Abstract
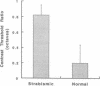
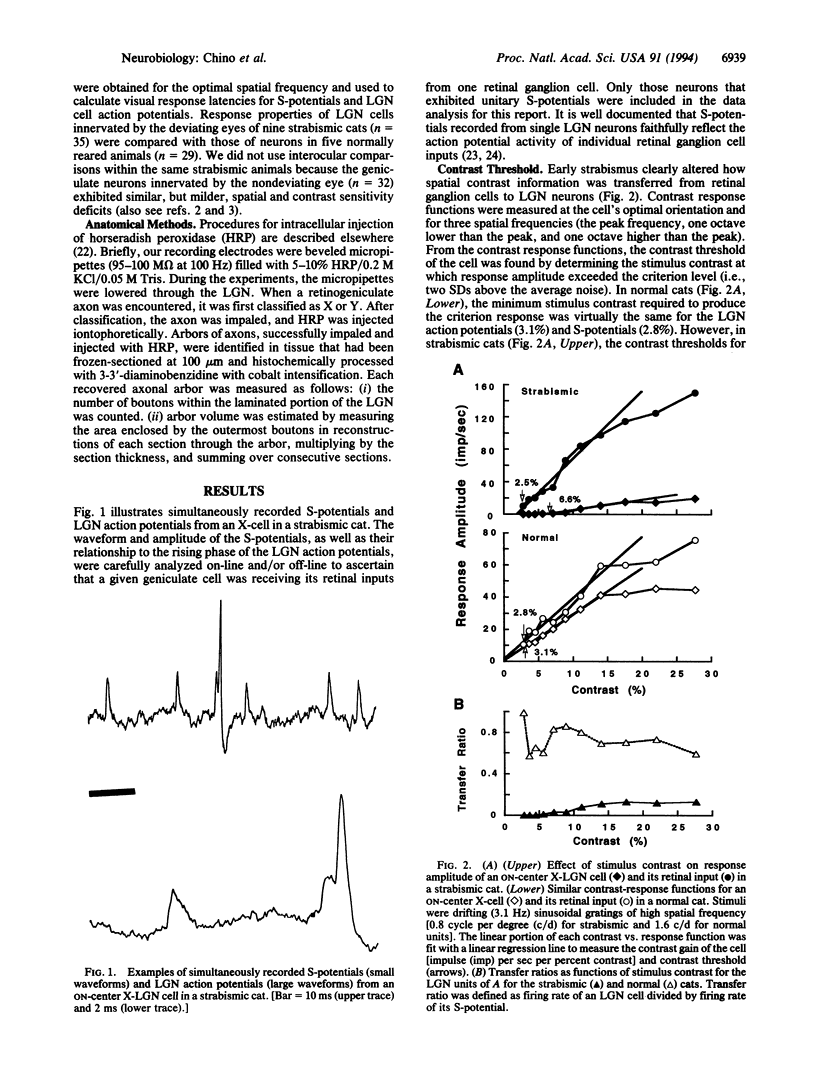
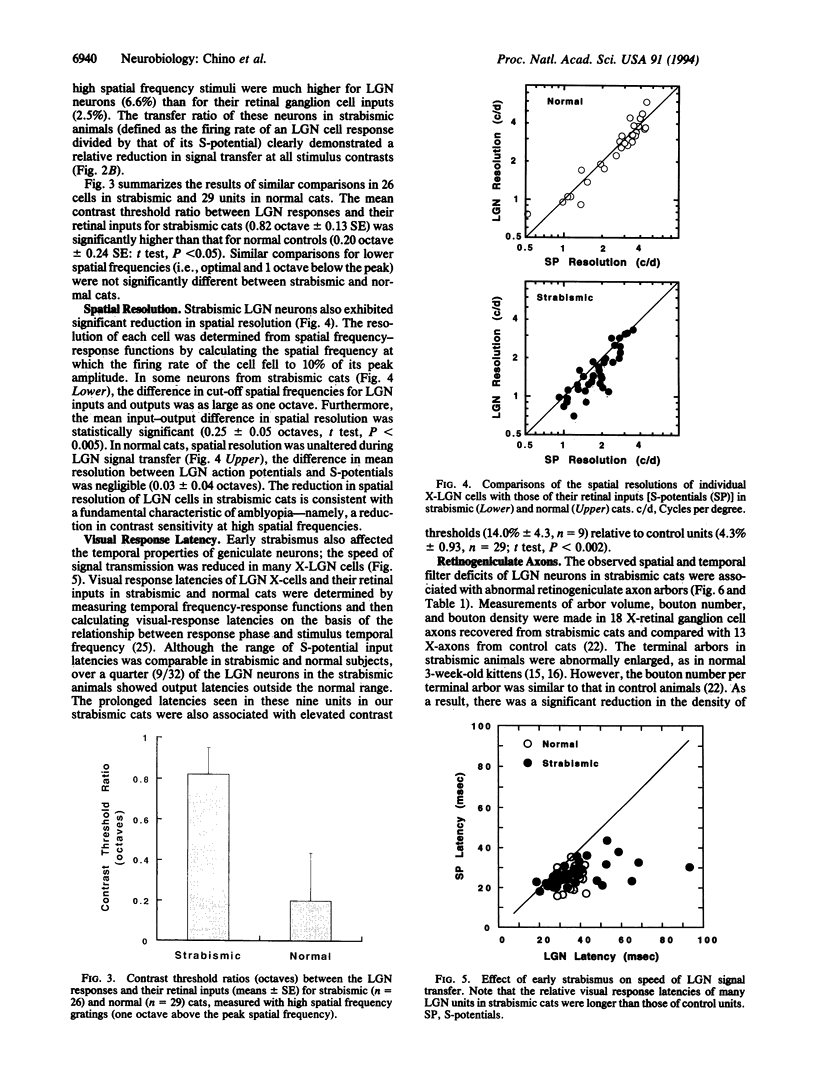
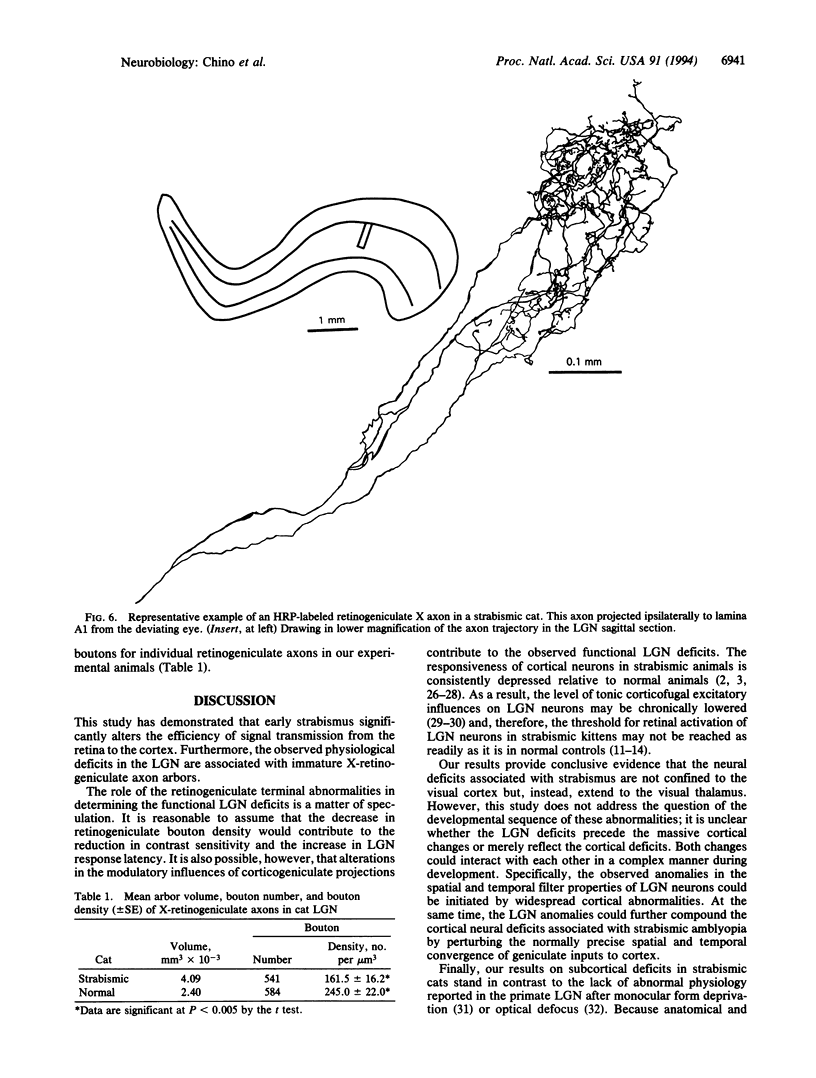
The mammalian lateral geniculate nucleus (LGN) is known to regulate signal transfer from the retina to the brain neocortex in a highly complex manner. Besides inputs from the brainstem, extraretinal inputs via corticogeniculate projections and local inhibitory neurons modulate signal transfer in the LGN. However, very little is known about whether the postnatal development of LGN signal-transfer mechanisms is influenced by early discordant binocular vision. By intraunit comparisons of responses between individual X-LGN cells and their direct retinal inputs, the efficiency of signal transfer was found permanently reduced due to an early interocular misalignment (strabismus). The contrast sensitivity and spatial resolution of cat LGN cells were significantly lower relative to their retinal inputs, and there was substantial decrease in signal-transfer speed. The observed physiological deficits were associated with immature X-retinogeniculate axon arbors. Thus, contrary to previous ideas, conflicting binocular inputs can produce neural deficits in subcortical visual structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chino Y. M., Kaplan E. Abnormal orientation bias of LGN neurons in strabismic cats. Invest Ophthalmol Vis Sci. 1988 Apr;29(4):644–648. [PubMed] [Google Scholar]
- Chino Y. M., Shansky M. S., Jankowski W. L., Banser F. A. Effects of rearing kittens with convergent strabismus on development of receptive-field properties in striate cortex neurons. J Neurophysiol. 1983 Jul;50(1):265–286. doi: 10.1152/jn.1983.50.1.265. [DOI] [PubMed] [Google Scholar]
- Chino Y. M., Smith E. L., 3rd, Wada H., Ridder W. H., 3rd, Langston A. L., Lesher G. A. Disruption of binocularly correlated signals alters the postnatal development of spatial properties in cat striate cortical neurons. J Neurophysiol. 1991 Apr;65(4):841–859. doi: 10.1152/jn.1991.65.4.841. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Crewther D. P., Crewther S. G., Mitchell D. E. Normality of spatial resolution of retinal ganglion cells in cats with strabismic amblyopia. J Physiol. 1982 May;326:235–249. doi: 10.1113/jphysiol.1982.sp014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther S. G., Crewther D. P., Cleland B. G. Convergent strabismic amblyopia in cats. Exp Brain Res. 1985;60(1):1–9. doi: 10.1007/BF00237012. [DOI] [PubMed] [Google Scholar]
- Freeman R. D., Tsumoto T. An electrophysiological comparison of convergent and divergent strabismus in the cat: electrical and visual activation of single cortical cells. J Neurophysiol. 1983 Jan;49(1):238–253. doi: 10.1152/jn.1983.49.1.238. [DOI] [PubMed] [Google Scholar]
- Frishman L. J., Freeman A. W., Troy J. B., Schweitzer-Tong D. E., Enroth-Cugell C. Spatiotemporal frequency responses of cat retinal ganglion cells. J Gen Physiol. 1987 Apr;89(4):599–628. doi: 10.1085/jgp.89.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard-Crewther S., Crewther D. P. Neural site of strabismic amblyopia in cats: X-cell acuities in the LGN. Exp Brain Res. 1988;72(3):503–509. doi: 10.1007/BF00250595. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Properties of LGN cells in kittens reared with convergent squint: a neurophysiological demonstration of amblyopia. Exp Brain Res. 1976 May 10;25(1):63–77. doi: 10.1007/BF00237326. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Purpura K., Shapley R. M. Contrast affects the transmission of visual information through the mammalian lateral geniculate nucleus. J Physiol. 1987 Oct;391:267–288. doi: 10.1113/jphysiol.1987.sp016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. The origin of the S (slow) potential in the mammalian lateral geniculate nucleus. Exp Brain Res. 1984;55(1):111–116. doi: 10.1007/BF00240504. [DOI] [PubMed] [Google Scholar]
- Lal R., Friedlander M. J. Effect of passive eye position changes on retinogeniculate transmission in the cat. J Neurophysiol. 1990 Mar;63(3):502–522. doi: 10.1152/jn.1990.63.3.502. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989 Jul;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A., Frishman L. J., Jakiela H. G., Enroth-Cugell C. Receptive field properties of x and y cells in the cat retina derived from contrast sensitivity measurements. Vision Res. 1982;22(9):1173–1183. doi: 10.1016/0042-6989(82)90082-7. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Eggers H. M., Gizzi M. S., Hendrickson A. E., Kiorpes L., Boothe R. G. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. J Neurosci. 1987 May;7(5):1340–1351. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton T. T., Godwin D. W. Inhibitory GABAergic control of visual signals at the lateral geniculate nucleus. Prog Brain Res. 1992;90:193–217. doi: 10.1016/s0079-6123(08)63615-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P. Mechanisms of visual plasticity: Hebb synapses, NMDA receptors, and beyond. Physiol Rev. 1991 Apr;71(2):587–615. doi: 10.1152/physrev.1991.71.2.587. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res. 1986;63(1):1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Smith E. L., 3rd, Chino Y. M., Ridder W. H., 3rd, Kitagawa K., Langston A. Orientation bias of neurons in the lateral geniculate nucleus of macaque monkeys. Vis Neurosci. 1990 Dec;5(6):525–545. doi: 10.1017/s0952523800000699. [DOI] [PubMed] [Google Scholar]
- Sur M. Development and plasticity of retinal X and Y axon terminations in the cat's lateral geniculate nucleus. Brain Behav Evol. 1988;31(4):243–251. doi: 10.1159/000116592. [DOI] [PubMed] [Google Scholar]
- Sur M., Esguerra M., Garraghty P. E., Kritzer M. F., Sherman S. M. Morphology of physiologically identified retinogeniculate X- and Y-axons in the cat. J Neurophysiol. 1987 Jul;58(1):1–32. doi: 10.1152/jn.1987.58.1.1. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Freeman R. D. Effects of strabismus on development of cortico-geniculate projections in the kitten. Exp Brain Res. 1981;44(3):337–339. doi: 10.1007/BF00236572. [DOI] [PubMed] [Google Scholar]
- Varela F. J., Singer W. Neuronal dynamics in the visual corticothalamic pathway revealed through binocular rivalry. Exp Brain Res. 1987;66(1):10–20. doi: 10.1007/BF00236196. [DOI] [PubMed] [Google Scholar]
- Xue J. T., Ramoa A. S., Carney T., Freeman R. D. Binocular interaction in the dorsal lateral geniculate nucleus of the cat. Exp Brain Res. 1987;68(2):305–310. doi: 10.1007/BF00248796. [DOI] [PubMed] [Google Scholar]