Abstract
Introduction
Blue light information plays an important role in synchronising internal biological rhythm within the external environment. Circadian misalignment is associated with the increased risk of sleep disturbance, obesity, diabetes mellitus, depression, ischaemic heart disease, stroke and cancer. Meanwhile, blue light causes photochemical damage to the retina, and may be associated with age-related macular degeneration (AMD). At present, clear intraocular lenses (IOLs) and blue-blocking IOLs are both widely used for cataract surgery; there is currently a lack of randomised controlled trials to determine whether clear or blue-blocking IOLs should be used.
Methods and analysis
This randomised controlled trial will recruit 1000 cataract patients and randomly allocate them to receive clear IOLs or blue-blocking IOLs in a ratio of 1:1. The primary outcomes are mortality and the incidence of cardiovascular disease, cancer and AMD. Secondary outcomes are fasting plasma glucose, triglycerides, cholesterol, glycated haemoglobin, sleep quality, daytime sleepiness depressive symptoms, light sensitivity, the circadian rhythm of physical activity, wrist skin temperature and urinary melatonin metabolite. Primary outcomes will be followed until 20 years after surgery, and secondary outcomes will be assessed at baseline and 1 year after surgery.
Ethics and dissemination
Ethical approval has been obtained from the Institutional Review Board of Nara Medical University (No. 13-032). The findings of this study will be communicated to healthcare professionals, participants and the public through peer-reviewed publications, scientific conferences and the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) home page.
Trial registration number
UMIN000014680.
Keywords: ONCOLOGY
Strengths and limitations of this study.
This is the first randomised controlled trial comparing the effect of blue-blocking intraocular lens for cataract surgery on mortality, the incidence of cardiovascular disease, stroke, cancer and age-related macular degeneration.
To clarify the mechanism of the effects, we simultaneously measure circadian rhythm parameters such as urinary melatonin metabolite, wrist skin temperature and circadian physical activity rhythm.
A limitation of this study is the lack of information about light exposure at night.
Introduction
Circadian misalignment between the internal biological rhythm and the external environment, such as behaviour and light exposure cycle, results in a number of negative health consequences. Epidemiological studies among night shift workers have shown significant associations between circadian misalignment and systemic diseases such as sleep disturbance,1 hip fracture,2 obesity and dyslipidemia,3 4 diabetes mellitus,5 6 depression,7 ischaemic heart disease and stroke8–10 and cancer.11–14 The mechanism is partly explained by decreased melatonin among shift workers15 and the findings from experimental studies in controlled laboratory conditions showing that circadian misalignment induced by a 28 h sleep–wake cycle under dim light conditions increased glucose, insulin and blood pressure, and decreased leptin and sleep efficiency.16
Light is the most important cue of the circadian biological rhythm, because it regulates the timing of the internal biological rhythm according to the phase response curve to light.17 Amplitude change due to light exposure was also reported from mammalian cell culture.18 Non-visual light information, a specific signal for the circadian system, perceived by intrinsically photosensitive retinal ganglion cells (ipRGCs) that contain melanopsin, is transmitted to the master circadian oscillator located in the suprachiasmatic nucleus via the retinohypothalamic tract.19 The action spectrum of light information for the circadian biological rhythm shows a peak at a shorter wavelength (464 nm) than that for visual information (approximately 555 nm).20
According to the WHO, cataract is a leading cause of blindness (51% of cases worldwide)21 and a common eye condition in elderly individuals. Opacity of the lens due to cataract reduces the transmission of light, especially the shorter wavelengths,22 and may cause circadian misalignment and its related diseases. In fact, according to observational studies, cataract surgery to replace clouded lenses with artificial intraocular lenses (IOLs), which increase light transmission, may improve not only visual acuity and quality of life23 but also sleep quality,24–27 depressive moods28–31 and life expectancy.29 The effectiveness of bright light therapy to decrease depressive symptoms32 and increase sleep quality33 supports the mechanism of the improvement of circadian rhythm alignment after cataract surgery.
For cataract surgery, clear IOLs that block ultraviolet (UV) B (<320 nm) and UVA (320–400 nm) radiation have been used since the early 1880s. In the 1990s, blue-blocking IOLs were introduced to prevent retinal phototoxicity caused by shorter wavelength light. Blue-blocking IOLs reduce transmission of light at 460 nm by 64–77% compared with clear IOLs which allow >95% transmission of light at this wavelength.34 As the circadian timing system is most sensitive to light at 460 nm, several reviews have raised the possibility that the use of blue-blocking IOLs could reduce the benefit of cataract surgery in terms of the circadian biological rhythm.35 36
Experimental studies have shown that blue light causes photochemical damage to the retinal pigment epithelium (RPE) in the presence of lipofuscin, which accumulates with ageing. Fluorophore A2E, a major component of RPE lipofuscin, mediates cell apoptosis under blue light radiation but not under green light radiation.37–39 Several cohort studies have shown that patients undergoing cataract surgery have an increased risk of age-related macular degeneration (AMD) during follow-up.40–43 A dramatic increase in transmitted blue light radiation through artificial IOLs after cataract surgery may partly explain the increased risk of AMD. In an in vitro study of RPE cell cultures under blue light radiation, blue-blocking IOLs that were developed to protect against AMD decreased cell death by approximately 50%.44
Although previous research has suggested benefits and disadvantages of blue-blocking IOLs, clear and blue-blocking IOLs are both widely used for cataract surgery at present. It yet remains to be clarified whether clear or blue-blocking IOLs should be used for routine cataract surgery. The purpose of the present randomised controlled study is to compare all-cause mortality, the incidence of cardiovascular disease (CVD) and the incidence of cancer associated with circadian rhythm misalignment between participants after cataract surgery with implantation of clear or blue-blocking IOLs, and to determine whether blue-blocking IOLs reduce the incidence of AMD after cataract surgery compared with clear IOLs.
The CLOCK-IOL colour (Cataract Surgery and Circadian Biological Rhythm among Japanese Older People with Cataract in Nara, Kansai Region: Influence of Intraocular Lens Implantation) study is a parallel group, open label, randomised controlled study. After baseline assessment, all participants will be allocated to receive either clear IOLs (clear IOL group) or blue-blocking IOLs (blue-blocking IOL group) in a 1:1 ratio. The outcomes among both groups will be followed at 1 year intervals.
Materials and methods
Participants
All procedures will be conducted at the Nara Medical University Hospital in Japan.
Ophthalmologists will assess the eligibility of patients diagnosed as having cataracts in Nara Medical University Hospital for the present study according to the following inclusion and exclusion criteria.
Inclusion criteria:
Patients scheduled for the first cataract surgery
Age ≥60 years
Cataract with grade ≥2 nuclear opacification according to Lens Opacities Classification System III.45
Exclusion criteria:
Severe mental illness or dementia
Severe corneal opacities with difficulty in assessment of lens opacity or fundal examination
Glaucoma with a visual field deficit with least mean deviation >14 dB (Humphrey perimeter)
Vitreous haemorrhage
Proliferative diabetic retinopathy
Macular oedema
AMD
Patients needing immediate cataract surgery
Patients needing combined cataract and glaucoma surgery or combined cataract surgery and vitrectomy.
Participants requiring cataract surgery for one eye or both eyes will be included. For participants requiring surgery in both eyes, the intervention will be completed within 1–2 weeks, and the same type of IOL will be used in both eyes.
Intervention
The intervention in the present study is phacoemulsification with a small incision and implantation of an IOL. Participants will be randomly allocated to the clear or blue-blocking IOL group in a ratio of 1:1. In the clear IOL group, a clear spherical IOL (SA60AT, Alcon, Fort Worth, USA) will be implanted. In the blue-blocking IOL group, a spherical blue-blocking IOL (SN60AT) or an aspherical blue-blocking IOL (SN60WF, Alcon, Fort Worth, USA) will be implanted in a randomly allocated 1:1 ratio. Blue-blocking lenses with +20.0 D transmit 34%, 47% and 64% of light at 420, 440 and 460 nm, respectively.34 Before cataract surgery, the axial length of the eye will be measured with an A-scan UD-6000 (Tomey, Nagoya, Japan). The appropriate power of each IOL will be estimated using the SRK/T formula.46
Primary outcomes
The primary outcomes of the present study are mortality and the incidence of CVD, cancer and AMD after surgery.
Secondary outcomes
The secondary outcomes are listed below:
Glucose/lipid metabolism indicators including glycated haemoglobin (HbA1c), fasting plasma glucose (FPG), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C)
Obesity as determined by body mass index and abdominal circumference
Indicators of circadian rhythm, including urinary melatonin metabolite (6-sulfatoxymelatonin (aMT6-s)), wrist skin temperature and the circadian rhythm of physical activity
Sleep quality based on actigraphic sleep quality, the Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS)
The presence of depressive symptoms assessed using the short version of the Geriatric Depression Scale (GDS-15)
Light sensitivity assessed by the post-illumination pupil response (PIPR)
Ophthalmic parameters including visual acuity, the amplitude of pseudoaccommodation, the thickness of the retina and choroid measured using spectral-domain optical coherence tomography (SD-OCT), density of the macular pigment, aberration and subjective visual function assessed using the National Eye Institute Visual Function Questionnaire (NEI VFQ25).
Participant timeline
After baseline assessment and the surgical intervention, all participants will be requested to visit Nara Medical University Hospital annually (table 1). All outcomes will be assessed at baseline and 1 year after surgery. At annual follow-up visits from 2 to 20 years after surgery, details of mortality, the incidence of CVD, cancer and AMD will be recorded; the questionnaire survey will be administered; SD-OCT will be performed and density of the macular pigment will be measured.
Table 1.
Schedule of participants visit and data collection
| Enrolment | Allocation/ baseline | Intervention* | 1 year after intervention | Annual follow-up from 2 to 20 years after intervention | |
|---|---|---|---|---|---|
| Eligibility screen | |||||
| Slit-lamp examination | ✓ | ||||
| Fundal examination | ✓ | ||||
| Intraocular pressure | ✓ | ||||
| Outcomes assessment | |||||
| Mortality | ✓ | ✓ | ✓ | ||
| Incidence of CVD, cancer | ✓ | ✓ | ✓ | ||
| PSQI, ESS, GDS-15, NEI VFQ25 | ✓ | ✓ | ✓ | ||
| Fundal examination | ✓ | ✓ | ✓ | ||
| SD-OCT | ✓ | ✓ | ✓ | ||
| Density of the macular pigment | ✓ | ✓ | ✓ | ||
| Pseudoaccommodation aberration | ✓ | ✓ | |||
| Glucose, HbA1c, TG, LDL/HDL C | ✓ | ✓ | |||
| BMI, abdominal circumference | ✓ | ✓ | |||
| Actigraphy, wrist skin temperature | ✓ | ✓ | |||
| Urinary 6-sulfatoymelatonin | ✓ | ✓ | |||
| PIPR | ✓ | ✓ |
*The intervention of the present study is cataract surgery using a clear IOL versus blue-blocking IOL.
BMI, body mass index; CVD, cardiovascular disease; ESS, Epworth Sleepiness Scale; GDS-15, Geriatric Depression Scale; HbA1c, glycated haemoglobin; IOL, intraocular lens; LDL/HDL C, low-density/high-density lipoprotein cholesterol; NEI VFQ25, National Eye Institute Visual Function Questionnaire; PIPR, post-illumination pupil response; PSQI, Pittsburgh Sleep Quality Index; SD-OCT, spectral-domain optical coherence tomography; TG, triglyceride.
Mortality and disease incidence
The incidence of CVD and cancer will be assessed using a self-administered questionnaire, and the diagnosis will be confirmed based on medical records. When a participant dies, the cause of death will be determined from the medical records and the death certificate. The presence of AMD at each visit will be investigated by fundus examination and SD-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany).
Ocular examinations
Slit-lamp examinations with photographic documentation will be performed at baseline. The diagnosis will be confirmed and the grade of cataract determined by two independent ophthalmologists. The amplitude of pseudoaccommodation will be measured by the lens-loading method in an examination room under 350 lux illumination. Ocular higher order aberrations will be measured with a Pentacam (Oculus, Wetzler, Germany) and a KW-9000 aberrometer (Topcon, Tokyo, Japan). The measurements will be made through a 4 mm pupil diameter and repeated at least three times to acquire well focused and properly aligned images. The retinal and choroidal thickness will be measured by SD-OCT (RS-3000; NIDEK, Gamagori, Japan). The optical density of the macular pigment will be measured using a macular pigment screener (MPS III; Electron Technology, Cambridge, UK).
Post-illumination pupil response
Following pharmacological blockage of rod and cone cells in vitro, the melanopsin-associated ganglion cell response can be isolated as a slow, maintained depolarisation to light stimulation, which repolarises slowly after light offset.47 This PIPR is an index of the sensitivity of the melanopsin-containing ipRGC pathway.48 The PIPR will be measured using a pupillometer (RAPDx; Konan Medical Inc, Tokyo, Japan). The baseline pupil diameter will be measured after 10 min of dark adaptation. The diameters at baseline, the peak and during sustained pupillary constriction, will be analysed after 10 s of blue light (440 nm) and red light (605 nm) stimulation. Baseline pupil diameter is the average pupil diameter during a 7 s period before light onset. Sustained pupil diameter is the average from 10 to 40 s after light offset. The PIPR (mm), PIPR change (%), net PIPR (mm) and net PIPR change (%) will be calculated as follows.49
 |
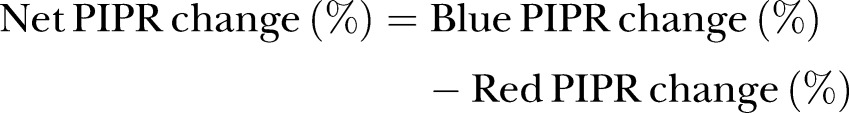 |
Self-reported questionnaires
Depressive symptoms will be assessed using the GDS-15, which is a self-administered questionnaire consisting of 15 items.50 The validity of the questionnaire has been established previously.51 52 Subjective sleep quality and daytime sleepiness will be assessed using the PSQI53 and the ESS,54 respectively. Chronotype and subjective visual function will be determined using the Morningness–Eveningness Questionnaire,55 the Munich Chronotype Questionnaire56 and the NEI VFQ25.57 58
Analysis of venous blood sample and morning spot urine
Overnight fasting venous blood samples and morning spot urine samples will be obtained at baseline and 1 year after surgery and will be analysed at a commercial laboratory (SRL Co, Inc, Tokyo, Japan) using standard clinical chemistry analysis to determine the concentrations of HbA1c, FPG, TG, LDL-C and HDL-C. The urinary aMT6-s concentration will be measured using an ELISA kit (RE54031; IBL International, Hamburg, Germany). Peak nocturnal plasma melatonin is significantly associated with aMT6-s in subsequent morning spot urine (r=0.69).59 60
Actigraphic sleep and circadian activity rhythm
Participants will wear an actigraph (ActiSleep-BT Monitor; ActiGraph Inc, Florida, USA) on the non-dominant arm for 5 days including weekdays and a weekend, and will keep a sleep diary logging bedtime and rising time. Total sleep time, sleep efficiency, sleep-onset latency and wake after sleep onset will be calculated with ActiLife 6 (ActiGraph Inc). Indices of sleep quality using this device show moderate-to-high agreement with sleep parameters measured by polysomnography.61 Actigraphic data show the circadian physical activity rhythm. According to large-scale prospective cohort studies, decreased amplitude, later phase and decreased robustness of circadian activity rhythm analysed using sigmoidally transformed cosine curves62 show a significantly higher HR for incidence of cognitive disorders, cancer mortality and all-cause mortality.63–65
Wrist skin temperature
Wrist skin temperature reveals a mirror image of core body temperature,66 67 and an evening increase in wrist temperature is significantly correlated with the time of dim light melatonin onset in real-life situations (r=0.76).68 Wrist skin temperature will be measured on the inside of the wrist, near the radial artery of the non-dominant arm at 3 min intervals using a temperature data logger (Thermochron iButton; Maxim/Dallas, Dallas, Texas, USA).
Sample size calculation
Of 2636 participants aged 60 years or older at enrolment in the Blue Mountains Eye Study,43 27% (n=713) died during 10 years of follow-up. To detect a 7.6% reduction in the risk of death over 10 years with a 95% two-sided α level of 5% with a power of 80%, 481 participants in each group would be required. Assuming a dropout rate of 3%, a total of 1000 participants would be needed.
Randomisation, masking
Central randomisation by an independent allocator maintains allocation concealment. Random sequence was generated by computer. The results of allocation will be open to the care providers and the participants, but to outcome assessors.
Statistical analyses
Outcomes will be compared between the clear IOL group and the blue-blocking IOL group based on the intention-to-treat principle. For missing values due to loss to follow-up after baseline measurement, baseline data will be imputed using the last-observation-carried-forward method. For continuous variables with normal distributions, the mean and SD will be reported. For variables not distributed normally, the median and IQR will be reported. Means, medians and proportions will be compared using the t test, the Mann-Whitney U test and the χ2 test, respectively. Analysis of covariance will be used to estimate adjusted mean values and 95% CIs. The prevalence of the two groups will be tested using multivariate logistic regression analysis. The Kaplan-Meier plot, the log-rank test and the Cox proportional hazard model will be used to compare the mortalities and disease incidence rates between the two groups.
Data monitoring
The frequency of the adverse events will be analysed according to medical records. A data monitoring committee consisting of researchers and external specialists in internal medicine, public health and ophthalmology will annually report the number of participants, adverse events and results of the interim analysis. The committee will make the final decision to terminate the trial. Annual audit will be conducted by the Institutional Review Board of Nara Medical University, independent of investigators.
Ethics and dissemination
All modifications to the protocol will be reported to the UMIN-CTR and communicated to the public. An appropriately trained ophthalmologist will obtain informed consent. To promote data quality, double data entry will be conducted. To assure confidentiality, all paper-based personal information, and blood and urine samples, will be coded by identification number without personal information and stored at Nara Medical University School of Medicine in locked cabinets or locked freezers with limited access. Electronic data will be stored on a secure password-protected server during the study. The findings of this study will be communicated to healthcare professionals, participants and the public through peer-reviewed publications, scientific conferences and the UMIN-CTR home page.
Discussion
The present study (CLOCK-IOL colour study) will be conducted simultaneously with another randomised controlled trial (the CLOCK-IOL study), which will investigate the influence of cataract surgery on circadian rhythm by comparing patients who receive cataract surgery with a control group at 3 months after baseline.69
There are two main limitations to this protocol. First, there is a lack of information about light exposure at night (LAN). If the amount of LAN is balanced between two groups due to random allocation, blue-blocking IOL may reveal a beneficial effect by reducing harmful influence of LAN according to recent cross-sectional evidence. The higher level of self-reported LAN asked by questionnaire was significantly associated with higher prevalence of obesity among over 100 000 women.70 Furthermore, objectively measured LAN in the bedroom also showed significant associations with a prevalence of obesity,71 depression,72 insomnia,73 nocturnal hypertension74 and atherosclerosis.75
Second, a seasonal effect may modify outcomes such as the prevalence of depression and circadian rhythm parameters, for instance, urinary aMT6-s, wrist skin temperature and the circadian rhythm of physical activity, because participants will be recruited throughout the year. Seasonal variables such as day length and outdoor temperature will be taken into account during data analysis.
Footnotes
Twitter: Follow Toyoaki Matsuura at @nekochan
Contributors: TN, KS and KO designed the study. TN and KS prepared the first draft of the manuscript. KM, MY, NM, YM, HT, MO, TH, SM, MK, TU and TM provided ophthalmic expertise for the protocol for this clinical trial. NT contributed to the engineering aspect of measuring outcomes. NK and NO are grant holders. All authors contributed to the refinement of the study protocol and approved the final manuscript.
Funding: The present study is supported by a grant for collaboration study from Nara Medical University.
Competing interests: None declared.
Ethics approval: The study protocol was approved by the Institutional Review Board of Nara Medical University (number 13-032) and was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; trial ID: UMIN000014680) on 28 July 2014, before the enrolment of the participants.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Flo E, Pallesen S, Akerstedt T et al. Shift-related sleep problems vary according to work schedule. Occup Environ Med 2013;70:238–45. 10.1136/oemed-2012-101091 [DOI] [PubMed] [Google Scholar]
- 2.Feskanich D, Hankinson SE, Schernhammer ES. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int 2009;20:537–42. 10.1007/s00198-008-0729-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 2001;58:747–52. 10.1136/oem.58.11.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietroiusti A, Neri A, Somma G et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med 2010;67:54–7. 10.1136/oem.2009.046797 [DOI] [PubMed] [Google Scholar]
- 5.Morikawa Y, Nakagawa H, Miura K et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health 2005;31:179–83. 10.5271/sjweh.867 [DOI] [PubMed] [Google Scholar]
- 6.Kroenke CH, Spiegelman D, Manson J et al. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol 2007;165:175–83. 10.1093/aje/kwj355 [DOI] [PubMed] [Google Scholar]
- 7.Bara AC, Arber S. Working shifts and mental health—findings from the British Household Panel Survey (1995–2005). Scand J Work Environ Health 2009;35:361–7. 10.5271/sjweh.1344 [DOI] [PubMed] [Google Scholar]
- 8.Fujino Y, Iso H, Tamakoshi A et al. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol 2006;164:128–35. 10.1093/aje/kwj185 [DOI] [PubMed] [Google Scholar]
- 9.Brown DL, Feskanich D, Sanchez BN et al. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol 2009;169:1370–7. 10.1093/aje/kwp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vyas MV, Garg AX, Iansavichus AV et al. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012;345:e4800 10.1136/bmj.e4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schernhammer ES, Laden F, Speizer FE et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 2001;93:1563–8. 10.1093/jnci/93.20.1563 [DOI] [PubMed] [Google Scholar]
- 12.Schernhammer ES, Laden F, Speizer FE et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst 2003;95:825–8. 10.1093/jnci/95.11.825 [DOI] [PubMed] [Google Scholar]
- 13.Kolstad HA. Nightshift work and risk of breast cancer and other cancers—a critical review of the epidemiologic evidence. Scand J Work Environ Health 2008;34:5–22. 10.5271/sjweh.1194 [DOI] [PubMed] [Google Scholar]
- 14.Poole EM, Schernhammer ES, Tworoger SS. Rotating night shift work and risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev 2011;20:934–8. 10.1158/1055-9965.EPI-11-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatti P, Mirick DK, Davis S. The impact of chronotype on melatonin levels among shift workers. Occup Environ Med 2014;71:195–200. 10.1136/oemed-2013-101730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheer FA, Hilton MF, Mantzoros CS et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–8. 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalsa SB, Jewett ME, Cajochen C et al. A phase response curve to single bright light pulses in human subjects. J Physiol 2003;549(Pt 3):945–52. 10.1113/jphysiol.2003.040477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulivarthy SR, Tanaka N, Welsh DK et al. Reciprocity between phase shifts and amplitude changes in the mammalian circadian clock. Proc Natl Acad Sci USA 2007;104:20356–61. 10.1073/pnas.0708877104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattar S, Liao HW, Takao M et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002;295:1065–70. 10.1126/science.1069609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brainard GC, Hanifin JP, Greeson JM et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 2001;21:6405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariotti SP. Gloval data on visual impairments 2010. World Health Organization, 2014:1–14. http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf [Google Scholar]
- 22.Kessel L, Lundeman JH, Herbst K et al. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg 2010;36:308–12. 10.1016/j.jcrs.2009.08.035 [DOI] [PubMed] [Google Scholar]
- 23.Danquah L, Kuper H, Eusebio C et al. The long term impact of cataract surgery on quality of life, activities and poverty: results from a six year longitudinal study in Bangladesh and the Philippines. PLoS ONE 2014;9:e94140 10.1371/journal.pone.0094140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asplund R, Ejdervik Lindblad B. The development of sleep in persons undergoing cataract surgery. Arch Gerontol Geriatr 2002;35:179–87. 10.1016/S0167-4943(02)00022-5 [DOI] [PubMed] [Google Scholar]
- 25.Ayaki M, Muramatsu M, Negishi K et al. Improvements in sleep quality and gait speed after cataract surgery. Rejuvenation Res 2013;16:35–42. 10.1089/rej.2012.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, She C, Chen D et al. Blue-light-blocking intraocular lens implantation improves the sleep quality of cataract patients. J Clin Sleep Med 2013;9:741–5. 10.5664/jcsm.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander I, Cuthbertson FM, Ratnarajan G et al. Impact of cataract surgery on sleep in patients receiving either ultraviolet-blocking or blue-filtering intraocular lens implants. Invest Ophthalmol Vis Sci 2014;55:4999–5004. 10.1167/iovs.14-14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray CS, Karimova G, Hildreth AJ et al. Recovery of visual and functional disability following cataract surgery in older people: Sunderland Cataract Study. J Cataract Refract Surg 2006;32:60–6. 10.1016/j.jcrs.2005.07.040 [DOI] [PubMed] [Google Scholar]
- 29.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Ophthalmol 2008;146:404–9. 10.1016/j.ajo.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 30.Fraser ML, Meuleners LB, Lee AH et al. Vision, quality of life and depressive symptoms after first eye cataract surgery. Psychogeriatrics 2013;13:237–43. 10.1111/psyg.12028 [DOI] [PubMed] [Google Scholar]
- 31.Meuleners LB, Hendrie D, Fraser ML et al. The impact of first eye cataract surgery on mental health contacts for depression and/or anxiety: a population-based study using linked data. Acta Ophthalmol 2013;91:e445–9. 10.1111/aos.12124 [DOI] [PubMed] [Google Scholar]
- 32.Golden RN, Gaynes BN, Ekstrom RD et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry 2005;162:656–62. 10.1176/appi.ajp.162.4.656 [DOI] [PubMed] [Google Scholar]
- 33.Lieverse R, Van Someren EJ, Nielen MM et al. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry 2011;68:61–70. 10.1001/archgenpsychiatry.2010.183 [DOI] [PubMed] [Google Scholar]
- 34.Tanito M, Okuno T, Ishiba Y et al. Transmission spectrums and retinal blue-light irradiance values of untinted and yellow-tinted intraocular lenses. J Cataract Refract Surg 2010;36:299–307. 10.1016/j.jcrs.2009.08.036 [DOI] [PubMed] [Google Scholar]
- 35.Mainster MA, Turner PL. Blue-blocking IOLs decrease photoreception without providing significant photoprotection. Surv Ophthalmol 2010;55:272–89. 10.1016/j.survophthal.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 36.Turner PL, Van Someren EJ, Mainster MA. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev 2010;14:269–80. 10.1016/j.smrv.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Ham WT Jr, Ruffolo JJ Jr, Mueller HA et al. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci 1978;17:1029–35. [PubMed] [Google Scholar]
- 38.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 2000;41:1981–9. [PubMed] [Google Scholar]
- 39.Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand 2006;84:4–15. 10.1111/j.1600-0420.2005.00627.x [DOI] [PubMed] [Google Scholar]
- 40.Klein R, Klein BE, Jensen SC et al. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol 1998;116:506–13. 10.1001/archopht.116.4.506 [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Klein BE, Wong TY et al. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Arch Ophthalmol 2002;120:1551–8. 10.1001/archopht.120.11.1551 [DOI] [PubMed] [Google Scholar]
- 42.Wang JJ, Klein R, Smith W et al. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology 2003;110:1960–7. 10.1016/S0161-6420(03)00816-9 [DOI] [PubMed] [Google Scholar]
- 43.Cugati S, Mitchell P, Rochtchina E et al. Cataract surgery and the 10-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology 2006;113:2020–5. 10.1016/j.ophtha.2006.05.047 [DOI] [PubMed] [Google Scholar]
- 44.Sparrow JR, Miller AS, Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg 2004;30:873–8. 10.1016/j.jcrs.2004.01.031 [DOI] [PubMed] [Google Scholar]
- 45.Chylack LT Jr, Wolfe JK, Singer DM et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831–6. 10.1001/archopht.1993.01090060119035 [DOI] [PubMed] [Google Scholar]
- 46.Sanders DR, Retzlaff J, Kraff MC. Comparison of empirically derived and theoretical aphakic refraction formulas. Arch Ophthalmol 1983;101:965–7. 10.1001/archopht.1983.01040010965024 [DOI] [PubMed] [Google Scholar]
- 47.Dacey DM, Liao HW, Peterson BB et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005;433:749–54. 10.1038/nature03387 [DOI] [PubMed] [Google Scholar]
- 48.Gamlin PD, McDougal DH, Pokorny J et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res 2007;47:946–54. 10.1016/j.visres.2006.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci 2010;51:2764–9. 10.1167/iovs.09-4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol 1991;4:173–8. 10.1177/089198879100400310 [DOI] [PubMed] [Google Scholar]
- 51.Wancata J, Alexandrowicz R, Marquart B et al. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand 2006;114:398–410. 10.1111/j.1600-0447.2006.00888.x [DOI] [PubMed] [Google Scholar]
- 52.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999;14:858–65. [DOI] [PubMed] [Google Scholar]
- 53.Buysee DJ, Reynolds CF III, Monk TH et al. The Pittsburgeh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1988;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 54.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992;15:376–81. [DOI] [PubMed] [Google Scholar]
- 55.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4:97–110. [PubMed] [Google Scholar]
- 56.Kitamura S, Hida A, Aritake S et al. Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiol Int 2014;31:845–50. 10.3109/07420528.2014.914035 [DOI] [PubMed] [Google Scholar]
- 57.Mangione CM, Lee PP, Gutierrez PR et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–8. 10.1001/archopht.119.7.1050 [DOI] [PubMed] [Google Scholar]
- 58.Suzukamo Y, Oshika T, Yuzawa M et al. Psychometric properties of the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25), Japanese version. Health Qual Life Outcomes 2005;3:65 10.1186/1477-7525-3-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graham C, Cook M, Kavet R et al. Prediction of nocturnal plasma melatonin form morning urinary measures. J Pineal Res 1998;24:230–8. 10.1111/j.1600-079X.1998.tb00538.x [DOI] [PubMed] [Google Scholar]
- 60.Schernhammer ES, Rosner B, Wilett W et al. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev 2004;13:936–43. [PubMed] [Google Scholar]
- 61.Cellini N, Burman M, McDevitt E et al. Direct Comparison of two actigraphy devices with polysomnographically recorded naps in healthy young adults. Chronobiology Int 2013;30:691–8. 10.3109/07420528.2013.782312 [DOI] [PubMed] [Google Scholar]
- 62.Marler MR, Gehrman P, Martin JL et al. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med 2006;25:3893–904. 10.1002/sim.2466 [DOI] [PubMed] [Google Scholar]
- 63.Tranah GJ, Blackwell T, Ancoli-Israel S et al. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc 2010;58:282–91. 10.1111/j.1532-5415.2009.02674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paudel ML, Taylor BC, Ancoli-Israel S et al. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int 2011;28:258–66. 10.3109/07420528.2011.553016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tranah GJ, Blackwell T, Stone KL et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011;70:722–32. 10.1002/ana.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarabia JA, Rol MA, Mendiola P et al. Circadian rhythm of wrist temperature in normal-living subjects a candidate of new index of the circadian system. Physiol Behav 2008;95:570–80. 10.1016/j.physbeh.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 67.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol 1994;267(3 Pt 2):R819–29. [DOI] [PubMed] [Google Scholar]
- 68.Bonmati-Carrion MA, Middleton B, Revell V et al. Circadian phase assessment by ambulatory monitoring in humans: correlation with dim light melatonin onset. Chronobiol Int 2014;31:37–51. 10.3109/07420528.2013.820740 [DOI] [PubMed] [Google Scholar]
- 69.Saeki K, Obayashi K, Nishi T et al. Short-term influence of cataract surgery on circadian biological rhythm and related health outcomes (CLOCK-IOL trial): study protocol for a randomized controlled trial. Trials 2014;15:514 10.1186/1745-6215-15-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McFadden E, Jones ME, Schoemaker MJ et al. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol 2014;180:245–50. 10.1093/aje/kwu117 [DOI] [PubMed] [Google Scholar]
- 71.Obayashi K, Saeki K, Iwamoto J et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab 2013;98:337–44. 10.1210/jc.2012-2874 [DOI] [PubMed] [Google Scholar]
- 72.Obayashi K, Saeki K, Iwamoto J et al. Exposure to light at night and risk of depression in the elderly. J Affect Disord 2013;151:331–6. 10.1016/j.jad.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 73.Obayashi K, Saeki K, Kurumatani N. Association between light exposure at night and insomnia in the general elderly population: the HEIJO-KYO cohort. Chronobiol Int 2014;31:976–82. 10.3109/07420528.2014.937491 [DOI] [PubMed] [Google Scholar]
- 74.Obayashi K, Saeki K, Iwamoto J et al. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol Int 2014;31:779–86. 10.3109/07420528.2014.900501 [DOI] [PubMed] [Google Scholar]
- 75.Obayashi K, Saeki K, Kurumatani N. Light exposure at night is associated with subclinical carotid atherosclerosis in the general elderly population: the HEIJO-KYO cohort. Chronobiol Int 2015;32:310–17 10.3109/07420528.2014.974809. [DOI] [PubMed] [Google Scholar]


