Erysipelothrix rhusiopathiae, a bacterium most commonly known from domestic swine and poultry, was recently associated with multiple unusual mortality events in muskoxen (Ovibos moschatus wardi) on 2 islands in the Canadian Arctic Archipelago. Muskoxen, 1 of the 2 main large herbivores in the Canadian Arctic, provide important ecosystem services and a healthy source of food, a focus for cultural activities, and a source of income through tourism and commercial and sport hunting. The detection of E. rhusiopathiae as a significant cause of mortality in muskoxen has potential implications for wildlife conservation, food safety, and food security.
In July/August 2012, approximately 150 dead muskoxen of all age classes were observed on Banks Island, Northwest Territories, Canada (Figure 1). Wildlife officials sampled 4 carcasses (1 bull and 2 calves found dead and 1 bull that was found recumbent and was euthanized) on July 14th and submitted tissues to the Canadian Wildlife Health Cooperative (CWHC) in Saskatoon. Animals were in good body condition and appeared to have died rapidly with terminal thrashing of legs. Liver, spleen, kidney, bone marrow, and/or intestine from the 2 bulls and 1 calf were cultured and all tissues were positive for E. rhusiopathiae, confirmed by 16sRNA sequencing (Prairie Diagnostic Services, Saskatoon, Saskatchewan). Tissues from the 2 calves and 1 bull were examined histologically but were too autolyzed to interpret. Tissues from the euthanized bull were relatively fresh and fibrin thrombi and/or small intravascular clusters of Gram-positive bacilli were seen in the heart, lung, and kidney. There was focal necrosis of the liver. In early August, tissues from 3 adult female carcasses were submitted and spleen swabs from 2 of these cultured positive for E. rhusiopathiae. Although most tissues were severely autolyzed, in some animals rod-shaped bacterial colonies were seen throughout sections of the kidney tissue in low numbers and around and within the tunica adventitia and tunica media of medium to large arteries in higher numbers. In 1 animal, bacterial colonies were present within collecting tubules in the medulla. Additional liver, kidney, and spleen samples from the culture-negative cow, and tissues from the calf submitted in July that were not initially cultured, were subsequently tested at the University of Calgary Faculty of Veterinary Medicine using a selective culture medium (1) and were culture-positive for E. rhusiopathiae. Based on the consistent necropsy, histological and bacteriological findings, E. rhusiopathiae was considered the cause of death in all 7 animals.
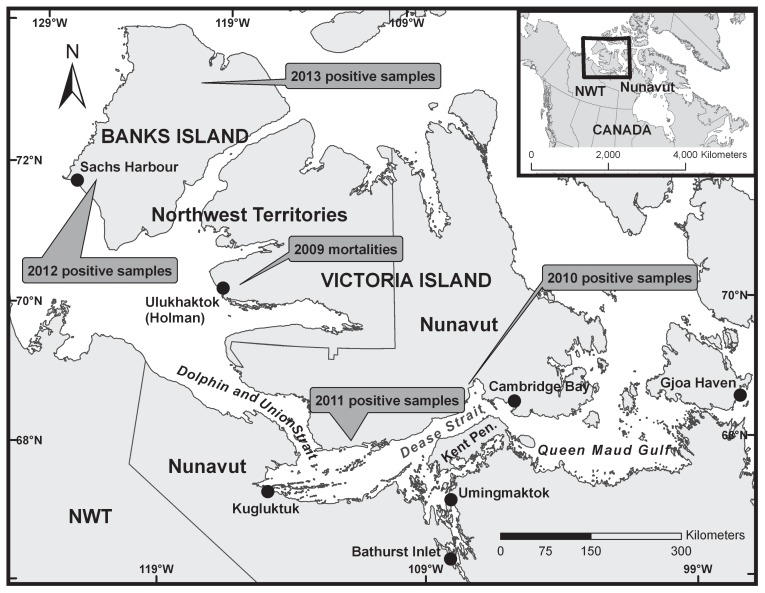
Figure 1.
Map demonstrating the general locations and years of the observed die-offs.
Erysipelothrix rhusiopathiae is a Gram-positive, facultative intracellular bacterium. It can cause a wide range of disorders, including skin lesions, fever, endocarditis, and septicemia, in a variety of domestic and wild vertebrates including, but not limited to, pigs, turkeys, cervids, dolphins, seals, rodents, and humans (2). It is also an important cause of disease in captive marine mammals (3) and is reported from the skin surface of asymptomatic freshwater and marine fish (4). The only previous published report of E. rhusiopathiae in wildlife in northern Canada is from 4 wood bison found dead (anthrax suspected but tested negative) near Fort Smith, Northwest Territories in 1973 to 1974, and from the mouth and rectum of 1 healthy wolf scavenging on a dead bison from this same area (5). Erysipelothrix rhusiopathiae can be maintained in carrier animals, may persist in aquatic and soil environments, meat products, and decomposing animal remains, and can be spread by ticks, mites, and flies (2). It tends to be opportunistic, with disease outbreaks following stress events in domestic animals (e.g., shipping, electrical storms). In free-ranging wildlife it is generally seen as isolated or small clusters of cases (6), but has occurred as epidemics in wild small mammals (7) and has been isolated as 1 of a variety of causative agents during large mortality events in wild birds (8).
The diagnosis of E. rhusiopathiae in muskoxen on Banks Island prompted a review and testing of archived tissues from muskox mortality events on nearby Victoria Island, Nunavut from 2009–2011, and a re-examination of previous recorded mortality events on Banks Island. In mid-August of 2009, 4 muskoxen were found dead near Ulukhaktok, Victoria Island, Northwest Territories. All had fat stores and appeared to have died suddenly with terminal paddling of their limbs. Tissues submitted to CWHC in Saskatoon were very autolyzed and bacterial culture revealed severe postmortem overgrowth; no further work-up was done. In mid-August 2010, hunting guides from the community of Cambridge Bay reported 9 dead muskoxen in the vicinity of north Wellington Bay, Victoria Island. A field investigation conducted 2 wk later from helicopter located 6 carcasses (likely different from those originally reported) and field postmortems were done on 4 of these (3 adult male, 1 adult female). Two of these bulls and the adult female were in good body condition and there was evidence of paddling prior to death as indicated by disturbance of the surrounding ground. There was no evidence of predation but some carcasses had been scavenged. Carcasses were in various states of decomposition with numerous maggots present. There was a variable amount of subcutaneous edema and ecchymotic and petechial hemorrhages throughout the carcasses and approximately 1 L serosanguinous fluid in the thorax and less in the abdomen. One animal had evidence of diarrhea based on recent fecal matting in the perianal region and no formed feces in the colon. The fourth muskox examined presented differently. It was an adult bull with no subcutaneous fat and which had chronic purulent abdominal fasciitis, peritonitis, and granulomatous typhlitis. The lungs of this animal were in good condition with no gross abnormalities. Tissues and long bones from all animals were submitted to CWHC in Saskatoon for further analyses. All tissues were severely autolyzed and a variety of bacterial organisms were isolated, indicating postmortem overgrowth. The ileum and other tissues from all animals were selectively cultured for enteric pathogens, including Yersinia pseudotuberculosis, as this bacterium had previously been isolated from a series of similar die-offs on Banks Island in 1986, and 1988 to 1990 (9,10). Neither Yersinia, nor any other enteric pathogens, were detected.
The third mortality event was in August 2011, during a ground-based muskox fecal survey for lungworms on southwest Victoria Island (11) (Figure 2). One dead adult male and 2 dead adult female muskoxen were found and field postmortems were performed. Findings were similar to those of the acute mortalities in 2010 with the exception that 1 animal had approximately 200 mL of serosanguinous fluid in the pericardium, and another animal had an intussusception and volvulus of the jejunum, extending approximately 20 cm with a few fibrinous tags but no gas in the intestine proximal to the intussusception, and no foreign bodies. The liver of this animal was tan, swollen, and had rounded edges. Samples from this outbreak were archived, but no further diagnostic work-up was done until 2012, following the Banks Island diagnosis of E. rhusiopathiae. At that time, the tissues from the 2011 outbreak, and additional archived samples from the 2010 Victoria Island outbreak were tested and E. rhusiopathiae was detected by direct polymerase chain reaction (PCR) and isolated from multiple tissues from all animals using a selective culture medium (1).
Figure 2.
Dead muskox found on Victoria Island in August 2011.
Finally, in late June/early July 2013, during a research expedition along Thomsen River in Aulavik National Park on northern Banks Island, several muskox carcasses estimated to be a few months to a few years old (little to no soft tissue present) were observed. Long bones were collected from 6 (adult males) of the more recent of these and submitted to the UCVM laboratory. Marrow from 2 were positive for E. rhusiopathiae by direct PCR, one of which, the “freshest” carcass, was also positive by culture. Four of the 6 had substantial fat reserves in their marrow. Subsequently, several recently dead muskoxen were reported by tourists canoeing in Aulavik National Park in July, and by hunters in other regions of the island during summer 2013, but no samples were obtained and postmortems were not done.
The broad geographic occurrence (over 200 000 km2) and the magnitude of the mortality events observed for muskoxen from 2009 to 2013 was an unusually severe presentation for E. rhusiopathiae in wildlife. However, large-scale mortality events for muskoxen have been reported sporadically on Banks Island. The first disease-associated documented die-off was in the summer of 1986 in Aulavik National Park when 67 carcasses and 52 skeletal remains of muskoxen were found (10). Animals were typically adults in good body condition. Severe enteritis was typical on postmortem examinations and Y. pseudotuberculosis was cultured and considered as the cause of death. Additional mortalities with similar presentations and pathology were recorded in this region from 1988 to 1991, with Y. pseudotuberculosis being isolated from most carcasses. However, in 1990, 4 of 25 Yersinia suspect animals had atypical pulmonary lesions (edema) and Y. pseudotuberculosis was not cultured, suggesting that another disease process may have appeared (9). In 1996, hunters on Banks Island reported an unusually high number of dead muskoxen in July. Subsequent surveys in August documented up to 85 carcasses (12). Age structure, body condition, and external presentation were similar to the previous reported mortality events; however, Y. pseudotuberculosis was not cultured from the ileum or feces of 12 animals sampled. Full postmortem examinations and testing were not done and the cause of the mortality event remained undetermined (12). Finally, in 2004 there was exceptionally high mortality of muskoxen on Banks Island that was attributed to a rain on snow icing event in October 2003, which prevented access to feed and lead to starvation. However, during the 2004 summer productivity survey additional mortalities were observed and some of these animals appeared to have died recently and acutely (13). The patterns of disease (acute summer mortality of animals in good body condition) observed in the 4 undiagnosed cases in 1990, the 1996 die-off, and perhaps in 2004, are consistent with our observations in 2009 to 2013, presenting E. rhusiopathiae as a possible causative agent.
The appearance of E. rhusiopathiae associated with significant acute mortality of muskoxen on these islands is enigmatic and begs the question of whether this is a result of a novel pathogen, changing ecological conditions/increased muskox vulnerability, or both. The pattern and severity of the recent mortality events are consistent with what would be expected with a new pathogen introduced into a naïve host population. Preliminary serology on small numbers of archived serum samples from 1990 to 1991, 2001, 2008, and 2012 indicate that E. rhusiopathiae was absent or very rare in 1990 to 1991 (W. Hutchins, S. Kutz, B. Elkin, unpublished data), but present at all other sampling periods, consistent with a hypothesis of a recent introduction. Full genome sequencing of isolates from muskoxen across this region is currently underway to further evaluate this hypothesis.
Alternatively, and consistent with the longer history of muskox die-offs on Banks Island (9,10,12,13) and more recent die-offs in a Norwegian population (14,15), the mortality events may reflect a more complex combination of host-environment-pathogen interactions. First, the hosts, muskoxen, have undergone historical bottlenecks, and more recent fragmentation and near extirpations (16,17). This has left them with exceptionally low genetic diversity (18,19), including low diversity of the Major Histocompatability Complex (MHC) (20), and likely influences their ability to respond to infectious disease. Second, the Arctic environment is warming rapidly; melting sea ice and extremes in heat are some of the key characteristics (21). High temperature extremes have previously lead to non-specific pneumonias and mortalities of muskoxen in Norway (14) and in captivity (22) and may have contributed to at least some of the recent mortality events in Canada. Temperatures on Victoria Island immediately prior to the 2010 and 2011 mortalities were 2°C to 3°C above average (Environment Canada). Finally, E. rhusiopathiae is a generalist pathogen that likely occurs, and may be maintained, in other host species in the Arctic. Exposure for muskoxen may be linked to population dynamics of these other species, additional environmental stressors, and low genetic “capital” may make muskoxen particularly vulnerable to this increased exposure.
The impact of the recent mortality events on muskox population dynamics remains undetermined. Surveys of Banks Island and the Nunavut side of Victoria Island in 2013/2014 suggest that both populations have declined by over 50% since 2010 and the late 1990s, respectively (T. Davison, Environment and Natural Resources, Government of the NWT, and L. Leclerc, Government of Nunavut — Department of Environment, unpublished data). Although it is unclear if these declines are due to disease outbreaks, natural cycles, or a combination of both, they have significant implications for food safety, food security, and economies. Given the low density of animals near the communities, the commercial harvests, a source of seasonal employment for communities on both islands, have been suspended; however, subsistence hunts for food continue. Additionally, E. rhusiopathiae has zoonotic potential, which adds an important layer of public health education and messaging to ensure that this nutritious food source continues to be harvested and handled in a manner that prevents human exposure.
In conclusion, the recent recognition of E. rhusiopathiae as a significant pathogen in muskoxen, and exploration of other unusual mortality events of multiple etiologies in this species, highlight the importance of understanding the diversity of pathogens, the resilience of their hosts, and the complex host-pathogen interactions in a changing Arctic. Climate warming and increasing mining, transport, and tourism in the Arctic are altering the landscape and may impact the future viability of unique arctic species that are vital components of the ecosystem and important sources of food and income for northerners. Only by understanding pathogen diversity and ecology, thoroughly investigating unusual wildlife mortalities, documenting local observations of unusual health, and maintaining effective community-engaged surveillance programs, will we be able to promote wildlife conservation and health, and food safety and security, in a rapidly changing Arctic.
Acknowledgments
We thank the hunters of Sachs Harbour, Cambridge Bay, and Ulukhaktok for reporting the incidents. We also thank Greg Elias, ENR in Sachs Harbour; Rob Harmer, Shane Sather, and Lisa-Marie Leclerc with the Government of Nunavut; Manigandan Lejeune-Virapin with the CWHC; Cindy Hague at University of Calgary; and Musangu Ngeleka and Prairie Diagnostic Services staff for microbiology diagnostics.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bender JS, Kinyon JM, Kariyawasam S, Halbur PG, Opriessnig T. Comparison of conventional direct and enrichment culture methods for Erysipelothrix spp. from experimentally and naturally infected swine. J Vet Diagn Invest. 2009;21:863–868. doi: 10.1177/104063870902100617. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Change BJ, Riley TV. Eryispelothrix rhusiopathiae. Vet Microbiol. 2010;140:405–417. doi: 10.1016/j.vetmic.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Leighton FA. Erysipelothrix infection. In: Williams ES, Barker IK, editors. Infectious Diseases of Wild Mammals. Ames, Iowa: Iowa State University Press; 2001. pp. 491–493. [Google Scholar]
- 4.Stenstrom JM, Norrung V, Ternstrom A, Molin G. Occurrence of different serotypes of Erysipelothrix rhusiopathiae in retail pork and fish. Acta Vet Scand. 1992;33:169–173. doi: 10.1186/BF03547323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langford EV, Dorward WJ. Erysipelothrix insidiosa recovered from sylvatic mammals in northwestern Canada during examinations for rabies and anthrax. Can Vet J. 1977;18:101–104. [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell GD, Addison EM, Barker IK, Rosendal S. Erysipelothrix rhusiopathiae, serototype 17, septicemia in moose (Alces alces) from Algonquin Park, Ontario. J Wildl Dis. 1994;30:436–438. doi: 10.7589/0090-3558-30.3.436. [DOI] [PubMed] [Google Scholar]
- 7.Wayson NE. An epizootic among meadow mice in California caused by the bacillus of mouse septicemia or of swine erysipelas. Public Health Reports. 1927;42:1489–1493. [Google Scholar]
- 8.Wolcott M. Ersyipelas. In: Thomas BJ, Hunter DB, Atkinson CT, editors. Infectious Diseases of Wild Birds. Ames, Iowa: Wiley-Blackwell Publishing; 2007. [Google Scholar]
- 9.McLean B. MSc thesis. University of Alaska Fairbanks; 1996. Yersiniosis as a mortality factor of muskoxen on Banks Island, Northwest Territories, Canada Fairbanks, AK, USA. [Google Scholar]
- 10.Blake JE, McLean B, Gunn A. Yersiniosis in free-ranging muskoxen on Banks Island, Northwest Territories, Canada. J Wildl Dis. 1991;27:527–533. doi: 10.7589/0090-3558-27.4.527. [DOI] [PubMed] [Google Scholar]
- 11.Kutz SJ, Checkley S, Verocai GG, et al. Invasion, establishment, and range expansion of two protostrongylid nematodes in the Canadian Arctic. Glob Chang Biol. 2013;19:3254–3262. doi: 10.1111/gcb.12315. [DOI] [PubMed] [Google Scholar]
- 12.Larter NC, Nagy JA. Manuscript Report No. 117. Government of the Northwest Territories; 1999. Muskox mortality survey, Banks island, August 1996. [Google Scholar]
- 13.Nagy JA, Gunn A. Manuscript Report No. 204. Yellowknife, Northwest Territories, Canada: Government of the Northwest Territories; 2009. Productivity of Peary caribou and muskoxen on Banks and Melville Islands, NT, July 2004. [Google Scholar]
- 14.Ytrehus B, Bretten T, Bergsjo B, Isaksen K. Fatal pneumonia epizootic in muskox (Ovibos moschatus) in a period of extraordinary weather conditions. EcoHealth. 2008;5:213. doi: 10.1007/s10393-008-0166-0. [DOI] [PubMed] [Google Scholar]
- 15.Handeland K, Tengs T, Kokotovic B, et al. Mycoplasma ovipneumoniae — A primary cause of severe pneumonia epizootics in the Norwegian muskox (Ovibos moschatus) population. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0106116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos PF, Willerslev E, Sher A, et al. Ancient DNA analyses exclude humans as the driving force behind late Pleistocene muskox (Ovibos moschatus) population dynamics. Proc Natl Acad Sci USA. 2010;107:5675–5680. doi: 10.1073/pnas.0907189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lent PC. Muskoxen and their Hunters. Norman, Oklahoma: University of Oklahoma Press; 1999. p. 324. [Google Scholar]
- 18.Groves P. Intraspecific variation in mitochondrial DNA of muskoxen, based on control-region sequences. Can J Zool. 1997;75:568–575. [Google Scholar]
- 19.van Coeverden de Groot PJ, Boag P. Optimization of novel polymorphic microsatellites in muskox (Ovibos moschatus) leads to an increased estimate of muskox microsatellite diversity. Mol Ecol Notes. 2004;4:713–715. [Google Scholar]
- 20.Mikko S, Schmutz S, Andersson L. Monomorphism and polymorphism at Mhc DRB loci in domestic and wild ruminants. Immunol Rev. 1999;167:169–178. doi: 10.1111/j.1600-065x.1999.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 21.Post E, Bhatt U, Bitz C, et al. Ecological consequences of sea-ice decline. Science. 2013;341:519–524. doi: 10.1126/science.1235225. [DOI] [PubMed] [Google Scholar]
- 22.Seidel KB, Rowell JE. Canadian muskoxen in Central Europe — A zoo veterinary review. Rangifer. 1996;16:79–85. [Google Scholar]