Abstract
Ongoing injection drug use contributes to the HIV and HCV epidemics in people who inject drugs. In many places, pharmacies are the primary source of sterile syringes for people who inject drugs; thus, pharmacies provide a viable public health service that reduces blood-borne disease transmission.
Replacing the supply of high dead space syringes with low dead space syringes could have far-reaching benefits that include further prevention of disease transmission in people who inject drugs and reductions in dosing inaccuracies, medication errors, and medication waste in patients who use syringes.
We explored using pharmacies in a structural intervention to increase the uptake of low dead space syringes as part of a comprehensive strategy to reverse these epidemics.
There are approximately 1.1 million people living with HIV in the United States.1 Over the past decade, the HIV incidence rate among people who inject drugs (PWID) has decreased; however, PWID remain disproportionately affected by HIV. It is estimated that 8% of new HIV cases in 2010 were among PWID.1 Co-occurring is the HCV epidemic; approximately 2.7 million people are chronically infected with HCV.2 Studies estimate that the prevalence of HCV among PWID ranges from 40% to 90%.3,4 Ongoing injection drug use and injection risk behaviors contribute to both epidemics.
Although effective therapies exist, ultimately, preventing the transmission of HIV and HCV is essential to ending these epidemics, particularly in high-risk populations. PWID constitute a vulnerable population that faces numerous economic and personal barriers (e.g., comorbidities) that prevent them from receiving appropriate medical care.5,6 Public health resources and interventions that focus on the prevention of HIV and HCV in PWID are needed.
Multiperson use of needles and syringes (i.e., direct sharing) and multiperson use of drug preparation materials (i.e., indirect sharing) are important risk factors for infection acquisition and transmission among PWID.7 An estimated 50% to 80% of PWID acquire HCV infection within the first year of injection drug use.8 Recommended interventions to discourage injection drug use include risk-reduction programs and substance abuse treatment.9 However, because of limited awareness of available programs, lack of access to treatment facilities, need for program referral, and cost of treatment, many PWID are unable to stop injecting drugs.7 A well-known public health measure to reduce the spread of infection is to promote the use of sterile syringes.10 We explored using pharmacies in a structural intervention to help prevent the transmission of HIV and HCV through syringes.
SOURCES OF SYRINGES FOR PEOPLE WHO INJECT DRUGS
Syringe services programs (SSPs) are an important source of sterile syringes and needles for PWID in some US states and in many countries. These programs often provide other materials (e.g., sterile water, filters, and metal caps for heating drug solutions). Most programs offer referrals to substance abuse treatment and HIV testing. Some programs offer testing for HIV, HCV, and other services onsite. As of 2012, there were 203 SSPs operating in 34 states, the District of Columbia, Puerto Rico, and the Indian Nations.11 They can be effective in reducing syringe sharing and the transmission of HIV, HCV, and other blood-borne pathogens.12–14 However, SSPs are illegal in many states in the United States and in many countries (Table 1). In many places, pharmacies are the primary source of sterile syringes for PWID and, thus, are a vital element for reducing blood-borne disease transmission.15,16
TABLE 1—
Restrictions on Nonprescription Sale of Syringes and Authorization of Syringe Service Programs, by State
| State Restrictions on NPSS |
||||||||
| State | Maximum Quantity of Syringes | Minimum Age, Years | Provide Information on Drug Addiction and Safe Disposal | Transaction Records | Dispensed by Pharmacist | Legitimate Medical Use | Other | Explicitly Authorizes SSPs |
| Alabama | ||||||||
| Alaska | ||||||||
| Arizona | ||||||||
| Arkansas | ||||||||
| California | 30 | ≥ 18 | X | Authorizes | ||||
| Colorado | Authorizes | |||||||
| Connecticut | 10 | Authorizes | ||||||
| Delaware | ≥ 18 | Authorizes | ||||||
| Florida | ≥ 18 | Xa | ||||||
| District of Columbia | X | Authorizes | ||||||
| Georgia | X | X | ||||||
| Hawaii | X | Authorizes | ||||||
| Idaho | ||||||||
| Illinois | 20 | ≥ 18 | Authorizes (certain locations) | |||||
| Indiana | ≥ 18 | X | X | Xb | ||||
| Iowa | ||||||||
| Kansas | ||||||||
| Kentucky | X | |||||||
| Louisiana | ||||||||
| Maine | ≥ 18 | X | Authorizes | |||||
| Maryland | X | X | Xc | Authorizes (certain locations) | ||||
| Massachusetts | ≥ 18 | X | Authorizes | |||||
| Michigan | ||||||||
| Minnesota | 10 | |||||||
| Mississippi | ||||||||
| Missouri | ||||||||
| Montana | ||||||||
| Nebraska | ||||||||
| Nevada | Authorizes | |||||||
| New Hampshire | 10 | ≥ 18 | X | |||||
| New Jersey | 10 | ≥ 18 | X | Authorizes | ||||
| New Mexico | Authorizes | |||||||
| New York | 10 | ≥ 18 | Authorizes | |||||
| North Carolina | ||||||||
| North Dakota | ||||||||
| Ohio | ||||||||
| Oklahoma | ||||||||
| Oregon | ||||||||
| Pennsylvania | Authorizes (certain locations) | |||||||
| Rhode Island | X | Authorizes | ||||||
| South Carolina | X | X | X | |||||
| South Dakota | ||||||||
| Tennessee | X | |||||||
| Texas | ||||||||
| Utah | ||||||||
| Vermont | Authorizes | |||||||
| Virginia | ≥ 16 | X | X | |||||
| Washington | X | Authorizes (certain locations) | ||||||
| West Virginia | ||||||||
| Wisconsin | ||||||||
| Wyoming | ||||||||
Note. NPSS = nonprescription sale of syringes; SSP = syringe service programs.
Some Florida counties prohibit NPSS.
Volume restrictions on concurrent opioids in Indiana.
Proper identification required in Maryland.
Pharmacy nonprescription sale of syringes (NPSS) allows customers and patients to purchase a syringe without a prescription. Presently, all states permit NPSS in pharmacies with varying levels of restrictions, including proof of identification, maximum number of syringes for sale, and medical need (Table 1). Pharmacy NPSS is further governed by state regulations, which prohibit dispensing or possessing syringes without a valid prescription, and by drug paraphernalia laws, which criminalize the distribution and possession of syringes intended for injection drug use. Pharmacy NPSS has been successful at reducing HIV transmission.17,18 In the 1990s, HIV infection rates doubled in US metropolitan areas that prohibited pharmacy NPSS.18,19 In 2000, New York State enacted the Expanded Syringe Access Demonstration Program to permit NPSS in pharmacies.20 The program resulted in a significant reduction of receptive syringe sharing over a two-year study period (from 13.4% to 3.6%; P < .001).20
Other factors influence NPSS in pharmacies. Chain and independently owned pharmacies often institute their own policies regarding NPSS.21 It is not uncommon for pharmacists to employ their own discretion with customers to determine the extent to which they will allow NPSS, for example, requiring picture identification, requiring proof of diabetes diagnosis, and selling syringes only in large quantities (e.g., packs of 100 syringes).21,22 A 2012 literature review of 47 studies found that pharmacists often cite that safety concerns (e.g., staff safety, theft, improper syringe disposal) are greater than legal or health concerns (e.g., prevention of blood-borne illness).22 The author concluded that although pharmacists’ personal opinions about PWID and HIV influence their attitudes about NPSS, pharmacists generally support sterile syringe distribution to PWID.22
Pharmacies provide a viable public health service for PWID. In fact, pharmacies are believed to be more accessible and reliable than are SSPs—particularly in states that do not authorize SSPs—because of their convenient location in many communities, longer hours of operation (some pharmacies are open 24 hours a day), and anonymity.15
SYRINGE DEAD SPACE
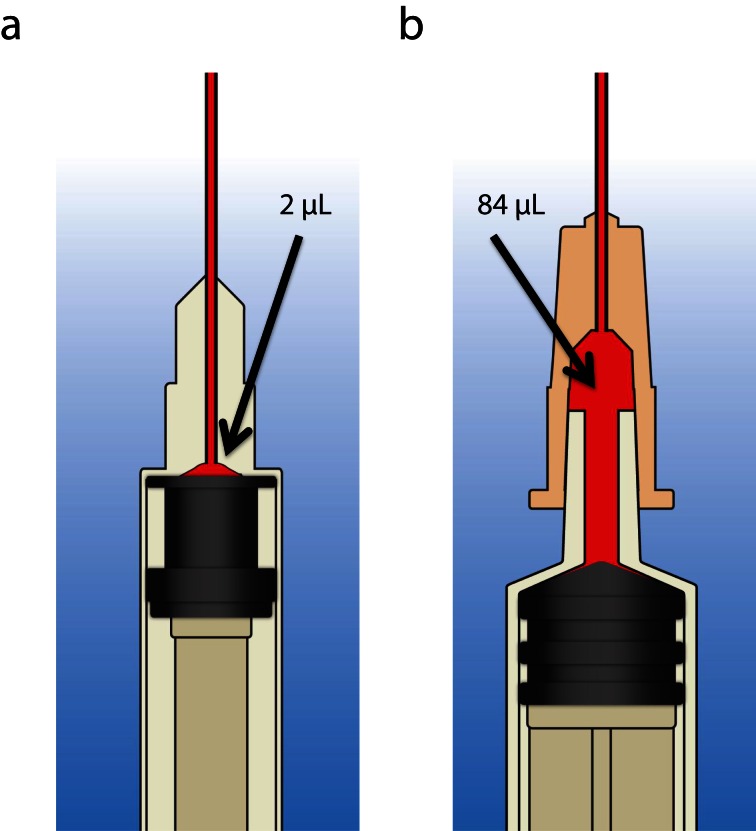
A proposed biomedical intervention to reduce infection transmission focuses on syringe design. Syringe “dead space” is the fluid that remains in the syringe after the plunger is fully depressed.23 The terms “high dead space syringes” (HDSS) and “low dead space syringes” (LDSS) describe the relative amount of excess dead space in the syringe (Figure 1). Most HDSS have a detachable needle, whereas most LDSS have a permanently attached needle. Problems attributed to syringe dead space include inadvertent digoxin overdose in neonates as a result of unaccounted digoxin in syringes24 and loss of diabetic control in patients with diabetes because of insulin dosing errors.25 The first insulin LDSS were introduced in 1969 and their use has reduced insulin waste and improved dosing accuracy in patients with diabetes.26,27 LDSS are a practical alternative to HDSS for avoiding such medication administration errors and dosing discrepancies.
FIGURE 1—
Comparison of (a) low dead space syringe and (b) high dead space syringe.
LDSS are purported to have public health benefits because they reduce the risk of infection transmission in PWID, as a reduced amount of dead space translates to a reduced amount of infected blood that is exchanged or transmitted.28–30 Through modeling simulations of needle and syringe sharing among PWID, Zule et al. demonstrated that syringes with detachable needles retain 40 times as much fluid as do syringes with permanently attached needles.31 Per their simulations, HDSS with detachable needles retained 84 microliters of excess fluid, whereas LDSS with integrated cannula retained only two microliters of excess fluid.31
In a series of experiments assessing the viability of HIV-1 from used syringes, HDSS with detachable needles were associated with longer survival time and harbored a greater amount of blood than did LDSS with attached cannulae.32 In a separate simulation study, viable HCV was recoverable for up to 63 days in used HDSS.33 The amount of viable HCV in LDSS declined sharply and was no longer recoverable after 7 days. As virus survival is a function of infectivity, these findings suggest that replacing HDSS with LDSS may reduce HIV and HCV transmission.
Beyond laboratory experiments, few studies have investigated the impact of LDSS in reducing HIV and HCV transmission. Two studies have been conducted internationally and in the United States.30,34,35 The use of LDSS in these studies led to a large reduction in the likelihood of HIV and HCV infection. Pooled analysis by the World Health Organization showed that the risk of HIV infection in PWID was decreased by 71% in LDSS users compared with HDSS users (relative risk [RR] = 0.29; 95% confidence interval [CI] = 0.18, 0.46).36 Similarly, the risk of HCV infection was reduced by 51% in LDSS users (RR = 0.49; 95% CI = 0.44, 0.55).36 Of note, the observational design of these studies is a limitation of these findings.
In 2014, the World Health Organization released a guidance document acknowledging the potential for LDSS to reduce HCV transmission in PWID.37 The document recommends that LDSS be made available for distribution in SSPs as part of a comprehensive strategy to reduce disease transmission.37
A drawback of LDSS with a permanently attached needle is that the needle cannot be removed and used with syringe barrels of different sizes. Needles and syringes vary in needle length, needle gauge, and syringe barrel size. PWID often use shorter needles (one half to five eighths inch), higher gauge needles (27–29 gauge), and smaller syringes (one mL).38 Most LDSS with permanently attached needles are one milliliter and may not be appropriate for all PWID, particularly those who inject larger volumes (five to 10 mL).37 However, with the possible exception of people who inject prescription opioids that may require larger volumes of fluid, most PWID in the United States inject volumes of fluid of one milliliter or less.
LDSS DISTRIBUTION IN PHARMACIES
As a major source of syringes for PWID, pharmacies have the potential to play a critical role to increase access to LDSS. One potential strategy to reduce the availability of HDSS is to make LDSS an industry standard in pharmacies through changes in syringe procurement and sales practices. Pharmacies could reduce the supply of HDSS by ordering, stocking, and distributing only LDSS, thereby regulating the availability of syringe type for all patients and customers. It has been demonstrated that PWID can adapt to changes in needle and syringe type on the basis of availability and accessibility.39,40
In a Texas case study of PWID, the transition from HDSS to LDSS was gradually implemented by syringe distributors to pharmacies between the 1970s and the 1980s.40 Syringe distributors marketed LDSS as a more efficient delivery system for insulin than HDSS. PWID who had purchased syringes from pharmacies during this time subsequently transitioned to using LDSS.
A 2012 LDSS social marketing campaign in Vietnam involved monitoring the distribution and sales of syringes to PWID in 18 pharmacies.41 Interviews with PWID at one study site revealed that individuals were unaware of the risks of dead space and HIV transmission and expressed strong interest in learning how to purchase LDSS in place of HDSS. These findings demonstrate that it is possible to implement a change in syringes stocked by pharmacies. However, there has been no push for syringe manufacturers to make LDSS the industry standard.
Ideally, the reduced demand for HDSS, created by a reduction in the quantity of HDSS that pharmacies supply, and the rising demand for LDSS would encourage manufacturers to manufacture LDSS instead of HDSS. The ultimate goal is to promote the manufacture of LDSS for other indications, beyond insulin administration, and in all syringe sizes, not just one milliliter. The Texas case study suggests that syringe preference can be influenced if HDSS are difficult to acquire and LDSS are more readily available.40
Following the introduction of integral cannula syringes (i.e., LDSS) in 1969 and through the early 1990s, reported use of syringes with detachable needles declined among PWID, a change that was noted by pharmacists who were interviewed for the case study.40 From 2003 to 2005, Zule and Bobashev conducted a study of syringe use in the metropolitan area of Raleigh–Durham, North Carolina.30 Of 194 participants who had used a HDSS since 2000, 41% reported obtaining a HDSS from a pharmacy, which was more than from any other single source. The majority of participants (62%) reported using a HDSS because of ease of access; therefore, the availability of syringe type in pharmacies influences syringe use in PWID.
Replacing the supply of HDSS with LDSS could have far-reaching benefits, beyond helping PWID and decreasing the transmission of HIV and HCV, to include reductions in dosing inaccuracies, medication errors, and medication waste among patients who use syringes. Most LDSS are available only as one-milliliter syringes. Selling LDSS in sizes larger than one milliliter would also benefit PWID who inject larger quantities of liquid drugs and patient populations who need larger syringe sizes who also purchase syringes in pharmacies. Examples include patients requiring cyanocobalamin (vitamin B12) injections and those requiring injectable testosterone replacement therapy. However, to our knowledge, a pharmacy-level intervention to promote widespread LDSS availability in the United States has not been attempted.
Feasibility Concerns
There are feasibility concerns when considering pharmacies in a structural intervention for increasing the use of LDSS and decreasing the use of HDSS. First, the literature is unclear about the preference of PWID for HDSS over LDSS. Ibragimov and Latypov found that PWID preferred LDSS; however, the limited availability of syringe size and lack of a removable needle limited uptake of LDSS in their study.42 Some manufacturers are producing detachable needles for LDSS that overcome these barriers to uptake, for example, those that can be attached to different barrel sizes.
Second, data demonstrating the effectiveness of LDSS in preventing HIV and HCV infection are limited. Much of the existing evidence is from laboratory experiments,29,32,33 mathematical modeling,29,43 and biobehavioral studies.30,34,35,40 Gyarmathy et al. conducted two studies to investigate the likelihood of HIV infection stratified by syringe type (HDSS vs LDSS).34,35 However, these were cross-sectional studies and provide little evidence of the causal relationship between the use of LDSS and the reduction in HIV and HCV transmission.
Another concern is the cost of LDSS compared with the cost of HDSS. No research has been conducted domestically comparing the cost of the syringes. Studies in Central Asia and Eastern Europe have shown that the costs of LDSS and HDSS are similar.39 Nonetheless, according to the World Health Organization, the cost difference between LDSS and HDSS is considered a low barrier to stocking LDSS.36
Current Research Needs
Much research is needed regarding the transition to LDSS in US community pharmacies. There is no systematic method for pharmacy personnel to distinguish between LDSS and HDSS that are stocked. Research is needed to develop resources to help pharmacy personnel identify LDSS products. The extent to which LDSS versus HDSS are sold and distributed in community pharmacies has not been evaluated. Data regarding syringe sales are also needed to determine whether there are cost differences between LDSS and HDSS.
The degree to which pharmacy personnel are aware of the harms associated with syringe dead space has not been assessed. Initiatives are needed to inform and educate pharmacy personnel regarding the benefits of LDSS and to determine the feasibility and acceptability of such an intervention among pharmacy personnel. It is imperative to identify intervention implementation practices that limit disruptions to pharmacy workflow and that are conducted in accordance with pharmacy-specific policies and state regulations governing NPSS in pharmacies.
The effect of such a pharmacy-based intervention among PWID and patients who use syringes is unknown. Research should focus on knowledge and attitudes regarding the perceived benefits associated with LDSS, perceptions regarding receiving health information from pharmacy personnel, and the preferred method for receiving injection-related health information from pharmacy personnel. Lastly, SSPs can also serve as the basis for a structural intervention, as they are widely used in jurisdictions that permit them and are likely to be amenable to promoting LDSS and educating PWID.
CONCLUSIONS
Continued injection drug use remains a risk factor for HIV and HCV transmission in PWID. Using pharmacies to increase access to LDSS and decrease access to HDSS is a structural intervention to increase the uptake of a biomedical intervention (LDSS) as part of a comprehensive strategy to reverse the HIV and HCV epidemics. The benefits of LDSS extend beyond disease transmission, as they reduce medication waste and prevent dosing inaccuracies. Pharmacies are a common source of sterile syringes for PWID and patients requiring syringes for medication administration; therefore, pharmacies have the potential to play a critical role in promoting the widespread availability of LDSS. Much research is still needed to determine the feasibility of a pharmacy-level intervention to promote the transition from HDSS to LDSS. Nevertheless, further exploration into implementing this intervention is warranted.
Human Participant Protection
No protocol approval was necessary because this work was not considered human participant research.
References
- 1.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 US dependent areas—2011. HIV Surveillance Supplemental Report. 2013;18(5) [Google Scholar]
- 2.Denniston MM, Jiles RB, Drobeniuc J et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagan H, Pouget ER, Williams IT et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201(3):378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 4.Page K, Hahn JA, Evans J et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200(8):1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno Y, Purcell DW, Knowlton AR, Wilkinson JD, Gourevitch MN, Knight KR. Syndemic vulnerability, sexual and injection risk behaviors, and HIV continuum of care outcomes in HIV-positive injection drug users. AIDS Behav. doi: 10.1007/s10461-014-0890-0. 2014; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56(6):806–816. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Substance abuse treatment for injection drug users: a strategy with many benefits. Available at: http://www.cdc.gov/idu/facts/treatment.htm. Accessed November 10, 2014.
- 8.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2012;61(RR–5):1–40. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Syringe exchange programs—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488–1491. [PubMed] [Google Scholar]
- 11.North American Syringe Exchange Network. Available at: http://www.nasen.org. Accessed November 5, 2014.
- 12.Des Jarlais DC, Marmor M, Paone D et al. HIV incidence among injecting drug users in New York City syringe-exchange programmes. Lancet. 1996;348(9033):987–991. doi: 10.1016/s0140-6736(96)02536-6. [DOI] [PubMed] [Google Scholar]
- 13.Hagan H, Thiede H. Changes in injection risk behavior associated with participation in the Seattle needle-exchange program. J Urban Health. 2000;77(3):369–382. doi: 10.1007/BF02386747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtzman D, Barry V, Ouellet LJ et al. The influence of needle exchange programs on injection risk behaviors and infection with hepatitis C virus among young injection drug users in select cities in the United States, 1994–2004. Prev Med. 2009;49(1):68–73. doi: 10.1016/j.ypmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph AE, Crawford ND, Ompad DC et al. Comparison of injection drug users accessing syringes from pharmacies, syringe exchange programs, and other syringe sources to inform targeted HIV prevention and intervention strategies. J Am Pharm Assoc (2003) 2010;50(2):140–147. doi: 10.1331/JAPhA.2010.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammett TM, Phan S, Gaggin J et al. Pharmacies as providers of expanded health services for people who inject drugs: a review of laws, policies, and barriers in six countries. BMC Health Serv Res. 2014;14:261. doi: 10.1186/1472-6963-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller CM, Galea S, Caceres W, Blaney S, Sisco S, Vlahov D. Multilevel community-based intervention to increase access to sterile syringes among injection drug users through pharmacy sales in New York City. Am J Public Health. 2007;97(1):117–124. doi: 10.2105/AJPH.2005.069591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman SR, Perlis T, Des Jarlais DC. Laws prohibiting over-the-counter syringe sales to injection drug users: relations to population density, HIV prevalence, and HIV incidence. Am J Public Health. 2001;91(5):791–793. doi: 10.2105/ajph.91.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86(5):642–654. doi: 10.2105/ajph.86.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouget ER, Deren S, Fuller CM et al. Receptive syringe sharing among injection drug users in Harlem and the Bronx during the New York State Expanded Syringe Access Demonstration Program. J Acquir Immune Defic Syndr. 2005;39(4):471–477. doi: 10.1097/01.qai.0000152395.82885.c0. [DOI] [PubMed] [Google Scholar]
- 21.Harbke CR, Fisher DG, Cagle HH, Trubatch BN, Fenaught AM, Johnson ME. Telephone survey of Alaskan pharmacists’ nonprescription needle-selling practices. J Urban Health. 2000;77(1):113–120. doi: 10.1007/BF02350967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janulis P. Pharmacy nonprescription syringe distribution and HIV/AIDS: a review. J Am Pharm Assoc (2003) 2012;52(6):787–797. doi: 10.1331/JAPhA.2012.11136. [DOI] [PubMed] [Google Scholar]
- 23.Strauss K, van Zundert A, Frid A, Costigliola V. Pandemic influenza preparedness: the critical role of the syringe. Vaccine. 2006;24(22):4874–4882. doi: 10.1016/j.vaccine.2006.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhambhani V, Beri RS, Puliyel JM. Inadvertent overdosing of neonates as a result of the dead space of the syringe hub and needle. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F444–F445. doi: 10.1136/adc.2004.070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann NP, Johnston DI. Insulin syringe dead-space and diabetic control. Lancet. 1981;318(8255):1120–1121. doi: 10.1016/s0140-6736(81)91332-5. [DOI] [PubMed] [Google Scholar]
- 26.Shainfeld FJ. Errors in insulin doses due to the design of insulin syringes. Pediatrics. 1975;56(2):302–303. [PubMed] [Google Scholar]
- 27.Berne C, Agenäs I, Eriksson G, Wibell L. Insulin wastage in ambulant practice. Diabetes Care. 1984;7(4):343–346. doi: 10.2337/diacare.7.4.343. [DOI] [PubMed] [Google Scholar]
- 28.Gaughwin MD, Gowans E, Ali R, Burrell C. Bloody needles: the volumes of blood transferred in simulations of needlestick injuries and shared use of syringes for injection of intravenous drugs. AIDS. 1991;5(8):1025–1027. [PubMed] [Google Scholar]
- 29.Bobashev GV, Zule WA. Modeling the effect of high dead-space syringes on the human immunodeficiency virus (HIV) epidemic among injecting drug users. Addiction. 2010;105(8):1439–1447. doi: 10.1111/j.1360-0443.2010.02976.x. [DOI] [PubMed] [Google Scholar]
- 30.Zule WA, Bobashev G. High dead-space syringes and the risk of HIV and HCV infection among injecting drug users. Drug Alcohol Depend. 2009;100(3):204–213. doi: 10.1016/j.drugalcdep.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zule WA, Ticknor-Stellato KM, Desmond DP, Vogtsberger KN. Evaluation of needle and syringe combinations. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(3):294–295. doi: 10.1097/00042560-199703010-00015. [DOI] [PubMed] [Google Scholar]
- 32.Abdala N, Stephens PC, Griffith BP, Heimer R. Survival of HIV-1 in syringes. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(1):73–80. doi: 10.1097/00042560-199901010-00011. [DOI] [PubMed] [Google Scholar]
- 33.Paintsil E, He H, Peters C, Lindenbach BD, Heimer R. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis. 2010;202(7):984–990. doi: 10.1086/656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gyarmathy VA, Neaigus A, Li N et al. Liquid drugs and high dead space syringes may keep HIV and HCV prevalence high—a comparison of Hungary and Lithuania. Eur Addict Res. 2010;16(4):220–228. doi: 10.1159/000320287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gyarmathy VA, Neaigus A, Mitchell MM, Ujhelyi E. The association of syringe type and syringe cleaning with HCV infection among IDUs in Budapest, Hungary. Drug Alcohol Depend. 2009;100(3):240–247. doi: 10.1016/j.drugalcdep.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guidance on Prevention of Viral Hepatitis B and C Among People Who Inject Drugs. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 37.Walsh N, Verster A, Rodolph M, Akl EA. WHO guidance on the prevention of viral hepatitis B and C among people who inject drugs. Int J Drug Policy. 2014;25(3):363–371. doi: 10.1016/j.drugpo.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Harm Reduction Coalition. A safety manual for injection drug users. Available at: http://harmreduction.org/wp-content/uploads/2011/12/getting-off-right.pdf. Accessed October 19, 2014.
- 39.Zule WA, Cross HE, Stover J, Pretorius C. Are major reductions in new HIV infections possible with people who inject drugs? The case for low dead-space syringes in highly affected countries. Int J Drug Policy. 2013;24(1):1–7. doi: 10.1016/j.drugpo.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Zule WA, Desmond DP, Neff JA. Syringe type and drug injector risk for HIV infection: a case study in Texas. Soc Sci Med. 2002;55(7):1103–1113. doi: 10.1016/s0277-9536(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 41.Gray R, Tuan NM, Neukom J. Rapid Assessment Report on Needle and Syringe Types Used by People Who Inject Drugs in Hanoi and Ho Chi Minh City, Vietnam. Washington, DC: Population Services International; 2012. [Google Scholar]
- 42.Ibragimov U, Latypov A. Needle and Syringe Types Used by People Who Inject Drugs in Eastern Europe and Central Asia: Key Findings From a Rapid Situational Assessment. Vilnius, Lithuania: Eurasian Harm Reduction Network; 2012. [Google Scholar]
- 43.Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr. 1992;5(11):1116–1118. [PubMed] [Google Scholar]