Abstract
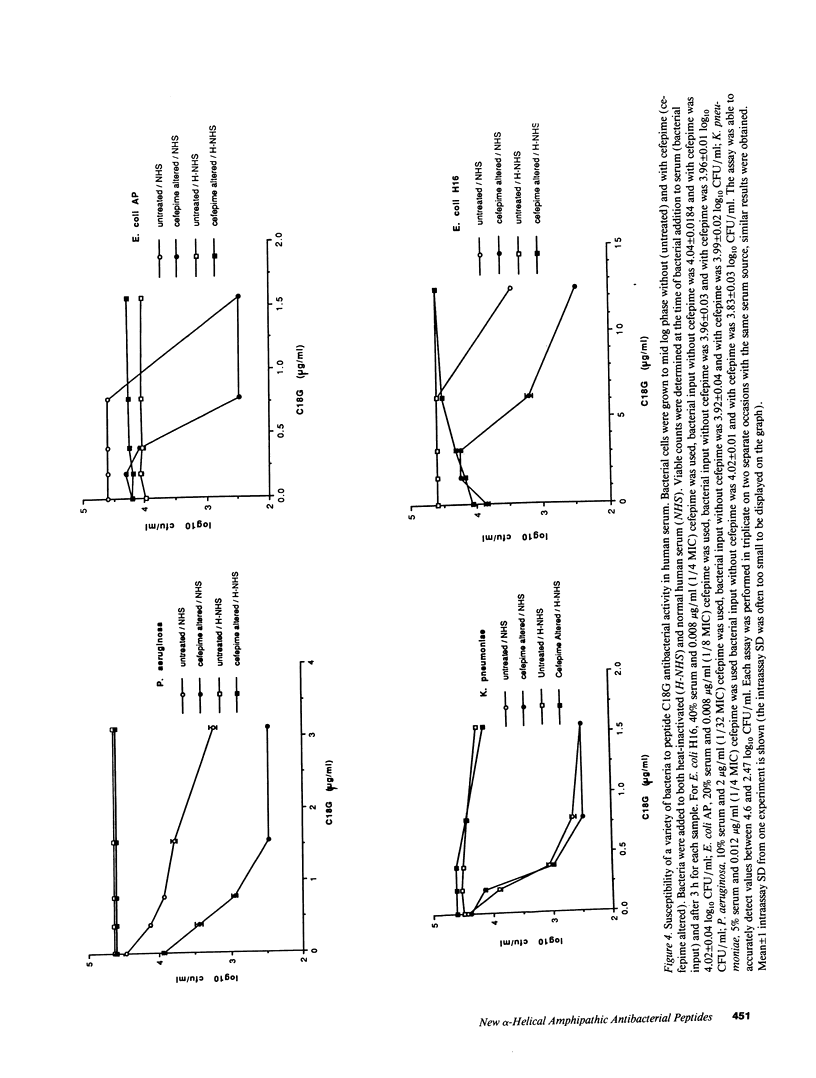
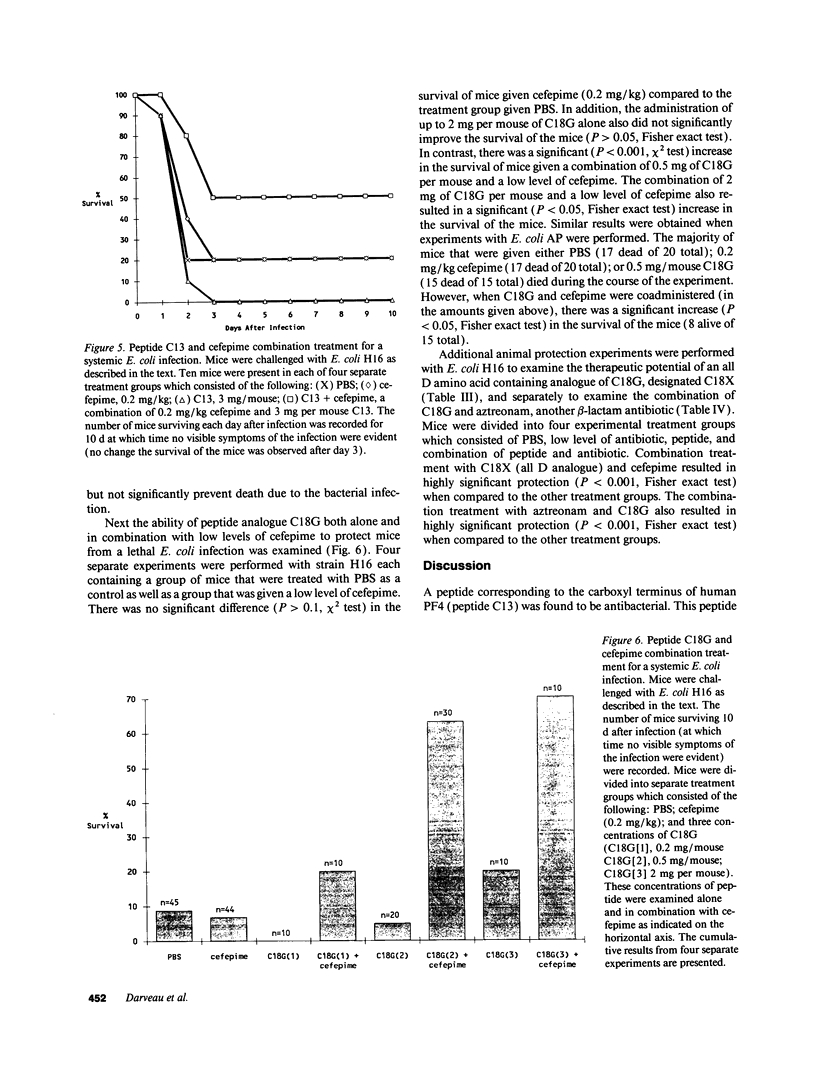
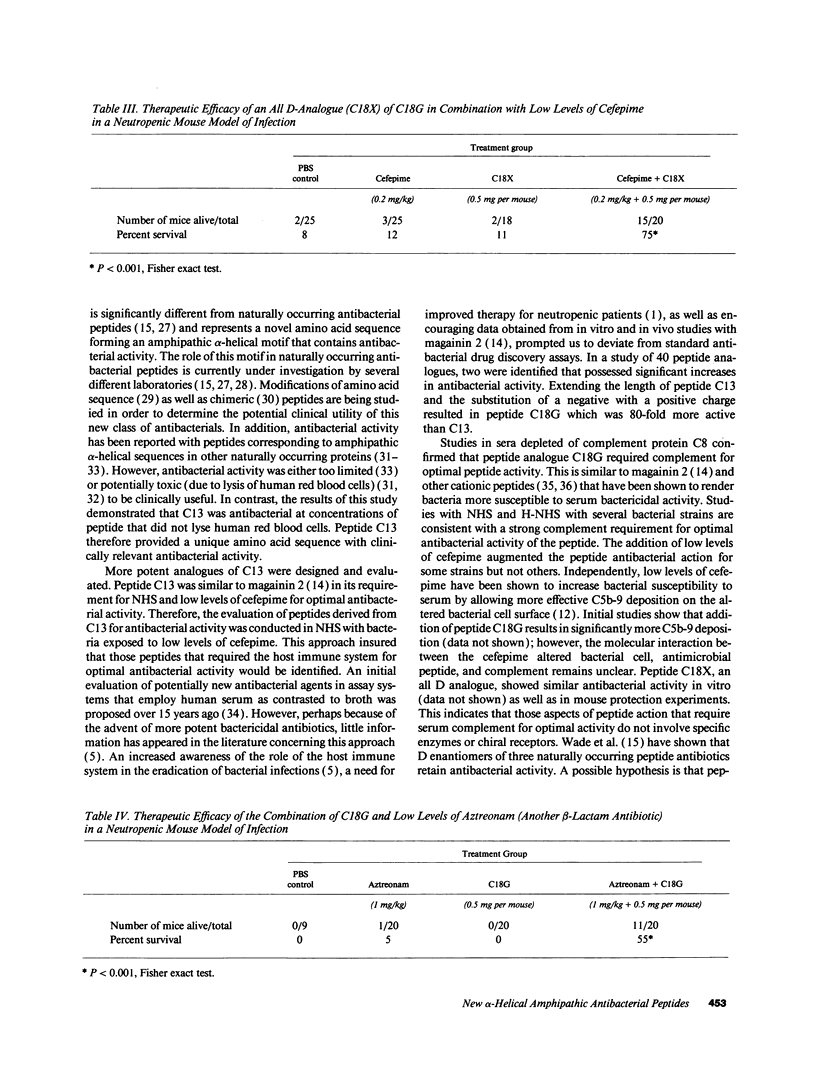
A peptide (C13) corresponding to the last 13 amino acids of the carboxyl terminus of human platelet factor IV was found to be antibacterial. Amino acid substitutions predicted to disrupt either the amphipathic or alpha-helical nature of C13 rendered the peptide inactive. Antibacterial activity was demonstrated in normal human serum on bacteria which had been previously exposed to low levels of cefepime, a beta-lactam antibiotic. Peptide analogues were examined for more potent antibacterial activity in an antibacterial assay that employed normal human serum and low levels of cefepime. A peptide analogue (C18G) with 80-fold more antibacterial activity than C13 was identified. Studies in C8-deficient sera confirmed an essential role of human serum complement for optimal antibacterial activity. Additional studies showed low levels of cefepime, although not essential, enhanced the antibacterial activity of C18G. Animal protection experiments demonstrated that either peptide C18G or an analogue with all D amino acids (C18X) significantly increased the survival of neutropenic mice when coadministered with a low level of cefepime. This work has resulted in the identification of a new group of antibacterial peptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Wade D., Boman I. A., Wåhlin B., Merrifield R. B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989 Dec 18;259(1):103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- Clas F., Loos M. Requirement for an additional serum factor essential for the antibody-independent activation of the classical complement sequence by Gram-negative bacteria. Infect Immun. 1982 Sep;37(3):935–939. doi: 10.1128/iai.37.3.935-939.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Three-dimensional structure of interleukin 8 in solution. Biochemistry. 1990 Feb 20;29(7):1689–1696. doi: 10.1021/bi00459a004. [DOI] [PubMed] [Google Scholar]
- Cuervo J. H., Rodriguez B., Houghten R. A. The Magainins: sequence factors relevant to increased antimicrobial activity and decreased hemolytic activity. Pept Res. 1988 Nov-Dec;1(2):81–86. [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., Turner K. A., White A. J. Inhibition of penicillin-binding protein 3 of Escherichia coli K-12. Effects upon growth, viability and outer membrane barrier function. J Antimicrob Chemother. 1985 Sep;16(3):287–296. doi: 10.1093/jac/16.3.287. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Cunningham M. D. Influence of subinhibitory concentrations of cephalosporins on the serum sensitivity of Pseudomonas aeruginosa. J Infect Dis. 1990 Oct;162(4):914–921. doi: 10.1093/infdis/162.4.914. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Cunningham M. D., Seachord C. L., Cassiano-Clough L., Cosand W. L., Blake J., Watkins C. S. Beta-lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob Agents Chemother. 1991 Jun;35(6):1153–1159. doi: 10.1128/aac.35.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilove J. L., Jakubowski A., Scher H., Sternberg C., Wong G., Grous J., Yagoda A., Fain K., Moore M. A., Clarkson B. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988 Jun 2;318(22):1414–1422. doi: 10.1056/NEJM198806023182202. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Hammer M. C., Baltch A. L., Smith R. P., Conroy J. V., Bishop M., Michelsen P., Hill L. Human granulocyte activity against moxalactam-induced filamentous forms of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1988 Oct;32(10):1565–1570. doi: 10.1128/aac.32.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jit Sud I., Feingold D. S. Detection of agents that alter the bacterial cell surface. Antimicrob Agents Chemother. 1975 Jul;8(1):34–37. doi: 10.1128/aac.8.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Ihara I., Suzuki A., Harada Y. Properties of a new complement-dependent bactericidal factor specific for Ra chemotype salmonella in sera of conventional and germ-free mice. J Immunol. 1982 Nov;129(5):2198–2201. [PubMed] [Google Scholar]
- Klastersky J., Zinner S. H., Calandra T., Gaya H., Glauser M. P., Meunier F., Rossi M., Schimpff S. C., Tattersall M., Viscoli C. Empiric antimicrobial therapy for febrile granulocytopenic cancer patients: lessons from four EORTC trials. Eur J Cancer Clin Oncol. 1988;24 (Suppl 1):S35–S45. [PubMed] [Google Scholar]
- Lee S., Mihara H., Aoyagi H., Kato T., Izumiya N., Yamasaki N. Relationship between antimicrobial activity and amphiphilic property of basic model peptides. Biochim Biophys Acta. 1986 Nov 6;862(1):211–219. doi: 10.1016/0005-2736(86)90485-2. [DOI] [PubMed] [Google Scholar]
- Lee S., Yoshida M., Mihara H., Aoyagi H., Kato T., Yamasaki N. The spectroscopic analysis for binding of amphipathic and antimicrobial model peptides containing pyrenylalanine and tryptophan to lipid bilayer. Biochim Biophys Acta. 1989 Sep 4;984(2):174–182. doi: 10.1016/0005-2736(89)90213-7. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989 Aug;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D., Ravetch J. V. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J Exp Med. 1987 Oct 1;166(4):1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Garry R. F., Jaynes J. M., Montelaro R. C. A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res Hum Retroviruses. 1991 Jun;7(6):511–519. doi: 10.1089/aid.1991.7.511. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Qu X. M., Natori S. Interaction between liposomes and sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina (flesh fly). J Biol Chem. 1987 Feb 5;262(4):1665–1669. [PubMed] [Google Scholar]
- Pruul H., Lewis G., McDonald P. J. Enhanced susceptibility of gram-negative bacteria to phagocytic killing by human polymorphonuclear leucocytes after brief exposure to aztreonam. J Antimicrob Chemother. 1988 Nov;22(5):675–686. doi: 10.1093/jac/22.5.675. [DOI] [PubMed] [Google Scholar]
- Pruul H., McDonald P. J. Damage to bacteria by antibiotics in vitro and its relevance to antimicrobial chemotherapy: a historical perspective. J Antimicrob Chemother. 1988 Jun;21(6):695–698. doi: 10.1093/jac/21.6.695. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Shimonishi Y., Kishida Y., Okada M., Sugihara H. Use of anhydrous hydrogen fluoride in peptide synthesis. I. Behavior of various protective groups in anhydrous hydrogen fluoride. Bull Chem Soc Jpn. 1967 Sep;40(9):2164–2167. doi: 10.1246/bcsj.40.2164. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler J. M., Malech H. L., White C. J., Gallin J. I. Recombinant human interferon-gamma reconstitutes defective phagocyte function in patients with chronic granulomatous disease of childhood. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4874–4878. doi: 10.1073/pnas.85.13.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram N., Nagaraj R. A synthetic 13-residue peptide corresponding to the hydrophobic region of bovine seminalplasmin has antibacterial activity and also causes lysis of red blood cells. J Biol Chem. 1990 Jun 25;265(18):10438–10442. [PubMed] [Google Scholar]
- Stoeckle M. Y., Barker K. A. Two burgeoning families of platelet factor 4-related proteins: mediators of the inflammatory response. New Biol. 1990 Apr;2(4):313–323. [PubMed] [Google Scholar]
- Tanaka T., Okamura S., Okada K., Suga A., Shimono N., Ohhara N., Hirota Y., Sawae Y., Niho Y. Protective effect of recombinant murine granulocyte-macrophage colony-stimulating factor against Pseudomonas aeruginosa infection in leukocytopenic mice. Infect Immun. 1989 Jun;57(6):1792–1799. doi: 10.1128/iai.57.6.1792-1799.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Gaunt H., Unger F. M. Effect of subinhibitory concentrations of mecillinam on the serum susceptibility of Escherichia coli strains. Antimicrob Agents Chemother. 1981 May;19(5):786–788. doi: 10.1128/aac.19.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh V. L., Duncan R. L., Jr, Morrison D. C. The interaction of Escherichia coli with normal human serum: the kinetics of serum-mediated lipopolysaccharide release and its dissociation from bacterial killing. J Immunol. 1986 Aug 15;137(4):1329–1335. [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]