeine syrup, alcohol, or other street drugs. We also documented a detailed history of binging on these drugs. Drug history also included questions on injecting drugs, age at first injection, frequency and duration of injections, type of drug injected, use of clean needles, and sharing of drug use equipment with others19 (data available as a supplement to the online version of this article at http://www.ajph.org).
We performed blood draws for viral markers along with interviews at 0, 6, 12, 18, and 24 months. We tested the blood specimens for HBsAg, anti-HBs, anti-HBc, and anti-HCV antibodies using Abbott’s AxSYM system (Abbott Laboratories, Chicago, IL) and anti-HIV antibodies using the Abbott PPC Commander system.
HBV infection was considered when any participant from the 707 HBV susceptible drug users seroconverted from a negative to a positive test for anti-HBc antibody, HBsAg, or both at any time between enrollment and 24 months of follow-up. Thus, our analysis time began at enrollment for incidence of HBV infection calculations. We only calculated anti-HBs antibody loss (anti-HBs < 10 mIU/mL) for 339 participants who generated a positive response to vaccine (anti-HBs ≥ 10 mIU/mL and free from HBV infection after completion of 3 vaccine doses) at the 12-month follow-up. Thus, our analysis time began at 12 months for antibody loss calculations. Chronic HBV infection was defined as testing HBsAg positive for more than 6 months.
Statistical Analyses
We used STATA version 11.0 (STATA Corporation, College Station, TX) to complete all data analyses. After we examined preliminary data for consistency and accuracy, we calculated incidence densities of anti-HBs antibody loss and HBV infection from person–time data. We computed life tables to calculate the cumulative incidences for each outcome by using the Kaplan–Meier method, and we performed comparisons using Wilcoxon tests. We compared failure rates for the 2 vaccination schedules using a likelihood ratio test. We calculated unadjusted and adjusted incidence density ratios and 95% confidence intervals (CIs) for various exposures of interest by a Cox proportional hazards model. We retained variables in the final multiple regression model based on their statistical significance or relevance to hepatitis B infection. We also stratified incidence densities for HBV infection and antibody loss by age, gender, race, education, marital status, housing status, intravenous drug use, and illicit drug use; and traded sex for money or drugs, men who have sex with men, and total number of sexual partners in past 30 days. We compared Kaplan–Meier failure curves for each outcome between the 2 vaccination schedules using the Breslow–Gehan–Wilcoxon tests. A 2-tailed P < .05 was considered statistically significant for measures of association.
RESULTS
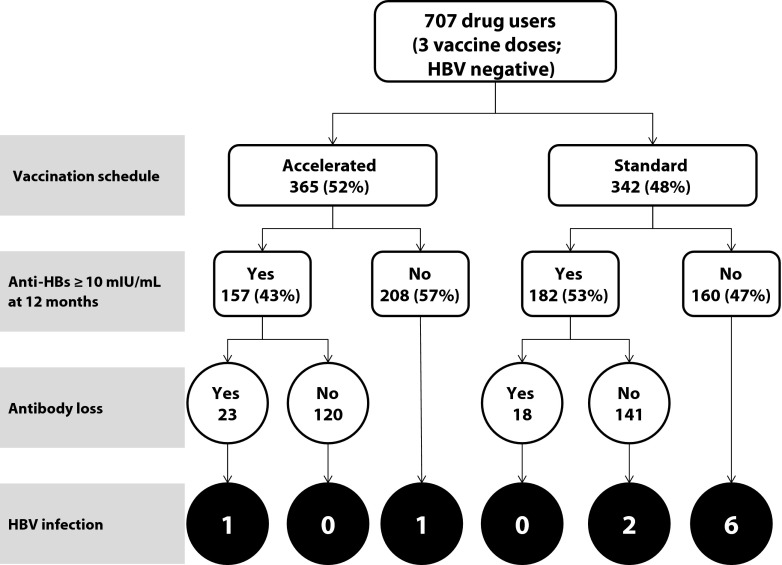
The follow-up rates at each time point for 707 HBV susceptible drug users was 92% (650) at 6 months, 81% (576) at 12 months, 73% (519) at 18 months, and 67% (473) at 24 months. Baseline characteristics for this study population, stratified by the 2 vaccination schedules, were described in detail in our earlier study.20 These 707 participants contributed approximately 1166 person-years from the time of their enrollment. We show the outcomes for the our study population in Figure 1. A large proportion of participants did not develop the desired anti-HBs antibody level at 12-month follow-up (57% and 47%), and these nonresponders had the highest incidence of HBV infection (1 and 6 cases) in accelerated and standard schedule groups, respectively.
FIGURE 1—
Outcomes stratified by vaccination schedules: Long-Term Effectiveness of Accelerated Hepatitis B Vaccination Schedule.
Note. HBs = hepatitis B; HBV = hepatitis B virus. Data on antibody loss were not available for 14 (9%) drug users in the accelerated schedule group and 23 (13%) drug users in the standard schedule group.
Anti-Hepatitis B Antibody Loss and Persistence
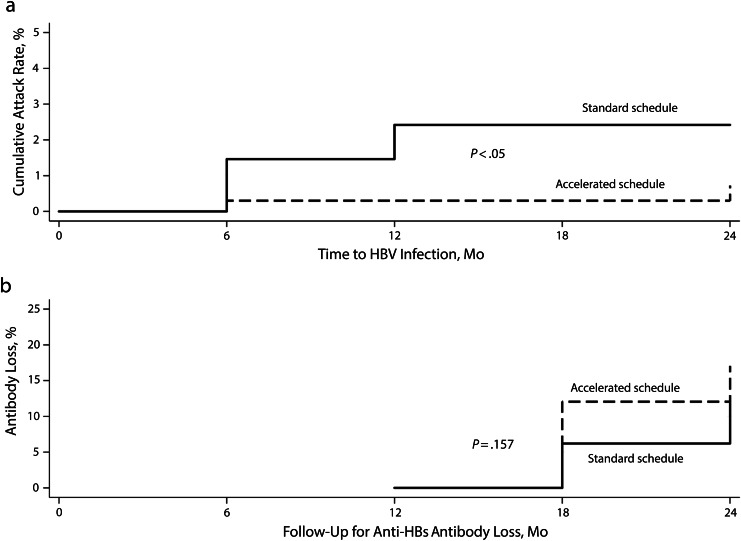
Of the 339 drug users who developed an adequate seroprotective response after completion of 3 vaccine doses and were free of infection at 6 months, a total of 41 lost detectable anti-HBs antibodies, including 23 in the accelerated group and 18 in the standard group (Figure 2). The cumulative incidence of antibody loss for the entire subgroup was 20 cases per 100 drug users (cumulative incidence of antibody persistence was 80%). We could not identify any statistically significant difference for anti-HBs antibody loss between accelerated and standard vaccine schedules (22 vs 18 cases per 100 drug users, respectively; likelihood ratio test: P = .223). The geometric mean titers (GMT) were 116, 105, 58, 67, and 70 milli-international units per milliliter for the accelerated schedule and 128, 64, 185, 159 and 150 milli-international units per milliliter for the standard schedule at 2, 6, 12, 18, and 24 months, respectively. We found no significant difference between the incidence density for anti-HBs antibody loss for the accelerated and standard schedules (186 and 127 cases per 1000 person-years, respectively).
FIGURE 2—
Kaplan-Meier failure curves, stratified by vaccination schedules, for (a) hepatitis B virus (HBV) infection and (b) antibody to hepatitis B surface antigen (anti-HBs) loss: Long-Term Effectiveness of Accelerated Hepatitis B Vaccination Schedule.
Note. The sample sizes were n = 707 HBV-susceptible drug users in part a and n = 339 vaccine responders at 12 months in part b. P values were determined by Breslow–Gehan–Wilcoxon test of homogeneity.
The effects of various exposures on incidence rate of antibody loss are demonstrated in Table 1. None of the exposures listed in the Table 1 significantly predicted antibody loss, except for age. Drug users who were aged 40 years or older at enrollment had a 57% decreased incidence of antibody loss compared with those younger than 40 years (P < .05).
TABLE 1—
Effect of Selected Exposures on the Incidence Rate of Anti-Hepatitis B Antibody Loss Among Successfully Vaccinated Drug Users Over the 2-year Follow-Up, Long-Term Effectiveness of Accelerated Hepatitis B Vaccination Schedule
| Predictor Variable | Total (No.) | No. With Anti-HBs Loss (No.) | Person-Years | Incidence Density | Adjusted Relative Risk (95% CI)a | P |
| Overall | 339 | 41 | 265 | 154.7 | . . . | |
| Vaccination schedule | .168 | |||||
| Standard | 182 | 18 | 141.5 | 127.2 | 1.00 (Ref) | |
| Accelerated | 157 | 23 | 123.5 | 186.2 | 1.55 (0.83, 2.89) | |
| Age, y | .014 | |||||
| < 40 | 126 | 21 | 93.5 | 224.6 | 1.00 (Ref) | |
| ≥ 40 | 213 | 20 | 171.5 | 116.6 | 0.43 (0.22, 0.84) | |
| Gender | . . . | |||||
| Male | 248 | 32 | 186 | 172.0 | ||
| Female | 91 | 9 | 79 | 113.9 | ||
| Race | .258 | |||||
| Non-African Americans | 38 | 3 | 25.5 | 117.6 | 1.00 (Ref) | |
| African American | 301 | 38 | 239.5 | 158.7 | 2.1 (0.58, 7.57) | |
| Education | . . . | |||||
| ≤ high school | 263 | 32 | 206 | 155.3 | ||
| > high school | 76 | 9 | 59 | 152.5 | ||
| HCV at enrollment | 57 | 8 | 42 | 190.5 | 2.13 (0.83, 5.48) | .116 |
| MSM | 24 | 4 | 17 | 235.3 | 1.68 (0.59, 4.83) | .332 |
| Drug use in past 30 d | ||||||
| IDU | 13 | 1 | 10 | 99.9 | . . . | |
| Crack | 311 | 37 | 244 | 151.6 | . . . | |
| Cocaine | 51 | 8 | 38 | 210.5 | . . . | |
| Methamphetamine | 10 | 1 | 8.5 | 117.6 | . . . | |
| Fry | 10 | 2 | 7.5 | 266.7 | . . . | |
| Marijuana | 179 | 20 | 138 | 145.5 | . . . | |
| Alcohol | 235 | 28 | 187 | 150.1 | . . . | |
| Heroin | 9 | 1 | 4.5 | 222.2 | . . . | |
| Speedball | 5 | 0 | 2 | 0 | . . . | |
| Ever used injecting drugs | 60 | 6 | 44 | 136.4 | 0.77 (0.27, 2.17) | .616 |
| Multidrug use | . . . | |||||
| < 2 | 59 | 7 | 46 | 152.2 | ||
| ≥ 2 | 280 | 34 | 219 | 155.3 |
Note. CI = confidence interval; HBs = hepatitis B; IDU = injecting drug use; MSM = men who have sex with men.
Cox proportional hazard regression.
Hepatitis B Virus Infection
During the 2-year follow-up period, we detected HBV infection in 10 drug users who had completed their vaccination doses, thus giving a cumulative incidence of 2 cases per 100 drug users. Of these 10 infection cases, 8 had received vaccination according to the standard schedule compared with 2 cases who received it according to the accelerated schedule (cumulative incidence of 3 cases vs 1 case per 100 drug users, respectively). This was a statistically significant difference in the incidence of HBV infection between the 2 vaccination schedules (likelihood ratio test χ2 = 4; P < .05). The incidence density for the accelerated vaccination schedule (3 cases per 1000 person-years) was significantly lower than that for the standard schedule (14 cases per 1000 person-years). Incidence densities stratified for various exposures of interest are shown in Table 2. None of the exposures listed in Table 2 significantly predicted acquisition of HBV infection, except for HCV seropositivity at enrollment. Having HCV at enrollment increased the risk for acquiring HBV infection during the follow-up (adjusted risk ratio = 6.54; 95% CI = 1.49, 28.76; P = .013). Although not statistically significant, the risk for HBV infection increased by 60% for participants who did not develop protective anti-HBs level at 12 months. Similarly, the risk of HBV infection was increased by 80% when participants were vaccinated using the standard schedule compared with the accelerated schedule. Kaplan-Meier failure curves demonstrated the significant difference in cumulative incidence rate for HBV infection for the 2 vaccination schedules (P < .05 for Breslow–Gehan–Wilcoxon test; Figure 2).
TABLE 2—
Effect of Selected Exposures on the Incidence Rate of Hepatitis B Virus Infection Among Successfully Vaccinated Drug Users Over the 2-year Follow-Up, Long-Term Effectiveness of Accelerated Hepatitis B Vaccination Schedule
| Predictor Variable | Total (No.) | No. With HBV Infection | Person-Years | Incidence Density | Adjusted Relative Risk (95% CI)a | P |
| Overall | 707 | 10 | 1166 | 8.6 | . . . | |
| Vaccination schedule | .053 | |||||
| Standard | 342 | 8 | 576 | 13.9 | 1.00 (Ref) | |
| Accelerated | 365 | 2 | 590 | 3.4 | 0.21 (0.04, 1.02) | |
| Age, y | .422 | |||||
| < 40 | 250 | 3 | 394 | 7.6 | 1.00 (Ref) | |
| ≥ 40 | 457 | 7 | 772 | 9.1 | 0.53 (0.11, 2.5) | |
| Gender | . . . | |||||
| Male | 543 | 8 | 881 | 9.1 | ||
| Female | 164 | 2 | 286 | 7.0 | ||
| Race | .293 | |||||
| Non-African | 59 | 1 | 130 | 7.7 | 1.00 (Ref) | |
| African American | 616 | 9 | 1036 | 8.7 | 3.5 (0.34, 36.27) | |
| Education | . . . | . . . | ||||
| ≤ high school | 534 | 9 | 885 | 10.2 | ||
| > high school | 173 | 1 | 281 | 3.6 | ||
| HCV at enrollment | 139 | 6 | 218 | 27.5 | 6.54 (1.49, 28.76) | .013 |
| MSM | 47 | 1 | 71 | 14.2 | 1.63 (0.19, 13.96) | .658 |
| Past 30 d | ||||||
| IDU | 36 | 0 | 52 | 0 | . . . | . . . |
| Crack | 656 | 9 | 1090 | 8.3 | . . . | |
| Cocaine | 98 | 2 | 152 | 13.2 | . . . | |
| Methamphetamine | 22 | 0 | 39 | 0 | . . . | |
| Fry | 16 | 0 | 27 | 0 | . . . | |
| Marijuana | 345 | 4 | 563 | 7.1 | . . . | |
| Alcohol | 491 | 8 | 809 | 9.9 | . . . | |
| Heroin | 19 | 0 | 28 | 0 | . . . | |
| Speedball | 7 | 0 | 10 | 0 | . . . | |
| Ever used injecting drugs | 146 | 4 | 233 | 17.2 | 1.75 (0.38, 7.95) | .47 |
| No. drug use | . . . | |||||
| < 2 | 138 | 0 | 224 | 0 | ||
| ≥ 2 | 569 | 10 | 942 | 10.6 | ||
| Anti-HBs antibody (mIU/ml)b | .176 | |||||
| < 10 | 368 | 7 | 552.5 | 12.7 | 1.00 (Ref) | |
| ≥ 10 | 339 | 3 | 613.5 | 4.9 | 0.39 (0.1, 1.53) |
Note. CI = confidence interval; HBs = hepatitis B; IDU = injecting drug use; MSM = men who have sex with men.
Cox proportional hazard regression.
At 12 months postenrollment.
A detailed description for 10 HBV infected drug users including age, gender, vaccination schedule, anti-HBc test results, and anti-HBs status at 12 months is provided in Table 3. The majority of these users were male (80%), aged 40 years or older (70%), had 12 month anti-HBs levels less than 10 milli-international units per milliliter (70%), received vaccination according to the standard schedule (80%), and acquired HBV infection by 6 months of enrollment (60%). HBsAg was detected in 2 cases, with both the vaccination groups having 1 case each. No chronic infection was identified in either of the vaccination schedule groups over the 2-year period.
TABLE 3—
Timeline for Hepatitis B Virus Infected Drug Users: Long-Term Effectiveness of Accelerated Hepatitis B Vaccination Schedule
| Anti-HBc at Follow-Up, Months |
||||||||
| Patient | Age, Years | Gender | Anti-HBs Status at 12 Months (mIU/mL) | Vaccination Schedule | 6 | 12 | 18 | 24 |
| 1 | 53 | Male | < 10 | Standard | + | — | — | + |
| 2 | 54 | Male | < 10 | Standard | + | + | + | + |
| 3 | 43 | Male | < 10 | Standard | + | — | + | + |
| 4 | 54 | Female | < 10 | Standard | — | + | + | + |
| 5 | 41 | Male | < 10 | Standard | + | + | — | — |
| 6 | 52 | Male | ≥ 10 | Standard | — | + | + | + |
| 7 | 29 | Female | ≥ 10 | Standard | — | + | — | + |
| 8 | 28 | Male | < 10 | Standard | +a | — | — | + |
| 9 | 43 | Male | < 10 | Accelerated | + | + | — | — |
| 10 | 27 | Male | ≥ 10 | Accelerated | — | — | — | +a |
Note. HBc = hepatitis B core; HBs = hepatitis B; HBsAg = hepatitis B surface antigen; — = missing.
HBsAg positive.
DISCUSSION
Important conclusions can be drawn from our study, which was aimed at comparing the long-term effectiveness of accelerated and standard hepatitis B vaccine schedules in drug users. Compared with a standard vaccination schedule, drug users in an accelerated schedule had improved adherence and a significantly lower incidence of HBV infection, despite no significant difference in the rates of anti-HBs antibody loss during the 2-year follow-up.
Compared with the general population, which has excellent response rates to HBV vaccinations, drug users have suboptimal antibody response.15,23–26 The incidence of antibody loss during the follow-up period was not significantly different between the 2 vaccination schedules. To our knowledge, ours was the first study to examine the rate of antibody loss for a follow-up period of 2 years following HBV vaccination by an accelerated schedule. Therefore, we did not have comparative rates of antibody loss for this schedule from the literature. Loss of seroprotective antibody levels was not significantly associated with any host characteristics, except for age. Drug users aged 40 years or older were significantly less likely to lose seroprotection following HBV vaccination compared with younger vaccinated drug users. This was in contrast to a decreased seroprotection achievement by older drug users, as reported by previous studies.20,24 Thus, it could be speculated that although achieving seroprotection is difficult with increasing age, seroprotection was probably long term when achieved at an older age and less likely to be lost because of immune maturity. Increasing age is an important factor in immune response to HBV vaccination and should be examined further.
We observed a significantly lower incidence of HBV infection rates in the group that received vaccines per the accelerated schedule compared with the standard schedule. This might be explained by the fact that most of the drug users in the standard vaccination schedule were identified as infected at the 6-month follow-up visit, when they had not yet completed their third vaccine dose and developed a seroprotective immune response. The long time required for completion of vaccine doses according to the standard schedule that led to a delayed seroprotective immune response, coupled with continued exposure to HBV infection because of their high-risk behavior, made the accelerated vaccination schedule an ideal choice for vaccinating the drug-using population.
Limitations
The Breslow-Wilcoxon-Gehan test demonstrated a statistically significant difference in the HBV infection rate between the 2 vaccination schedules (P = .043), but the Cox proportional hazards regression analysis did not show a significant difference in this risk (adjusted P = .053). The Cox proportional hazards regression model might be lacking in adequate power to adjust for various confounders, because only 10 drug users developed HBV infection over the entire study period. As per our clinical trial reports, IDUs were more likely to benefit from the accelerated schedules,19 but we did not observe any difference with respect to drug use factors. Similarly, no host factors were significantly associated with acquiring HBV infection, except for being HCV positive at the time of enrollment. Other studies also documented that HCV infection at enrollment might be a risk factor for lack of seroconversion following hepatitis B vaccination.24,27,28 This might be explained by the fact that the participant might have high-risk behaviors for acquiring HBV and HCV infections, or a compromised immune status because of HCV infection that makes them more vulnerable to developing HBV infection. We could not explore this association further because of the secondary nature of this study. Therefore, we recommend future studies to examine this. Another limitation of our study was the loss to follow-up of drug users who might have lost their seroprotection or developed HBV infection. Unfortunately, this is an unavoidable limitation for any study that examines drug users. However, there were no differences in loss to follow-up rates between the 2 vaccination schedule groups, thus minimizing a potential bias.
Compared with the general population, overall anti-HBs antibody response rate was low; however, there was adequate seroprotective response in vaccinated drug users. We observed no significant difference in terms of antibody loss between the 2 schedules, but there was significantly higher incidence of HBV infection rate in the standard vaccination group. We observed no chronic HBV carriers in either of the vaccination schedule groups. Because this is a hard-to-reach population with documented low follow-up rates and adherence to vaccination schedules, an accelerated schedule might be more beneficial in this population. An accelerated schedule might help prevent HBV infections by completing 3 doses of vaccination earlier, unlike the standard schedule, in which an adequate seroprotective immune response might not be achieved until 6 months. A shorter schedule would also ensure better compliance rates in this difficult to follow-up population.19 In addition, if the participants are available at 6 months follow-up, a booster dose might be given at 6 months if a waning immune response is observed. An accelerated HB vaccination schedule was recommended in alcoholic patients29 and hemodialysis patients.30 An accelerated vaccination schedule in drug users would be one of the steps to decrease HBV infection and transmission in this high-risk population, who are still reported to have the highest rates of HBV infection despite availability of an effective vaccine. Because drug users do not have normal immune response to HBV vaccine, more immunogenic vaccines are needed. Addition of vaccine adjuvants, such as the immunostimulatory DNA sequence, might be one of the possible mechanisms to increase the immunogenicity of HBV vaccines and thus reduce the number of required doses.31 A recent review demonstrated that 1018 immunostimulatory DNA sequence plus recombinant HBsAg was safe and successful in the vaccine-hyporesponsive population.31 Another possible solution to increase the response rates and antibody titers in drug users could be the administration of 4 intramuscular or intradermal HBV vaccine double doses at 0, 1, 2, and 6 months, a strategy similar to that reported in HIV-infected adults.32,33 These methods should be further evaluated in the drug-using population.
Conclusions
An accelerated HBV vaccination schedule might be more preferable than a standard vaccination schedule in preventing HBV infections in drug users. Because compliance of a longer time period and continued high-risk behavior during that time were the 2 disadvantages involved with the standard vaccination schedule, we recommend the accelerated vaccination schedule as a potential solution in the drug-using population. Our study should be repeated in different cohorts to validate our findings and establish the role of an accelerated schedule in hepatitis B vaccination guidelines for this high-risk population.
Acknowledgments
This study was funded by the National Institute of Drug Abuse, National Institutes of Health (NIDA# 1R01DA017505).
Part of this study was presented at the 140th American Public Health Association (APHA) Annual Meeting, San Francisco, 2012.
Human Participant Protection
All data collection procedures and laboratory methods were approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center institutional review board.
References
- 1. World Health Organization. Hepatitis B. 2013. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/index.html. Accessed April 6, 2013.
- 2.Lok ASF. Chronic hepatitis B. N Engl J Med. 2002;346(22):1682–1683. doi: 10.1056/NEJM200205303462202. [DOI] [PubMed] [Google Scholar]
- 3.Wasley A, Kruszon-Moran D, Kuhnert W et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202(2):192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 4.Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154(5):319–328. doi: 10.7326/0003-4819-154-5-201103010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. 2009. Available at: http://www.cdc.gov/hepatitis/b/bfaq.htm#. Accessed August 15, 2014.
- 6.Hwang LY, Ross MW, Zack C, Bull L, Rickman K, Holleman M. Prevalence of sexually transmitted infections and associated risk factors among populations of drug abusers. Clin Infect Dis. 2000;31(4):920–926. doi: 10.1086/318131. [DOI] [PubMed] [Google Scholar]
- 7.Inciardi JA. Crack, crack house sex, and HIV risk. Arch Sex Behav. 1995;24(3):249–269. doi: 10.1007/BF01541599. [DOI] [PubMed] [Google Scholar]
- 8.Kral AH, Bluthenthal RN, Booth RE, Watters JK. HIV seroprevalence among street-recruited injection drug and crack cocaine users in 16 US municipalities. Am J Public Health. 1998;88(1):108–113. doi: 10.2105/ajph.88.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heimer R, Clair S, Grau LE, Bluthenthal RN, Marshall PA, Singer M. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction. 2002;97(10):1277–1287. doi: 10.1046/j.1360-0443.2002.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- 10.Murrill CS, Weeks H, Castrucci BC et al. Age-specific seroprevalence of HIV, hepatitis B virus, and hepatitis C virus infection among injection drug users admitted to drug treatment in 6 US Cities. Am J Public Health. 2002;92(3):385–387. doi: 10.2105/ajph.92.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mast EE, Weinbaum CM, Fiore AE et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33. quiz CE31–34. [PubMed] [Google Scholar]
- 12.Neaigus A, Atillasoy A, Friedman SR . Trends in the non-injected use of heroin and factors associated with the transition to injecting. In: Inciardi J, Harrison LD, editors. Heroin in the Age of Crack Cocaine. Thousand Oaks, CA: Sage; 1998. pp. 131–159. [Google Scholar]
- 13.Gyarmathy VA, Neaigus A, Miller M, Friedman SR, Des Jarlais DC. Risk correlates of prevalent HIV, hepatitis B virus, and hepatitis C virus infections among noninjecting heroin users. J Acquir Immune Defic Syndr. 2002;30(4):448–456. doi: 10.1097/00042560-200208010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Neaigus A, Miller M, Friedman SR, Des Jarlais DC. Sexual transmission risk among noninjecting heroin users infected with human immunodeficiency virus or hepatitis C virus. J Infect Dis. 2001;184(3):359–363. doi: 10.1086/322020. [DOI] [PubMed] [Google Scholar]
- 15.Budd J, Robertson R, Elton R. Hepatitis B vaccination and injecting drug users. Br J Gen Pract. 2004;54(503):444–447. [PMC free article] [PubMed] [Google Scholar]
- 16.Lugoboni F, Migliozzi S, Schiesari F et al. Immunoresponse to hepatitis B vaccination and adherence campaign among injecting drug users. Vaccine. 1997;15(9):1014–1016. doi: 10.1016/s0264-410x(96)00290-3. [DOI] [PubMed] [Google Scholar]
- 17.Seal KH, Edlin BR, Ochoa KC, Tulsky JP, Moss AR, Hahn JA. Risk of hepatitis B infection among young injection drug users in San Francisco: opportunities for intervention. West J Med. 2000;172(1):16–20. doi: 10.1136/ewjm.172.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine OS, Vlahov D, Koehler J, Cohn S, Spronk AM, Nelson KE. Seroepidemiology of hepatitis B virus in a population of injecting drug users. Association with drug injection patterns. Am J Epidemiol. 1995;142(3):331–341. doi: 10.1093/oxfordjournals.aje.a117639. [DOI] [PubMed] [Google Scholar]
- 19.Hwang LY, Grimes CZ, Tran TQ et al. Accelerated hepatitis B vaccination schedule among drug users: a randomized controlled trial. J Infect Dis. 2010;202(10):1500–1509. doi: 10.1086/656776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran TQ, Grimes CZ, Lai D, Troisi CL, Hwang L-Y. Effect of age and frequency of injections on immune response to hepatitis B vaccination in drug users. Vaccine. 2012;30(2):342–349. doi: 10.1016/j.vaccine.2011.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quaglio G, Lugoboni F, Mezzelani P, Des Jarlais DC, Lechi A. Hepatitis vaccination among drug users. Vaccine. 2006;24(15):2702–2709. doi: 10.1016/j.vaccine.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine. 2010;28(3):623–631. doi: 10.1016/j.vaccine.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 23.Ramasamy P, Lintzeris N, Sutton Y, Taylor H, Day CA, Haber PS. The outcome of a rapid hepatitis B vaccination programme in a methadone treatment clinic. Addiction. 2010;105(2):329–334. doi: 10.1111/j.1360-0443.2009.02765.x. [DOI] [PubMed] [Google Scholar]
- 24.Quaglio G, Talamini G, Lugoboni F et al. Compliance with hepatitis B vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction. 2002;97(8):985–992. doi: 10.1046/j.1360-0443.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 25.Rumi M, Colombo M, Romeo R et al. Suboptimal response to hepatitis B vaccine in drug users. Arch Intern Med. 1991;151(3):574–578. [PubMed] [Google Scholar]
- 26.Kamath GR, Shah DP, Hwang LY. Immune response to hepatitis B vaccination in drug using populations: a systematic review and meta-regression analysis. Vaccine. 2014;32(20):2265–2274. doi: 10.1016/j.vaccine.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 27.Lee SD, Chan CY, Yu MI, Lu RH, Chang FY, Lo KJ. Hepatitis B vaccination in patients with chronic hepatitis C. J Med Virol. 1999;59(4):463–468. [PubMed] [Google Scholar]
- 28.Wiedmann M, Liebert UG, Oesen U et al. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology. 2000;31(1):230–234. doi: 10.1002/hep.510310134. [DOI] [PubMed] [Google Scholar]
- 29.Rosman AS, Basu P, Galvin K, Lieber CS. Efficacy of a high and accelerated dose of hepatitis B vaccine in alcoholic patients: a randomized clinical trial. Am J Med. 1997;103(3):217–222. doi: 10.1016/s0002-9343(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 30.Medical Economics Co. Physicians’ Desk Reference. Oradell, NJ: Medical Economics Co; 1997. [Google Scholar]
- 31.Cooper C, Mackie D. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: a review of HEPLISAV™ safety and efficacy. Expert Rev Vaccines. 2011;10(4):417–427. doi: 10.1586/erv.10.162. [DOI] [PubMed] [Google Scholar]
- 32.Potsch DV, Camacho LA, Tuboi S et al. Vaccination against hepatitis B with 4-double doses increases response rates and antibodies titers in HIV-infected adults. Vaccine. 2012;30(41):5973–5977. doi: 10.1016/j.vaccine.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Launay O, van der Vliet D, Rosenberg AR et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305(14):1432–1440. doi: 10.1001/jama.2011.351. [DOI] [PubMed] [Google Scholar]