Abstract
Objectives. We estimated smoking-attributable mortality, assessed the impact of past smoking on recent mortality, and computed expected future losses in life expectancy caused by past and current smoking behavior in Latin America and the Caribbean.
Methods. We used a regression-based procedure to estimate smoking-attributable mortality and information for 6 countries (Argentina, Brazil, Chile, Cuba, Mexico, and Uruguay) for the years 1980 through 2009 contained in the Latin American Mortality Database (LAMBdA). These countries jointly comprise more than two thirds of the adult population in Latin America and the Caribbean and have the region’s highest rates of smoking prevalence.
Results. During the last 10 years, the impact of smoking was equivalent to losses in male (aged ≥ 50 years) life expectancy of about 2 to 6 years. These effects are likely to increase, particularly for females, both in the study countries and in those that joined the epidemic at later dates.
Conclusions. Unless innovations in the detection and treatment of chronic diseases are introduced soon, continued gains in adult survival in Latin America and the Caribbean region may slow down considerably.
Continuous progress in the remarkable mortality decline in Latin America and the Caribbean region1 may be difficult to sustain. This possibility is foreshadowed in a recent report showing that cancers of the respiratory tract, particularly lung cancers, are among the 3 most important forms of cancer in the region and are primary causes of adult mortality.2 It is known that these chronic illnesses are closely connected to smoking, but less is known about the actual contribution of past smoking on current and future adult mortality in these countries. It could well be that, if pervasive enough, past (and future) smoking behavior trumps long-term trends in adult mortality.
In response to the increasing vigilance and massive public health campaigns against tobacco consumption that began in the United States after the mid-1960s, the tobacco industry initiated an aggressive program to open new markets in Europe, Asia, and Latin America.3–5 A number of sociodemographic factors contributed to the higher numbers of potential smokers in Latin America and the Caribbean region beginning in the 1950s: the explosive growth in the populations of adolescents and young adults, who are at highest risk for smoking initiation; the spread of an urban lifestyle and the accelerated growth of cities; greater access to education; and the entry of women into the labor market.6,7 Increasing cigarette affordability,8–10 widespread legislative maneuvers,6,11–13 and a sophisticated publicity machine8,12–14 contributed to a massive market expansion for tobacco in all forms and cigarettes in particular. As a result, cigarette consumption increased first in countries in the vanguard of mortality decline (Argentina, Uruguay, Cuba, and Chile) and then in Mexico, Brazil, Colombia, Costa Rica, and Panama.3,15 Countries with higher mortality, such as, Peru, Ecuador, Bolivia, Paraguay, and Guatemala, still have low levels of smoking, but some of them (e.g., Bolivia) are catching up rapidly. The spread of smoking is known in public health circles as the “smoking epidemic”—a term we adopt here.16,17
According to a useful typology,18 countries in Latin America and the Caribbean span a broad range of experiences in the smoking epidemic, from those in the late stages (Argentina, Chile, Cuba, and Uruguay) to those of more recent onset (Mexico and Brazil).19 Table 1 contains key indicators for these 6 countries (plus the United States for comparative purposes).24 Males in 4 countries—Argentina, Cuba, Chile, and Uruguay—have higher rates of smoking than do US males, whereas the rates are lower in Brazil and Mexico. As we will show, Cuba’s unique position at the top of the ranking of smoking prevalence translates into the highest estimated excess adult mortality. Female rates lag behind male rates everywhere, but they have reached levels of around 20% in Argentina and Chile. Age-specific smoking prevalence rates for the 6 countries in this study (data available as a supplement to the online version of this article at http://www.ajph.org) display a high degree of heterogeneity and reflect characteristics typical of different stages of the epidemic. These age patterns reveal telling anomalies: an exceptionally high prevalence among the population younger than 25 years in Chile, signs of a recrudescence of the smoking epidemic, and unexpectedly low levels of adolescent smoking in Brazil, an indication of successful antismoking campaigns.19,26
TABLE 1—
Characteristics of the Smoking Epidemic Among Adults Aged 20–80 Years: Argentina, Brazil, Chile, Cuba, Mexico, Uruguay, and United States; 2005–2009
| Argentina, 2005 | Brazil, 2008 | Chile, 2006 | Cuba, 2009 | Mexico, 2009 | Uruguay, 2009 | United States, 2007 | |
| Males | |||||||
| No. | 16 647 | 15 995 | 7 981 | 5 350 | 2 360 | 2 228 | |
| Smoking prevalence/100 persons (SD)a | 35.7 (0.8) | 24.0 (0.4) | 37.8 (0.6) | 44.8c | 23.8 (0.7) | 32.5 (1.3) | 28.4 (45.1) |
| No. of cigarettes/d, mean (SD)a | 13.1 (0.3) | 15.3 (0.2) | 5.8 (0.1) | 10.3 (0.5) | 11.0 (0.5) | 16.5 (11.8) | |
| No. of cigarettes/y, mean (SD)a | 4 783.0 (109.3) | 5 583.7 (88.1) | 2 080.8 (48.3) | 3 752.9 (174.3) | 4 027.6 (166.6) | 6 040.7 (4322.1) | |
| Deaths/100 persons attributable to tobacco (all causes)b | 19 | 15 | 11 | 21 | 7 | 24 | 23 |
| Deaths/100 000 persons attributable to tobacco (trachea, bronchus, and lung cancers)b | 75 | 35 | 32 | 90 | 18 | 115 | 103 |
| Females | |||||||
| No. | 21 907 | 19 176 | 7 968 | 6 220 | 2 617 | 2 400 | |
| Smoking prevalence/100 persons (SD)a | 25.7 (0.7) | 14.5 (0.3) | 28.0 (0.6) | 29.6c | 7.7 (0.5) | 22.5 (1.0) | 21.5 (41.1) |
| No. of cigarettes/d, mean (SD)a | 9.6 (0.2) | 12.6 (0.2) | 4.9 (0.1) | 8.5 (0.5) | 10.9 (0.4) | 14.5 (10.1) | |
| No. of cigarettes/y, mean (SD)a | 3 507.1 (79.8) | 4 614 (83.4) | 1 757.6 (47) | 3 102.2 (200.0) | 3 962.7 (136.8) | 5 284.1 (3 702.1) | |
| Deaths/100 persons attributable to tobacco (all causes)b | 6 | 6 | 8 | 18 | 6 | 5 | 23 |
| Deaths/100 000 persons attributable to tobacco (trachea, bronchus, and lung cancers)b | 12 | 6 | 10 | 78 | 4 | 10 | 68 |
| Yearly consumption ratio, female–malea | 0.73 | 0.83 | 0.80 | 0.84 | 0.98 | 0.87 |
Note. Values in the table were computed from information contained in the original sources.
Source. National Risk Factors Study (Argentina),20 Global Adult Tobacco Survey (Brazil, Mexico, and Uruguay),21 Social Protection Study (Chile),22 and National Health and Nutrition Examination Survey (NHANES; Smoking Module).23
Population weighted and age standardized (Standard NHANES 2007–2008)23 for Argentina, Brazil, Chile, Mexico, and Uruguay.
World Health Organization (2012)24 estimated proportion of deaths attributable to tobacco and death rates correspond with 2004 and are totals for individuals aged 30 years and older.
Age standardized for individuals aged 15 years and older.25
The typology mentioned here is useful for comparing aggregate, country-specific conditions and is not informed by—nor does it intend to inform—individual psychological traits responsible for smoking-related behavior in the countries to which it is applied.
EFFECTS OF PAST SMOKING ON CURRENT MORTALITY
The causal connection between smoking and disease is well established, as are the mechanisms through which smoking affects mortality. There is considerable evidence that 2 conditions—lung cancer and chronic obstructive pulmonary disease—are more prevalent among smokers than nonsmokers.27–30 Empirical evidence also implicates smoking as a causative factor of other types of cancers and chronic illnesses, particularly cardiovascular disease.16–19,25–32
Cancer is rapidly becoming the most important cause of death among adults in Latin America and the Caribbean.2 Although its overall incidence is about one half that in the United States, its mortality rate is twice as high, a reflection of lower access to and use of early screening and effective treatment.2 Cancers of the respiratory tract—lung cancer in particular—are the second or third most important forms of cancer in the region.2 Although a fraction of these are related to environmental exposures, most are attributable to smoking.
Male lung cancer rates are highest (about 50%–75% of US rates) and steady or increasing in Cuba, Argentina, and Chile and steady or slowly decreasing in Uruguay, a country with a more vigorous antismoking campaign. The rates are lower but increasing in Brazil and Mexico, particularly among women.3,12,15 The rank order of countries according to the magnitude of lung cancer death rates is consistent with the stage of the smoking epidemic they are experiencing. In all cases, male rates are higher than female rates, but the pace of increase of smoking prevalence among females now exceeds that among males (data available as a supplement to the online version of this article at http://www.ajph.org).
EFFECTS OF PAST SMOKING ON FUTURE MORTALITY
Although the causal relation between smoking and lung cancer is not in question, assessing the magnitude of the effects of smoking on past, current, and future mortality is problematic. Estimating the impact of smoking would be a simple affair if we were in possession of longitudinal information about smoking behavior, health, and mortality. In the absence of such information, researchers must deploy indirect methods to estimate the fraction of adult deaths attributable to smoking. A number of questions can then be addressed with precision. First, how powerful is the decelerating power exerted by smoking-attributable mortality on recent mortality trends in Latin America and the Caribbean? Or, alternatively, how much larger would past gains in survival have been in the absence of smoking (i.e., forgone gains)? Second, how large are the likely short- and medium-term losses in life expectancy at 50 years as a result of past smoking?
METHODS
Estimation of smoking-attributable mortality—the counterfactual fraction of deaths that would be eliminated in the absence of smoking—is relatively unproblematic with longitudinal studies of cohorts of smokers and nonsmokers such as the Cancer Prevention Study II (CPS-II),33 or even with suitable cross-sectional data such as the National Health Interview Study linked to the National Death Index.34 In the absence of population-based cohort studies, we obtained country-based estimates of smoking-attributable mortality from techniques that combine aggregate information on age-, gender-, and cause-specific mortality and selected assumptions about the effects of smoking on selected conditions. The best known among these procedures was formulated by Peto et al.35 and subsequently modified by others.36–39 One of these variants, proposed by Preston et al.,40 is a generic regression-based procedure for aggregate cause-of-death data whereby observed lung cancer mortality rates become predictors of mortality rates from other causes of death. The technique was applied successfully to a sample of high-income countries to calculate the fraction of total intercountry differences in life expectancy at 50 years attributable to smoking.41 The method was modified to better capture gender variation,42 to reduce sensitivity to model dependency, and to tailor it for applications to defective vital statistics and census data.43 We applied the modified procedure to adjusted vital statistics while minimizing biases resulting from improper model selection and classification of causes of death (data available as a supplement to the online version of this article at http://www.ajph.org).
Data
We used a database for Latin American mortality (LAMbDA)25 that contains adjusted death rates from 1850 to 2010 for 18 countries of the Latin America–Caribbean region. Data on causes of death starting in 1950 are from World Health Organization databases.44 We focused on the 6 countries of interest during the period 1980 through 2007, and confined our analyses to ages 50 years and older.
Model and Estimates
The model we estimated is as follows:
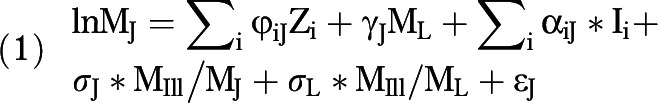 |
where ML and MJ are, respectively, the observed age-specific mortality rates from lung cancer and cause J in the population; Zi is a set of dummy variables including age, country, and calendar time; Ii’s are first-order interaction terms between ML and age, country, and calendar time; MIll is the mortality rate from ill-defined causes of death; and ɛJ is an error term. The parameters φiJ, γJ, αiJ, σJ, and σL are regression coefficients associated with the variables Zi, ML, Ιi, MIll/MJ, and MIll/ML, respectively, and are assumed to vary with cause of death (J). Instead of calendar years, we used years elapsed since 1980. We estimated models including dummy variables to identify individual countries and others with a single dummy variable to distinguish countries in the advanced stages of the smoking epidemic (Argentina, Chile, Cuba, and Uruguay) from those in earlier stages (Mexico and Brazil). The models with the single dummy fit as well as models with dummies to identify each country and were chosen as the most parsimonious. This result is consistent with the idea that relations between variables are more sensitive to shifts in stages of the smoking epidemic than to country-specific idiosyncrasies. We used a dummy variable with a value of 1 for Argentina, Chile, Cuba, and Uruguay, and zero for Mexico and Brazil.
Equation 1 includes 2 additional terms involving mortality rates from ill-defined causes (MIll). These variables control for differential propensities of deaths from lung cancer and from cause J to be classified as ill-defined (data available as a supplement to the online version of this article at http://www.ajph.org). To estimate the total number of deaths from cause J attributable to smoking, we used empirical estimates of the parameter γJ and an estimate of the fraction of all deaths from lung cancer not associated with smoking.
To estimate Equation 1 consistently, we used enough observations and time-varying covariates to capture time trends. We entered age, time period, and dummies for countries as well as 2- and 3-way interaction terms and applied goodness-of-fit tests to identify the most parsimonious specification. To estimate the parameters in Equation 1, there are 3 plausible models (ordinary least squares, Poisson, and negative binomial), 2 specifications (fixed effects and random effects), and 2 alternative groupings of causes of deaths, yielding 12 possible models. To reduce the number of parameters we needed to estimate, we concentrated on results from the negative binomial model, as this is the most appropriate for count variables with extra Poisson variation (data available as a supplement to the online version of this article at http://www.ajph.org).
We tested for differences between fixed-effects and random-effects specifications and for alternative groupings of causes of death by using the Hausman test statistic.45–47 Second, we assessed the sensitivity of estimates to alternative classifications of causes of deaths. The test consisted of comparing the proportions of deaths among adults older than 50 years that were attributable to smoking. These were predicted from the fixed-effects and random-effects models, employing 2 alternative classifications of causes: one with 2 causes (lung cancer; all other causes) and another with 4 causes (lung cancer; diseases of circulatory system; cancer other than lung; all other causes). We also carried out a systematic review of results for the entire period under investigation (1980-2005).
Assessing the Robustness of Empirical Estimates
Although the checks applied in the previous section were strict, we sought supplementary evidence of the robustness of estimates and applied 2 tests that gauged their external and internal validity. The first test compared the estimated effects of lung cancer death rates on mortality rates from all other causes with the effects estimated in a sample of 22 Organisation for Economic Co-Operation and Development (OECD) high-income countries for the same year.40,41
In a second test, we combined estimates of age-specific smoking prevalence from selected surveys with our empirical estimates of smoking prevalence (ρJ) to derive values of smoking relative risks (θJ) for selected causes of death J (data available as a supplement to the online version of this article at http://www.ajph.org). We relied on estimates from the random-effects model with 4 groups of causes of deaths.
Impact of Smoking on Adult Life Expectancy
We used yearly estimates of smoking-attributable mortality to calculate counterfactual trends in mortality rates—that is, those that would have been observed if smoking-attributable mortality were set to zero. We employed first principles to convert observed and counterfactual rates into life expectancies.
RESULTS
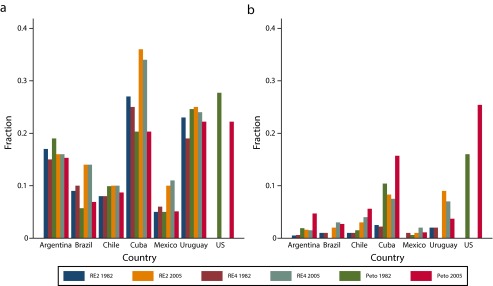
The Hausman test statistic indicated that the 2 models fit the data well and produced statistically similar results (data available as a supplement to the online version of this article at http://www.ajph.org). Results for the years 1980 and 2005 and 2 random-effects models, one for each classification of causes of deaths (2 and 4 causes), are displayed in Figure 1. This figure also includes results obtained by the procedure of Peto et al.35 The systematic review of the literature confirmed that the estimates were robust to both model specification (fixed effects vs random effects) and to alternative cause-of-death classifications (data available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 1—
Estimated smoking-attributable fraction of deaths, by country, year, and method, among (a) males and (b) females: Latin America and the Caribbean, 1980–2009.
Source. Peto et al.35
The Robustness of Empirical Estimates
Except for the first age group, the similarities between the 2 sets of estimates were striking (data available as a supplement to the online version of this article at http://www.ajph.org). As expected, because of the load of additional competing risks, the effects were somewhat lower in our sample of countries. This result could also be attributable to measurement errors or the different realities of the smoking epidemic. It is possible, for example, that conditions other than smoking had a heavier influence in Latin America and the Caribbean than in higher-income countries. Thus, cardiovascular diseases in low- to middle-income countries have etiologies implicating early conditions,48 and we should expect that a lower fraction of cardiovascular disease is associated with smoking. Similarly, lung cancer rates may be slightly understated, in which case our estimates are lower bounds.
Estimates of effects from the random-effects model were equivalent to the excess mortality risk attributable to smoking associated with each cause or group of causes. When these are combined with estimates of smoking prevalence by age, they yield the smoking relative risks for each group of causes of deaths. The estimates of relative risks can be compared with independent estimates, obtained from the most important longitudinal study on smoking, the CPS-II.49 The comparison is somewhat imprecise because the estimates available from the CPS-II are for the population aged 30 years and older (rather than 50 years and older) and correspond to disaggregated causes of death (whereas we only used figures for cancers other than lung and all circulatory diseases). In addition, the estimates from the CPS-II are appropriate for the United States,50 and the sample composition of the CPS-II does not necessarily mirror conditions in the Latin American and Caribbean countries we studied.
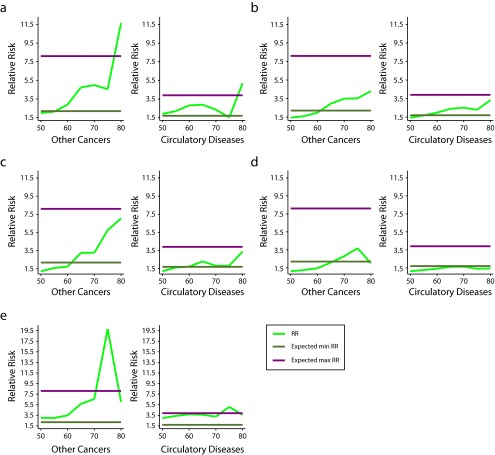
Despite these shortcomings, the results (for males only) plotted in Figure 2 are remarkable. Each panel displays estimated relative risks by age for chronic obstructive pulmonary disease plus cancers other than lung and circulatory diseases. The horizontal lines in each panel are the maximum and minimum relative risks estimated in the CPS-II for each group of diseases. The figure shows that our estimates are in close agreement with the much sturdier estimates derived from the CPS-II. If there are any slippages, they occur at very old ages—precisely the age groups that other research has found to be either intractable or problematic.35,41,51 This level of consistency would not have been possible if any of the key assumptions made to generate our estimates (data quality, relationship between lung cancer and other mortality as a result of other causes) had been violated.
FIGURE 2—
Estimated relative risks (RRs) among males by cause of death and age in (a) Argentina, (b) Brazil, (c) Chile, (d) Mexico, and (e) Uruguay: 1980–2009.
Impact of Smoking on Adult Life Expectancy
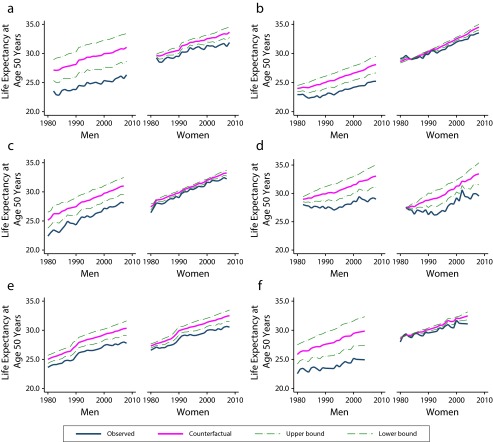
We used these results to answer the following question: how large is the impact of past smoking on conventional indicators of mortality such as life expectancy at adult ages? Figure 3 displays trends of observed and counterfactual life expectancy at 50 years for the period under examination. The counterfactual trend is plotted, with 95% confidence intervals obtained by bootstrap procedures. Differences between observed and counterfactual life expectancies were larger for males than for females. This is consistent with the idea that female smoking has not yet left a powerful imprint on female mortality. However, disparities between the observed and counterfactual trends among females are increasing over time and everywhere. Among males, the differences were large: 4 to 6 years in Argentina, Cuba, and Uruguay (about 20% of current life expectancy at 50 years), somewhat less than 4 years in Chile (about 15% of current life expectancy at 50 years), and less than 2 years in Brazil and Mexico (about 8% of current life expectancy at 50 years). During the most recent period (2000–2005), absolute and relative differences between observed and counterfactual life expectancies were steady in Argentina and Uruguay and increased in Cuba, Brazil, and Mexico. Cuba is a glaring example of the damaging power of smoking: the forgone gains in adult male life expectancy caused by smoking (20%–25% of current life expectancy at age 50 years) were equivalent to the observed total gains in survival experienced by Cuba in the last 20 years; it took 20 years of unprecedented progress to offset the losses that smoking was silently inflicting during the same period.
FIGURE 3—
Observed and counterfactual life expectancy at age 50 years, by gender and year in (a) Argentina, (b) Brazil, (c) Chile, (d) Cuba, (e) Mexico, and (f) Uruguay: 1980–2010.
Most of the differences between counterfactual and observed life expectancies (over 85%) everywhere were associated with reductions in heart and circulatory diseases as well as cancers other than lung cancer. The remaining differences were associated with lung cancer. As expected, their magnitude was larger in Argentina, Cuba, Chile, and Uruguay but growing steadily in Brazil and Mexico.
DISCUSSION
In the last 50 years, Latin America and the Caribbean has achieved a remarkable decline in mortality rates, punctuated by occasional setbacks caused by periodic economic crises. Between 1960 and 2000, these countries experienced increases in life expectancy at birth of about 0.25 years per year, a pace matched only by post–World War II Japan.1
At the outset, we conjectured that unless offsetting changes are quickly found, mortality decline will slow down as a result of unfavorable changes in smoking behavior among the adult population. The impacts of past smoking behavior are likely to be amplified in the short run even if older adults who currently smoke cease to do so in the near future. Worse yet, the spread of smoking in countries previously unaffected by it will increase the load of smoking-related deaths in the region. The issue is not if the impact of past smoking will be felt but rather when and how large it will be.
The smoking epidemic is well established in Latin America and the Caribbean, but countries are navigating through its various stages at different speeds, and health and mortality effects are heterogeneous. Additional cross-country variability is a result of diversity in the timing of onset and the success of tobacco control policies. Yet the threat of important shifts in mortality trends is there, particularly where smoking initiation began earlier and antismoking campaigns are more recent or feebler. Our estimates of the effects of smoking in the 6 Latin American–Caribbean countries with the highest smoking prevalence are consistent and remarkably robust to model specification. They suggest that during the last decade, the impact of smoking is equivalent to losses in male life expectancy at 50 years of 2 to 6 years. To date, these are only virtual, not observed, losses; they represent how much higher life expectancy at 50 years would be in the absence of smoking.
Forecasts are always risky, but there are 2 regularities we can count on. First, in countries with high smoking prevalence, current levels of smoking among men and women aged 20 to 50 years should translate into virtual losses equal to or larger than those calculated here. No sophisticated machinery is needed to predict this, because smoking behavior has a momentum that takes years to disappear. Second, given patterns of smoking initiation in countries in the early stages of the smoking epidemic, we expect the geographic spread and replication of forgone gains in longevity already experienced by countries that started earlier.
Will smoking behavior lead from virtual losses to real losses in adult life expectancy? Will the damage associated with smoking only slow down future gains? Or will past smoking become irrelevant? Uncertainty about these issues is rooted in 2 areas, and both are fertile grounds for policy interventions.
The first area is the future trajectory of smoking incidence and prevalence. Will countries now in the early stages of the epidemic contain it with timely and successful antismoking campaigns? Will countries at advanced stages reduce smoking prevalence and confine it to small pockets of risk populations? Although the outcome will depend on the efficacy of tobacco control policies, one priority looms large for all countries: the key target population ought to be its youngest members. It is among the youngest birth cohorts that the potential for averted future losses of life expectancy is largest.
The second area is attenuation of the effects of smoking and containment of non–smoking-related diseases. Do these countries have the wherewithal to count on a steady stream of future medical innovations for screening, detection, and treatment to reduce fatality rates from smoking- and non–smoking-related diseases alike? In Latin America and the Caribbean, there is limited room for survival gains derived from further reductions in infectious diseases. Only interventions to reduce mortality risks from chronic diseases will offer more than slight resistance to the harmful effects produced by smoking histories. What we do not know is whether the required stream of medical innovations will emerge at all and, if it does, whether it will be sufficient and timely enough to blunt the damage already inflicted by past smoking.
Acknowledgments
This research was supported by National Institute on Aging grants R37 AG025216, R01 AG016209, and R01 AG018016, and by core grants to the Center for Demography and Ecology (R24 HD047873) and the Center for Demography of Health and Aging (P30 AG017266) at the University of Wisconsin, Madison.
Human Participant Protection
Because this research involved secondary analysis of previously collected, anonymous data that met standards for public use, it did not require institutional review board approval.
References
- 1.Palloni A, Pinto G. Adult mortality in Latin America and the Caribbean. In: Rogers RG, Crimmins E, editors. International Handbook of Adult Mortality. New York, NY: Springer; 2011. pp. 101–132. [Google Scholar]
- 2.Goss PE, Lee B, Badovinac-Crnejic T et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14(5):391–436. doi: 10.1016/S1470-2045(13)70048-2. [DOI] [PubMed] [Google Scholar]
- 3.Bianco E, Champagne B, Barnoya J. The tobacco epidemic in Latin America and the Caribbean: a snapshot. Prev Control. 2005;1(4):311–317. [Google Scholar]
- 4. Le Monde. Comment le lobby du tabac a subventionne des labos francais. Le Monde Sciences et Techno. March 31, 2012. Available at: http://www.lemonde.fr/sciences/article/2012/05/31/guerre-du-tabac-la-bataille-de-la-nicotine_1710837_1650684.html. Accessed January 6, 2015.
- 5. Tobacco-Free Kids. Tobacco industry profile—Latin America. Report issued September 2009. Available at: http://global.tobaccofreekids.org/files/pdfs/en/IW_facts_countries_%20LatinAmerica.pdf. Accessed January 6, 2015.
- 6.Crosbie E, Sebrié EM, Glantz SA. Tobacco industry success in Costa Rica: the importance of FCTC Article 5.3. Salud Publica Mex. 2012;54(1):28–38. [PMC free article] [PubMed] [Google Scholar]
- 7.da Costa e Silva VL, Koifman S. Smoking in Latin America: a major public health problem. Cad Saude Publica. 1998;14(3):99–108. [PubMed] [Google Scholar]
- 8.Blecher EH, Walkbee P. An international analysis of cigarette affordability. Tob Control. 2004;13(4):339–346. doi: 10.1136/tc.2003.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blecher EH, Walkbee P. Cigarette affordability trends: an update and some methodological comments. Tob Control. 2009;18(3):167–175. doi: 10.1136/tc.2008.026682. [DOI] [PubMed] [Google Scholar]
- 10.Kan MY. Investigating cigarette affordability in 60 cities using the cigarette price-daily income ratio. Tob Control. 2007;16(6):429–432. doi: 10.1136/tc.2007.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebrié EM, Glantz SA. Tobacco industry “Youth Smoking Prevention” programs to undermine meaningful tobacco control in Latin America. Am J Public Health. 2007;97(8):1357–1367. doi: 10.2105/AJPH.2006.094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Projections of Tobacco Production, Consumption and Trade to the Year 2010. Rome, Italy: Food and Agriculture Organization of the United Nations; 2003. [Google Scholar]
- 13.Tavernise S. Tobacco firms’ strategy limits poorer nations’ smoking laws. New York Times. December 13, 2013. Available at: http://www.nytimes.com/2013/12/13/health/tobacco-industry-tactics-limit-poorer-nations-smoking-laws.html?pagewanted=all&_r=0. Accessed January 6, 2015. [Google Scholar]
- 14.Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health; 2012. [PubMed] [Google Scholar]
- 15.Valdés-Salgado R, Hernández Avila M, Sepúlveda Amor J. Tobacco use in the region of the Americas: elements for a program of action [in Spanish] Salud Publica Mex. 2002;44(suppl 1):S125–S135. [PubMed] [Google Scholar]
- 16.Champagne BM, Sebrié EM, Schargrodsky H, Pramparo P, Boissonnet C, Wilson E. Tobacco smoking in seven Latin American cities: the CARMELA study. Tob Control. 2010;19(6):457–462. doi: 10.1136/tc.2009.031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menezes AM, Lopez MV, Hallal PC et al. Prevalence of smoking and incidence of initiation in the Latin American adult population: the PLATINO study. BMC Public Health. 2009;9:151. doi: 10.1186/1471-2458-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3:242–247. [Google Scholar]
- 19.Ezzati M, Lopez AD. Smoking and oral tobacco use. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004. pp. 883–957. [Google Scholar]
- 20. Health Ministry, Argentina. National Risk Factors Study. ENFR 2005. Available at: http://www.msal.gov.ar/htm/Site/enfr/ index.asp. Accessed March 30, 2013.
- 21.World Health Organization. Tobacco Free Initiative. GATS (Global Adult Tobacco Survey). Available at: http://www.who.int/tobacco/surveillance/gats/en. Accessed March 30, 2013.
- 22. Sub-Secretary of Social Protection, Chile. Social Protection Study. EPS 2009 [in Spanish]. Available at: http://www.previsionsocial.gob.cl/subprev/?page_id=7185. Accessed April 2, 2012.
- 23. National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed June 25, 2012.
- 24.WHO Global Report: Mortality Attributable to Tobacco. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 25.Palloni A, Pinto G, Beltrán-Sánchez H. Latin American Mortality Database (LAMBdA) [Machine-readable database]. University of Wisconsin, Madison. 2014. Available at: http://www.ssc.wisc.edu/cdha/latinmortality. Accessed April 24, 2012.
- 26. Monteiro CA, Cavalcante TM, Moura EC, Moreira Claro R, Landmann Szwarcwald C. Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989–2003). Bull World Health Organ. 2007;85(7):527–534. [DOI] [PMC free article] [PubMed]
- 27.Glei DA, Meslé F, Vallin J. Diverging trends in life expectancy at age 50: a look at causes of death. In: Crimmins EM, Preston SH, Cohen B, editors. International Differences in Mortality at Older Ages: Dimensions and Sources. Washington, DC: National Academies Press; 2011. pp. 17–67. [PubMed] [Google Scholar]
- 28.Streppel MT, Boshuizen HC, Ocke MC, Kok FJ, Kromhout D. Mortality and life expectancy in relation to long-term cigarette, cigar and pipe smoking: the Zutphen Study. Tob Control. 2007;16(2):107–113. doi: 10.1136/tc.2006.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menezes AM, Perez-Padilla R, Jardim JR et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 30.Bosetti C, Malvezzi M, Chatenoud L, Negri E, Levi F, La Vecchia C. Trends in cancer mortality in the Americas, 1970–2000. Ann Oncol. 2005;16(3):489–511. doi: 10.1093/humrep/mdi086. [DOI] [PubMed] [Google Scholar]
- 31.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92(3):426–429. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Boreham J. Smoking and cardiovascular disease. Semin Vasc Med. 2002;2(3):243–252. doi: 10.1055/s-2002-35392. [DOI] [PubMed] [Google Scholar]
- 33. Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor air pollution from household use of solid fuels. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, eds. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004:1436–1493.
- 34.Rogers RG, Hummer RA, Krueger PM, Pampel FC. Mortality attributable to cigarette smoking in the United States. Popul Dev Rev. 2005;31(2):259–292. doi: 10.1111/j.1728-4457.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339(8804):1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- 36.Ezzati M, Lopez AD. Measuring the accumulated hazards of smoking: global and regional estimates for 2000. Tob Control. 2003;12(1):79–85. doi: 10.1136/tc.12.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112(4):489–497. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 38.Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 39.Parkin DM. Tobacco-attributable cancer burden in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S6–S13. doi: 10.1038/bjc.2011.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. Int J Epidemiol. 2010;39(2):430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston SH, Glei DA, Wilmoth JR. Contribution of smoking to international differences in life expectancy. In: Crimmins EM, Preston SH, Cohen B, editors. International Differences in Mortality at Older Ages: Dimensions and Sources. Washington, DC: The National Academies Press; 2011. pp. 105–131. [PubMed] [Google Scholar]
- 42.Rostron B. A modified new method for estimating smoking-attributable mortality in high-income countries. Dem Res. 2010;23:399–420. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palloni A, Novak B, Pinto G. Forecasting the effects of smoking in Latin American mortality. Paper presented at: Annual Meeting of the Population Association of America; April 11–13, 2013; New Orleans, LA.
- 44.World Health Organization. Statistics on mortality by cause of death. Available at: http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index.html. Accessed March 30, 2013.
- 45.Hausman JA. Specification tests in econometrics. Econometrica. 1978;46(6):1251–1271. [Google Scholar]
- 46.Hausman JA, Taylor WE. Panel data and unobservable individual effects. Econometrica. 1981;49(6):1377–1398. [Google Scholar]
- 47.Hausman JA, McFadden D. Specification tests for the multinomial logistic model. Econometrica. 1984;52:1219–1240. [Google Scholar]
- 48.Barker DJP. Mothers, Babies and Health in Later Life. Edinburgh, UK: Harcourt Brace; 1998. [Google Scholar]
- 49.Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA. 2000;284(6):706–712. doi: 10.1001/jama.284.6.706. [DOI] [PubMed] [Google Scholar]
- 50.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52(1):12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 51.Rostron BL, Wilmoth JR. Estimating the effect of smoking on slowdowns in mortality declines in developed countries. Demography. 2011;48(2):461–479. doi: 10.1007/s13524-011-0020-9. [DOI] [PubMed] [Google Scholar]