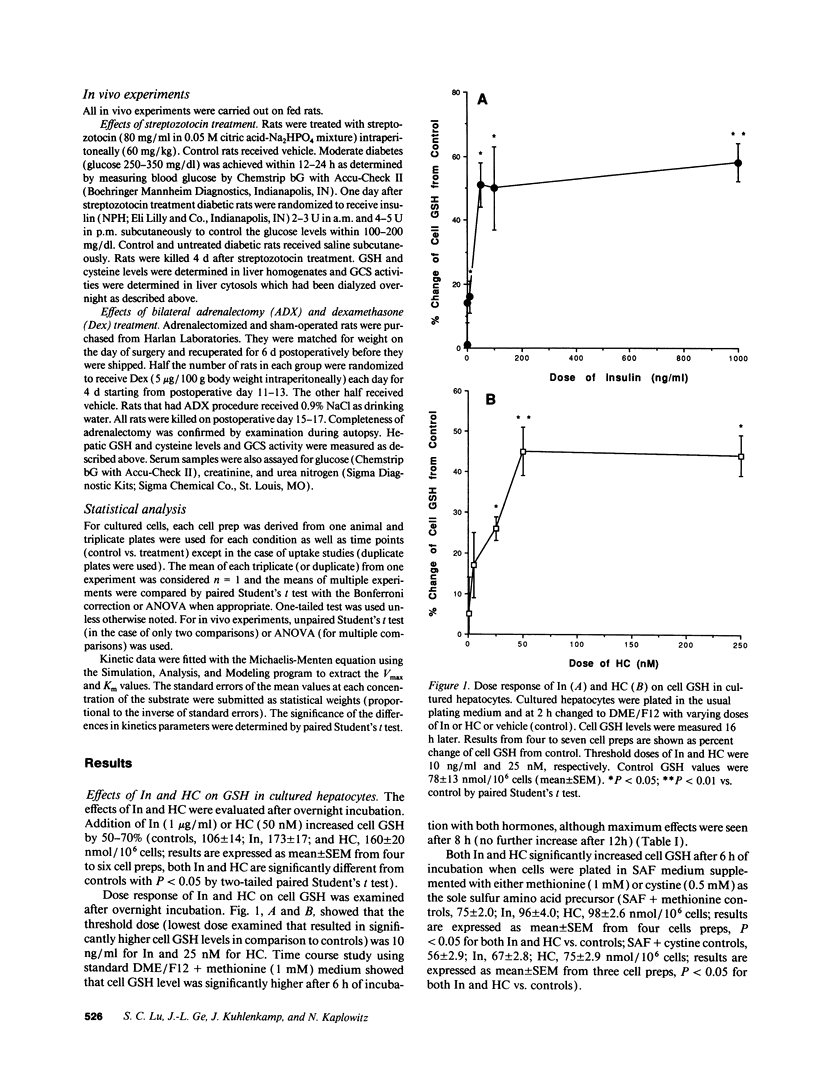
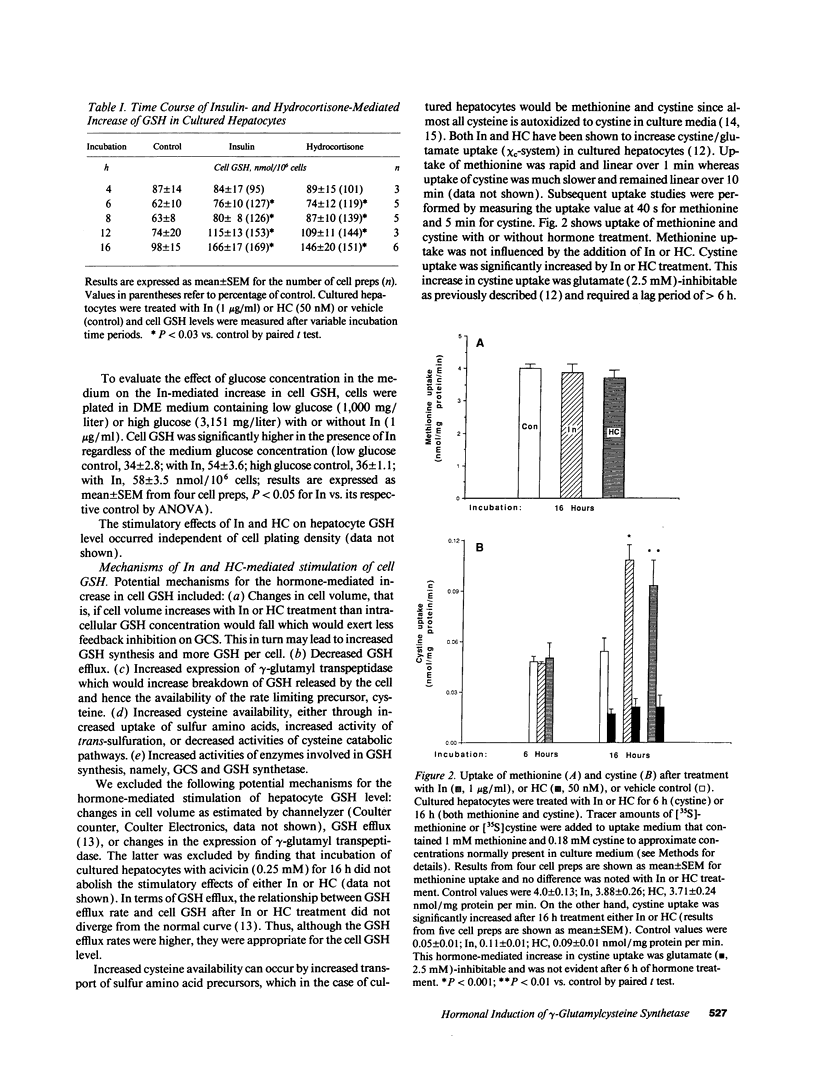
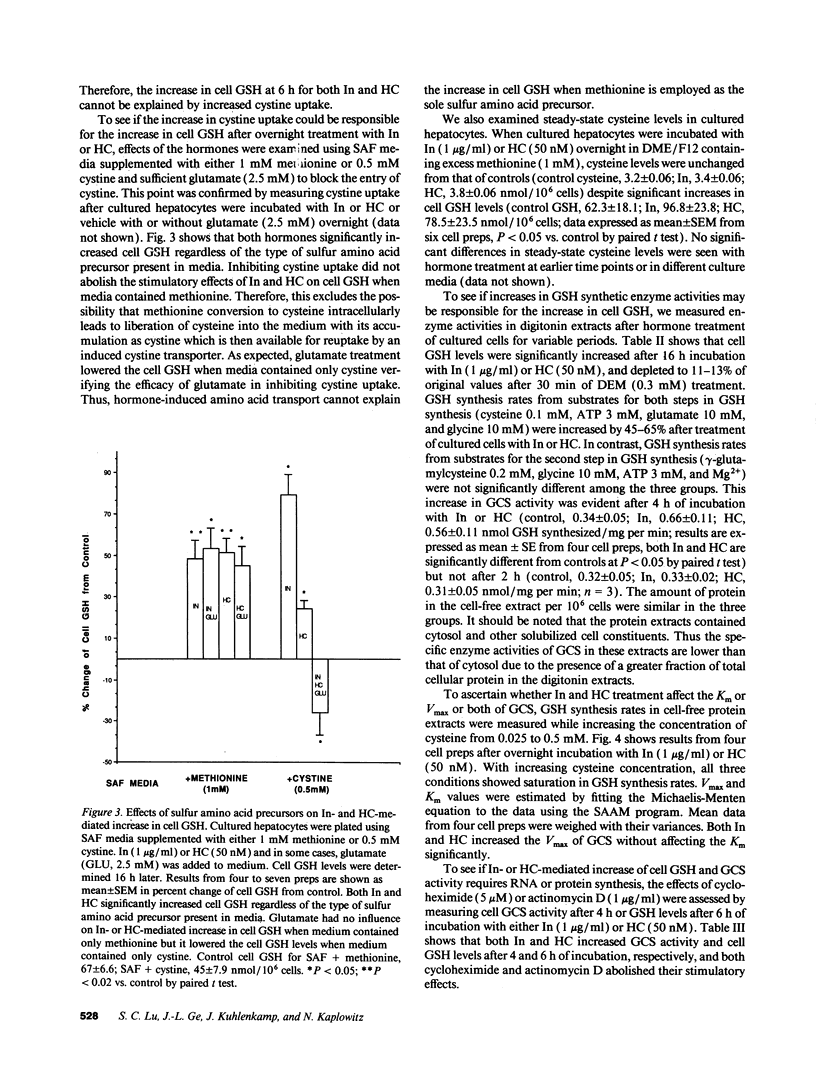
Abstract
We reported that glucagon and phenylephrine decrease hepatocyte GSH by inhibiting gamma-glutamylcysteine synthetase (GCS), the rate-limiting enzyme in GSH synthesis (Lu, S.C., J. Kuhlenkamp, C. Garcia-Ruiz, and N. Kaplowitz. 1991. J. Clin. Invest. 88:260-269). In contrast, we have found that insulin (In, 1 microgram/ml) and hydrocortisone (HC, 50 nM) increased GSH of cultured hepatocytes up to 50-70% (earliest significant change at 6 h) with either methionine or cystine alone as the sole sulfur amino acid in the medium. The effect of In occurred independent of glucose concentration in the medium. Changes in steady-state cellular cysteine levels, cell volume, GSH efflux, or expression of gamma-glutamyl transpeptidase were excluded as possible mechanisms. Both hormones are known to induce cystine/glutamate transport, but this was excluded as the predominant mechanism since the induction in cystine uptake required a lag period of greater than 6 h, and the increase in cell GSH still occurred when cystine uptake was blocked. Assay of GSH synthesis in extracts of detergent-treated cells revealed that In and HC increased the activity of GCS by 45-65% (earliest significant change at 4 h) but not GSH synthetase. In and HC treatment increased the Vmax of GCS by 31-43% with no change in Km. Both the hormone-mediated increase in cell GSH and GCS activity were blocked with either cycloheximide or actinomycin D. Finally, when studied in vivo, streptozotocin-treated diabetic and adrenalectomized rats exhibited lower hepatic GSH levels and GCS activities than respective controls. Both of these abnormalities were prevented with hormone replacement. Thus, both in vitro and in vivo, In and glucocorticoids are required for normal expression of GCS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S., Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89(1):1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstein K. L., Cidlowski J. A. Regulation of gene expression by glucocorticoids. Annu Rev Physiol. 1989;51:683–699. doi: 10.1146/annurev.ph.51.030189.003343. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Marcillat O., Pichard A. L., Henry J., Kahn A. Glucose-dependent and -independent effect of insulin on gene expression. J Biol Chem. 1991 Feb 25;266(6):3432–3438. [PubMed] [Google Scholar]
- Fernández-Checa J. C., Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990 Nov 1;190(2):212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Hutson S. M., Lloyd C. E., Taylor J. M., Shiman R., Jefferson L. S. Direct effect of insulin on albumin gene expression in primary cultures of rat hepatocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):E447–E453. doi: 10.1152/ajpendo.1985.249.5.E447. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynska E., Wegrzynowicz R. Structural and functional changes in organelles of liver cells in rats exposed to magnetic fields. Environ Res. 1991 Aug;55(2):188–198. doi: 10.1016/s0013-9351(05)80175-6. [DOI] [PubMed] [Google Scholar]
- Górska M., Lopaczynski W., Kinalska I. Effect of acute hyperthyroidism on insulin removal in the rats. Exp Clin Endocrinol. 1989;94(3):345–350. doi: 10.1055/s-0029-1210920. [DOI] [PubMed] [Google Scholar]
- Husson A., Renouf S., Fairand A., Buquet C., Benamar M., Vaillant R. Expression of argininosuccinate lyase mRNA in foetal hepatocytes. Regulation by glucocorticoids and insulin. Eur J Biochem. 1990 Sep 24;192(3):677–681. doi: 10.1111/j.1432-1033.1990.tb19275.x. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Stinson-Fisher C., Shiman R., Jefferson L. S. Regulation of albumin synthesis by hormones and amino acids in primary cultures of rat hepatocytes. Am J Physiol. 1987 Mar;252(3 Pt 1):E291–E298. doi: 10.1152/ajpendo.1987.252.3.E291. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Aw T. Y., Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Adams J. D., Mitchell J. R. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984 Jul-Aug;4(4):586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- Lou M. F., Dickerson J. E., Jr, Garadi R., York B. M., Jr Glutathione depletion in the lens of galactosemic and diabetic rats. Exp Eye Res. 1988 Apr;46(4):517–530. doi: 10.1016/s0014-4835(88)80009-5. [DOI] [PubMed] [Google Scholar]
- Loven D., Schedl H., Wilson H., Daabees T. T., Stegink L. D., Diekus M., Oberley L. Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozocin-induced diabetes. Diabetes. 1986 May;35(5):503–507. doi: 10.2337/diab.35.5.503. [DOI] [PubMed] [Google Scholar]
- Lu S. C., Kuhlenkamp J., Garcia-Ruiz C., Kaplowitz N. Hormone-mediated down-regulation of hepatic glutathione synthesis in the rat. J Clin Invest. 1991 Jul;88(1):260–269. doi: 10.1172/JCI115286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan S. V., Heffernan S., Wright L., Rae C., Fisher E., Yue D. K., Turtle J. R. Changes in hepatic glutathione metabolism in diabetes. Diabetes. 1991 Mar;40(3):344–348. doi: 10.2337/diab.40.3.344. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Howard G. Effects of insulin on gene transcription. Annu Rev Physiol. 1989;51:701–714. doi: 10.1146/annurev.ph.51.030189.003413. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Mordes J. P., Rossini A. A. Animal models of diabetes. Am J Med. 1981 Feb;70(2):353–360. doi: 10.1016/0002-9343(81)90772-5. [DOI] [PubMed] [Google Scholar]
- Murakami K., Kondo T., Ohtsuka Y., Fujiwara Y., Shimada M., Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989 Aug;38(8):753–758. doi: 10.1016/0026-0495(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Reul J. M., Pearce P. T., Funder J. W., Krozowski Z. S. Type I and type II corticosteroid receptor gene expression in the rat: effect of adrenalectomy and dexamethasone administration. Mol Endocrinol. 1989 Oct;3(10):1674–1680. doi: 10.1210/mend-3-10-1674. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Sherratt A. J., Banet D. E., Prough R. A. Glucocorticoid regulation of polycyclic aromatic hydrocarbon induction of cytochrome P450IA1, glutathione S-transferases, and NAD(P)H:quinone oxidoreductase in cultured fetal rat hepatocytes. Mol Pharmacol. 1990 Feb;37(2):198–205. [PubMed] [Google Scholar]
- Takada A., Bannai S. Transport of cystine in isolated rat hepatocytes in primary culture. J Biol Chem. 1984 Feb 25;259(4):2441–2445. [PubMed] [Google Scholar]
- Tateishi N., Higashi T., Naruse A., Nakashima K., Shiozaki H. Rat liver glutathione: possible role as a reservoir of cysteine. J Nutr. 1977 Jan;107(1):51–60. doi: 10.1093/jn/107.1.51. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M., Yoshikawa H., Itakura M., Yamashita K. Increased de novo purine synthesis by insulin through selective enzyme induction in primary cultured rat hepatocytes. Am J Physiol. 1990 May;258(5 Pt 1):C841–C848. doi: 10.1152/ajpcell.1990.258.5.C841. [DOI] [PubMed] [Google Scholar]