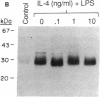
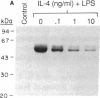
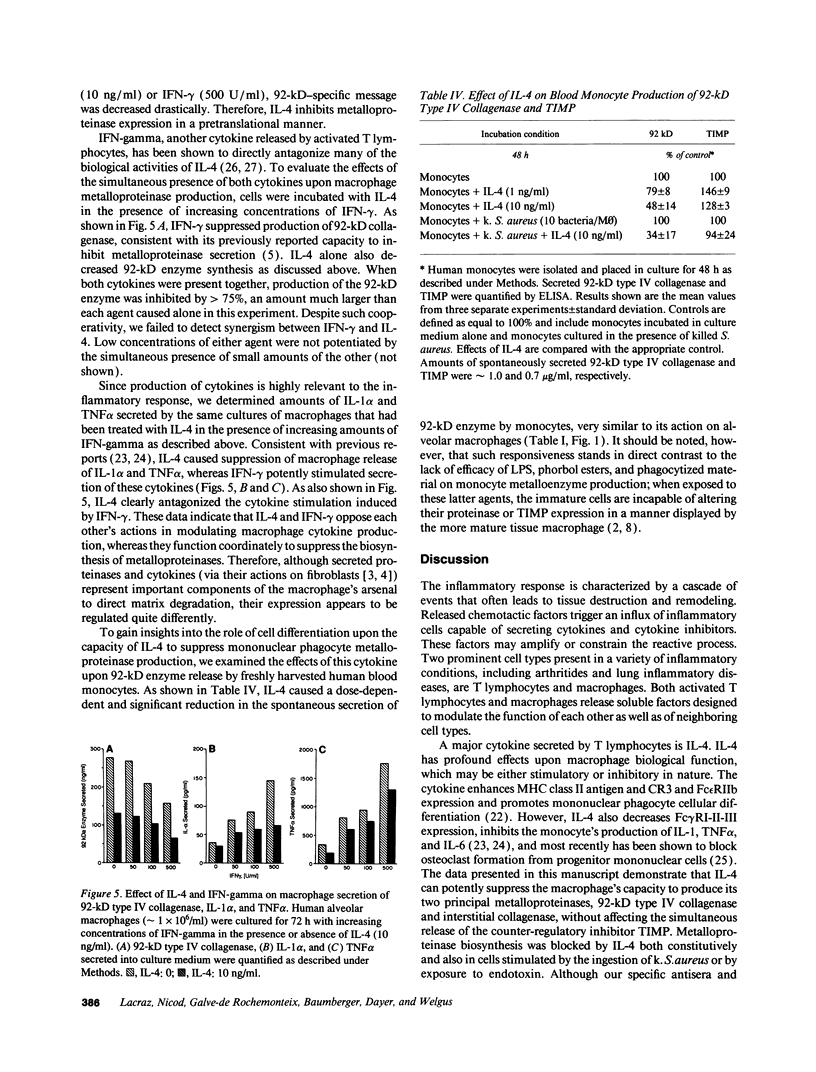
Abstract
To study the interaction of lymphocytes and macrophages in the control of extracellular matrix turnover, we determined the effects of several soluble T cell products on mononuclear phagocyte production of metalloproteinases. Cytokines including IL-2, IL-4, IL-6, tumor necrosis factor alpha (TNF alpha), GM-CSF, and IFN-gamma were each tested for capacity to modulate macrophage metalloproteinase and tissue inhibitor of metalloproteinases (TIMP) expression. The addition of IL-4 to cells cultured under basal conditions caused a dose-dependent suppression in the release of 92-kD type IV collagenase without affecting TIMP production. 92-kD enzyme secretion was inhibited by 50% with 1-2 ng/ml of IL-4 and by 90% with 10 ng/ml of IL-4. When cells were first exposed to killed Staphylococcus aureus to induce metalloproteinase production, IL-4 potently blocked the stimulated release of both interstitial collagenase and 92-kD type IV collagenase, again without effect upon TIMP. Metabolic labeling experiments and Northern hybridizations demonstrated that IL-4 exerted its action at a pretranslational level. Furthermore, IL-4 possessed the capacity to inhibit metalloproteinase expression even in the relatively immature peripheral blood monocyte. As reported previously (Shapiro, S. D., E. J. Campbell, D. K. Kobayashi, and H. G. Welgus. 1990. J. Clin. Invest. 86:1204), IFN-gamma suppressed constitutive macrophage production of 92-kD type IV collagenase. Despite the frequent antagonism observed between IL-4 and IFN-gamma in other systems, the combination of these two agents lowered metalloproteinase biosynthesis dramatically, whereas IL-4 opposed the IFN-gamma-stimulated production of cytokines (IL-1 and TNF alpha). IL-6 had only minimal effect upon metalloproteinase production, but appeared to specifically augment TIMP release. In summary, cytokines released by activated T cells may profoundly reduce the capacity of the macrophage to mediate extracellular matrix degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann U., Michaelis J., Oberhoff R., Knäuper V., Beckmann R., Tschesche H. Enzyme linked immunosorbent assays (ELISA) for the quantitative determination of human leukocyte collagenase and gelatinase. J Clin Chem Clin Biochem. 1989 Jun;27(6):351–359. doi: 10.1515/cclm.1989.27.6.351. [DOI] [PubMed] [Google Scholar]
- Bucker E. R., Martin S. E. Superoxide dismutase activity in thermally stressed Staphylococcus aureus. Appl Environ Microbiol. 1981 Feb;41(2):449–454. doi: 10.1128/aem.41.2.449-454.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Shapiro S. D., Goldberg G. I., Welgus H. G. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991 Feb 15;146(4):1286–1293. [PubMed] [Google Scholar]
- Clark S. D., Kobayashi D. K., Welgus H. G. Regulation of the expression of tissue inhibitor of metalloproteinases and collagenase by retinoids and glucocorticoids in human fibroblasts. J Clin Invest. 1987 Nov;80(5):1280–1288. doi: 10.1172/JCI113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-de Rochemonteix B., Nicod L. P., Junod A. F., Dayer J. M. Characterization of a specific 20- to 25-kD interleukin-1 inhibitor from cultured human lung macrophages. Am J Respir Cell Mol Biol. 1990 Oct;3(4):355–361. doi: 10.1165/ajrcmb/3.4.355. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Frost J. P., Karr R. W. All human monocytes have the capability of expressing HLA-DQ and HLA-DP molecules upon stimulation with interferon-gamma. J Immunol. 1986 Jul 15;137(2):519–524. [PubMed] [Google Scholar]
- Grassi J., Frobert Y., Pradelles P., Chercuitte F., Gruaz D., Dayer J. M., Poubelle P. E. Production of monoclonal antibodies against interleukin-1 alpha and -1 beta. Development of two enzyme immunometric assays (EIA) using acetylcholinesterase and their application to biological media. J Immunol Methods. 1989 Oct 24;123(2):193–210. doi: 10.1016/0022-1759(89)90223-8. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney A. M., Wakeley P. R., Davies M. J., Foster K., Hembry R., Murphy G., Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imschenetzky M., Puchi M., Pimentel C., Bustos A., Gonzales M. Immunobiochemical evidence for the loss of sperm specific histones during male pronucleus formation in monospermic zygotes of sea urchins. J Cell Biochem. 1991 Sep;47(1):1–10. doi: 10.1002/jcb.240470102. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Guerne P. A. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA). J Biol Chem. 1991 Feb 5;266(4):2017–2020. [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Faller D. V., Burakoff S. J. Interferon-gamma induction of LFA-1-mediated homotypic adhesion of human monocytes. J Immunol. 1986 Jul 1;137(1):108–113. [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D., Yang G., Muller-Sieburg C., Smith C., Arai N., Takabe Y., Gemmell L. Interleukin 4 (B-cell stimulatory factor 1) can enhance or antagonize the factor-dependent growth of hemopoietic progenitor cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6889–6893. doi: 10.1073/pnas.84.19.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Malefijt R. W., Slierendregt B., Aubry J. P., Bonnefoy J. Y., Defrance T., Banchereau J., de Vries J. E. Regulation of Fc receptor for IgE (CD23) and class II MHC antigen expression on Burkitt's lymphoma cell lines by human IL-4 and IFN-gamma. J Immunol. 1988 Apr 15;140(8):2625–2632. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Shapiro S. D., Campbell E. J., Kobayashi D. K., Welgus H. G. Dexamethasone selectively modulates basal and lipopolysaccharide-induced metalloproteinase and tissue inhibitor of metalloproteinase production by human alveolar macrophages. J Immunol. 1991 Apr 15;146(8):2724–2729. [PubMed] [Google Scholar]
- Shapiro S. D., Campbell E. J., Kobayashi D. K., Welgus H. G. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990 Oct;86(4):1204–1210. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Bar-Shavit Z., Senior R. M., Teitelbaum S. L. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest. 1985 Jul;76(1):219–224. doi: 10.1172/JCI111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde A. A., Huijbens R. J., Heije K., de Vries J. E., Figdor C. G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990 Oct 1;76(7):1392–1397. [PubMed] [Google Scholar]
- te Velde A. A., Huijbens R. J., de Vries J. E., Figdor C. G. IL-4 decreases Fc gamma R membrane expression and Fc gamma R-mediated cytotoxic activity of human monocytes. J Immunol. 1990 Apr 15;144(8):3046–3051. [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]