Abstract
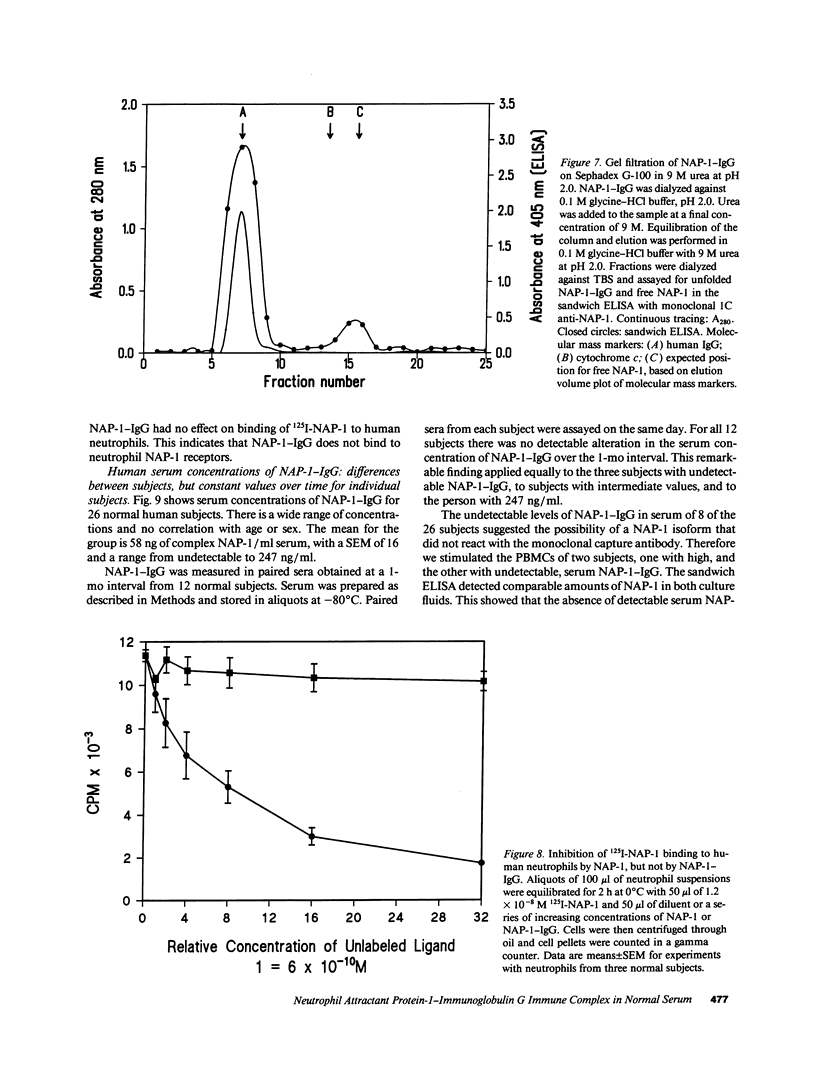
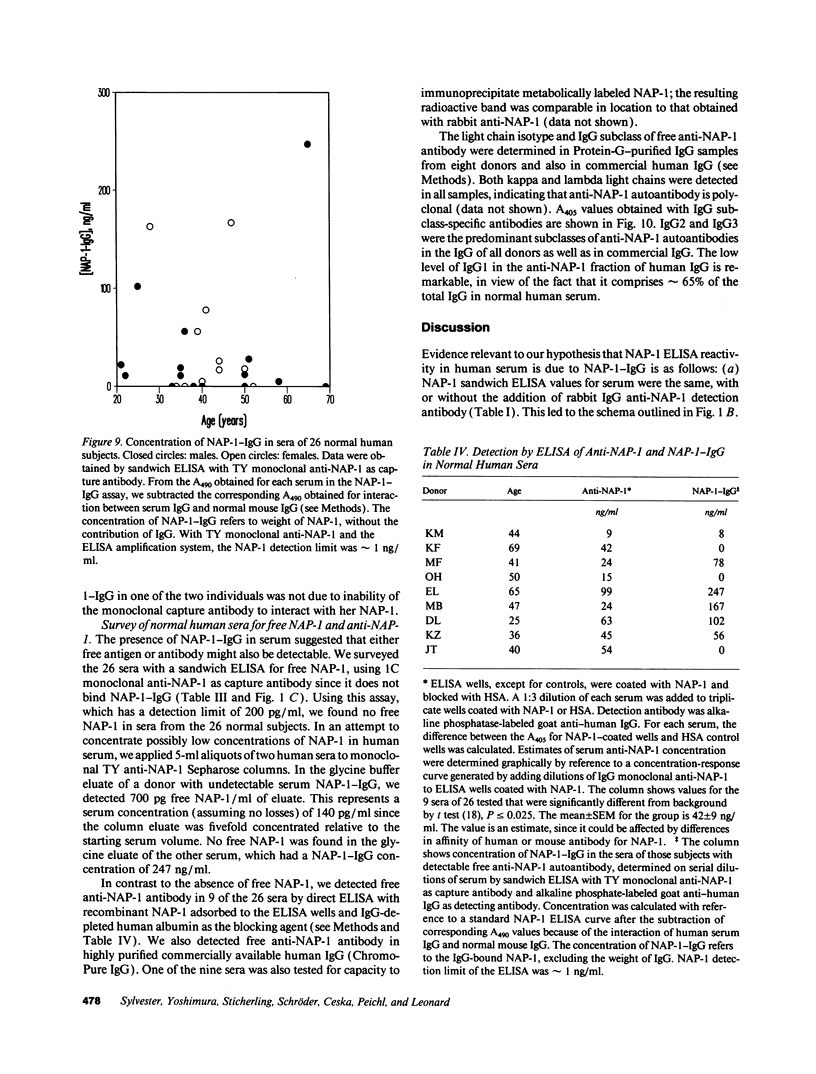
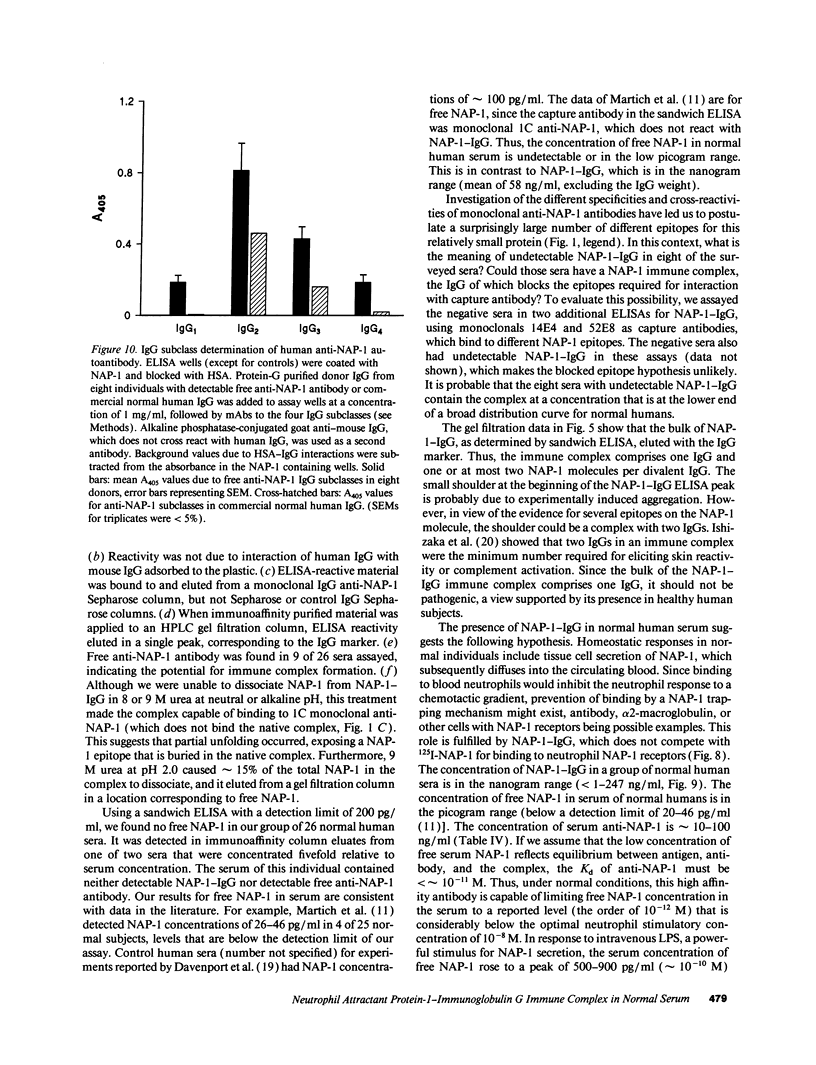
After obtaining data indicating the presence of a neutrophil attractant protein-1 (NAP-1)-IgG complex in normal human serum, we developed sandwich ELISAs that could quantify NAP-1 and NAP-1-IgG in mixtures of the two moieties. The ELISA for free NAP-1 used a monoclonal capture antibody that did not bind NAP-1-IgG. The ELISA for NAP-1-IgG was based on omission of the anti-NAP-1 detection antibody (required for the free NAP-1 ELISA) and on interaction of phosphatase-conjugated anti-human IgG with the human NAP-1-IgG complex. Gel filtration of immunoaffinity-purified NAP-1-IgG showed that the bulk of the complex comprised a single IgG. Binding between NAP-1 and antibody is strong, since 8 M urea at neutral or alkaline pH did not release NAP-1. However, at pH 2.0 in 9 M urea approximately 15% of the total NAP-1 could be dissociated from the complex. NAP-1-IgG was detected in 18 of 26 sera from normal humans. The mean serum concentration was 58 ng of IgG-bound NAP-1/ml, with an SEM of 16 and a range from undetectable to 247 ng/ml. NAP-1-IgG concentrations in paired sera drawn at a 1-mo interval were remarkably constant. Using an ELISA for free NAP-1 with a detection limit of 200 pg/ml, we found no free NAP-1 in the 26 sera. Free anti-NAP-1-IgG autoantibody was found in 9 of 26 sera by direct ELISA. IgG anti-NAP-1 of all nine sera was polyclonal, comprising both kappa and lambda isotypes; predominant subclasses were IgG2 and IgG3. NAP-1-IgG did not compete with 125I-NAP-1 for binding to neutrophils, which suggests that IgG anti-NAP-1 is a molecular trap that prevents binding of NAP-1 to neutrophils after it diffuses from production sites into the circulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Beaubien B. C., Collins P. D., Jose P. J., Totty N. F., Hsuan J., Waterfield M. D., Williams T. J. A novel neutrophil chemoattractant generated during an inflammatory reaction in the rabbit peritoneal cavity in vivo. Purification, partial amino acid sequence and structural relationship to interleukin 8. Biochem J. 1990 Nov 1;271(3):797–801. doi: 10.1042/bj2710797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscato L. M., Stuart M. C. Heterophilic antibodies: a problem for all immunoassays. Clin Chem. 1988 Jan;34(1):27–33. [PubMed] [Google Scholar]
- Brennan F. M., Zachariae C. O., Chantry D., Larsen C. G., Turner M., Maini R. N., Matsushima K., Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990 Sep;20(9):2141–2144. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- Buchanan D. R., Cromwell O., Kay A. B. Neutrophil chemotactic activity in acute severe asthma (status asthmaticus). Am Rev Respir Dis. 1987 Dec;136(6):1397–1402. doi: 10.1164/ajrccm/136.6.1397. [DOI] [PubMed] [Google Scholar]
- Darbonne W. C., Rice G. C., Mohler M. A., Apple T., Hébert C. A., Valente A. J., Baker J. B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991 Oct;88(4):1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R. D., Strieter R. M., Standiford T. J., Kunkel S. L. Interleukin-8 production in red blood cell incompatibility. Blood. 1990 Dec 15;76(12):2439–2442. [PubMed] [Google Scholar]
- ISHIZAKA K., ISHIZAKA T., BANOVITZ J. BIOLOGIC ACTIVITY OF SOLUBLE ANTIGEN-ANTIBODY COMPLEXES. IX. SOLUBLE COMPLEXES OF RABBIT ANTIBODY WITH UNIVALENT AND DIVALENT HAPTENS. J Immunol. 1964 Dec;93:1001–1007. [PubMed] [Google Scholar]
- Leonard E. J. NAP-1 (IL-8) Immunol Today. 1990 Jun;11(6):223–224. doi: 10.1016/0167-5699(90)90087-p. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A. Functional differences between resident and exudate peritoneal mouse macrophages: specific serum protein requirements for responsiveness to chemotaxins. J Reticuloendothel Soc. 1980 Nov;28(5):437–447. [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T., Noer K., Kutvirt S., Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990 Feb 15;144(4):1323–1330. [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]). Am J Respir Cell Mol Biol. 1990 Jun;2(6):479–486. doi: 10.1165/ajrcmb/2.6.479. [DOI] [PubMed] [Google Scholar]
- Martich G. D., Danner R. L., Ceska M., Suffredini A. F. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991 Apr 1;173(4):1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Identification and characterization of specific receptors for monocyte-derived neutrophil chemotactic factor (MDNCF) on human neutrophils. J Exp Med. 1989 Mar 1;169(3):1185–1189. doi: 10.1084/jem.169.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J. M., Christophers E. Identification of C5ades arg and an anionic neutrophil-activating peptide (ANAP) in psoriatic scales. J Invest Dermatol. 1986 Jul;87(1):53–58. doi: 10.1111/1523-1747.ep12523566. [DOI] [PubMed] [Google Scholar]
- Sticherling M., Schröder J. M., Christophers E. Production and characterization of monoclonal antibodies against the novel neutrophil activating peptide NAP/IL-8. J Immunol. 1989 Sep 1;143(5):1628–1634. [PubMed] [Google Scholar]
- Sylvester I., Rankin J. A., Yoshimura T., Tanaka S., Leonard E. J. Secretion of neutrophil attractant/activation protein by lipopolysaccharide-stimulated lung macrophages determined by both enzyme-linked immunosorbent assay and N-terminal sequence analysis. Am Rev Respir Dis. 1990 Mar;141(3):683–688. doi: 10.1164/ajrccm/141.3.683. [DOI] [PubMed] [Google Scholar]
- Van Zee K. J., DeForge L. E., Fischer E., Marano M. A., Kenney J. S., Remick D. G., Lowry S. F., Moldawer L. L. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991 May 15;146(10):3478–3482. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Appella E., Matsushima K., Showalter S. D., Skeel A., Leonard E. J. Three forms of monocyte-derived neutrophil chemotactic factor (MDNCF) distinguished by different lengths of the amino-terminal sequence. Mol Immunol. 1989 Jan;26(1):87–93. doi: 10.1016/0161-5890(89)90024-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]