Abstract
Background
Whether self-reported daytime napping is an independent predictor of cardiovascular or all-cause mortality remains unclear. The aim of this study was to investigate self-reported daytime napping and risk of cardiovascular or all-cause mortality by conducting a meta-analysis.
Material/Methods
A computerized literature search of PubMed, Embase, and Cochrane Library was conducted up to May 2014. Only prospective studies reporting risk ratio (RR) and corresponding 95% confidence intervals (CI) of cardiovascular or all-cause mortality with respect to baseline self-reported daytime napping were included.
Results
Seven studies with 98,163 subjects were included. Self-reported daytime napping was associated with a greater risk of all-cause mortality (RR 1.15; 95% CI 1.07–1.24) compared with non-nappers. Risk of all-cause mortality appeared to be more pronounced among persons with nap duration >60 min (RR 1.15; 95% CI 1.04–1.27) than persons with nap duration <60 min (RR 1.10; 95% CI 0.92–1.32). The pooled RR of cardiovascular mortality was 1.19 (95% CI 0.97–1.48) comparing daytime nappers to non-nappers.
Conclusions
Self-reported daytime napping is a mild but statistically significant predictor for all-cause mortality, but not for cardiovascular mortality. However, whether the risk is attributable to excessive sleep duration or napping alone remains controversial. More prospective studies stratified by sleep duration, napping periods, or age are needed.
MeSH Keywords: Meta-Analysis, Mortality, Sleep Bruxism
Background
Napping is a common practice in community-dwelling older adults [1,2], with prevalence up to 68.6% in the elderly population [3]. Daytime napping or siesta is considered to be a healthy habit and linked with good health. A short nap of less than 30 min duration during the afternoon is deemed to have an invigorating effect, helps to restores wakefulness, and improve performance [4,5].
Contrary to the popular view that naps are beneficial to health, longer or frequent naps might be associated with worse long-term health. Daytime napping may be a risk factor for morbidity and mortality in the elderly population. Particularly, these persons requiring naps might have an unrecognized disease or nighttime sleep deprivation. Many studies [6–9] suggested that daytime napping may increase mortality. One study [10] indicated that the greater risk of mortality was only significant in night sleep of more than 9 hours. Others [11,12] did not find these associations. These conflicting findings might be attributable to the different populations, geographic areas, duration of napping time, and cultural habits. Daytime napping as an independent predictor for cardiovascular or all-cause mortality remains controversial.
To our knowledge, no previous meta-analysis has quantitatively evaluated the association between self-reported daytime napping and risk of cardiovascular or all-cause mortality. The aim of this study was to evaluate the findings from the available prospective studies, to determine whether self-reported daytime napping is an independent predictor for cardiovascular or all-cause mortality.
Material and Methods
Literature search
This meta-analysis was conducted according to the reporting items for systematic reviews and meta-analyses (PRISMA) statement. A comprehensive computerized literature search was conducted through PubMed, Embase database, and Cochrane Library up to May 2014. Potentially relevant articles were identified using the following Medical Subject Headings (MeSH): “daytime napping” or “siesta” or “nappers” or “excessive daytime sleepiness” and “death” or “mortality” and “prospective” or “follow-up”. In addition, we searched the reference lists of all identified relevant studies to identify additional eligible studies.
Study selection
Studies meeting the following criteria were included in the meta-analysis: 1) prospective observational study; 2) investigating baseline daytime napping and subsequent risk of cardiovascular or all-cause mortality as outcomes; 3) reporting multivariate-adjusted risk ratio (RR) or hazard ratio(HR) and corresponding 95% confidence intervals (CI) of cardiovascular or all-cause mortality comparing daytime napping to the non-nappers; and 4) all-cause mortality was obtained from the medical records, or from official death certificates; cardiovascular mortality is grouped by medical diagnostic codes and clinical criteria. The question about napping status was phrased: ‘“Do you normally take a nap during the day?” Daytime napping was defined by the individual study, and the rest were defined as non-nappers. Articles were excluded if: 1) not a prospective observational study; and 2) non-nappers were not considered as control.
Data extraction and quality assessment
Data were extracted by 2 authors (XK Liu and Q Zhang) independently from each selected study. Data extracted for this study included the first author’s surname, publication year, geographic region, study design, sample size, male gender (%), age range of participants, assessment of daytime napping, adjusted RR and HR with 95% CI, duration of follow-up, and adjustment for covariates. Any disagreement was resolved by discussion. Study quality was judged by using the Newcastle-Ottawa Scale (NOS) [13] by 2 authors independently. Quality assessment was conducted in 3 domains: subject selection, comparability of groups, and the ascertainment of outcomes. Using the scale, the total NOS star count ranges from zero to 9. Studies achieving a rating of ≥5 stars were judged to be of high quality.
Data synthesis and analysis
Data analyses used most fully multivariate-adjusted RR or HR and 95% CI comparing daytime nappers to the non-nappers. If the publications reported separate RR for different napping duration, we pooled the separate RR for the different items from the individual study. The Cochrane Q statistic and the I2 statistic were used to assess the heterogeneity of RR across studies. A p-value <0.10 for the Q statistic or I2>50% were taken as indicator of severe heterogeneity using a random-effects model, otherwise a fixed-effects model was used [14]. As there was obvious clinical heterogeneity in the durations of napping across the different studies, a random-effects model was used to calculate the pooled RR.
Begg’s rank correlation test [15] and Egger linear regression test [16] were used to investigate any possible publication bias. Subgroup analysis was conducted as follows: geographic region (Asia versus No-Asia), follow-up duration (≤11 years versus >11 years), sample sizes (<5000 versus >5000), napping duration (<60 min versus >60 min), and gender (men versus women). Sensitivity analysis was conducted by excluding 1 study at a time using the metaninf algorithm command in STATA statistical software to investigate the influence of a single study on the overall effect size. All statistical analyses were performed using STATA statistical software, version 12.0 (STATA, College Station, TX, USA). A 2-tailed P value <0.05 was considered as statistically significant.
Results
Literature search and baseline characteristics
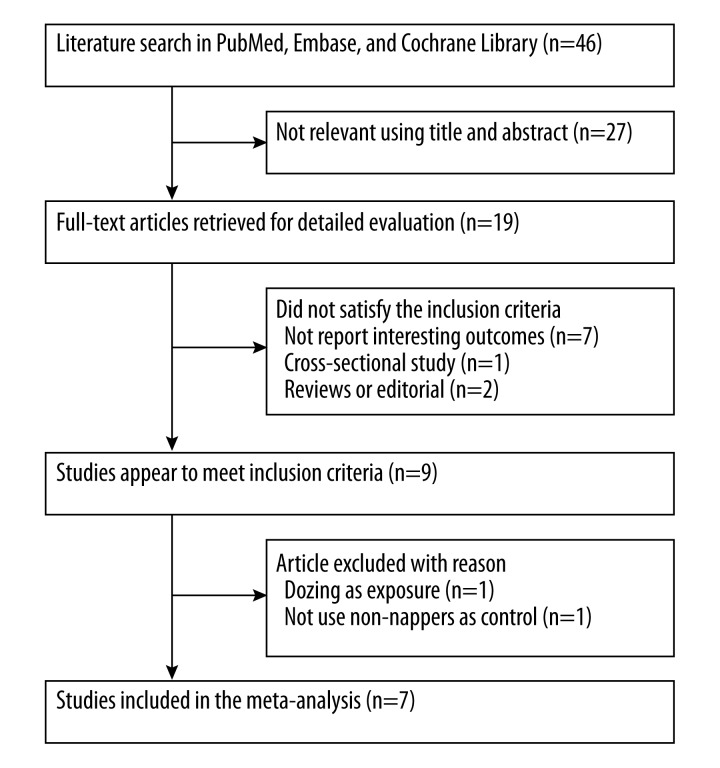
Our initial literature search produced 46 citations. Of these citations, 7 studies [6–12] met our predefined inclusion criteria. The detailed study selection process is listed in Figure 1. Table 1 lists the detail characters of the included studies. A total of 98,163 subjects were included. The age of the subjects was over 40 years. The sample sizes of the studies ranged from 455 to 67,129. One study [7] included only women, and others included both men and women. The follow-up duration ranged from 6.9 to 20 years. Overall quality of the included studies was good, and NOS scales ranged from 6 to 7 (Supplemental Table 1).
Figure 1.
Flow chart of study selection process.
Table 1.
Summary of clinical studies included in the meta-analysis.
| Study/year | Region | Type of study | Subjects (% men) age range | Assessment of daytime napping | Follow-up (years) | Outcome assessment | Outcome/events number/RR or HR (95% CI) | Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|
| Burazeri et al. [11] 2003 | Israel | Prospective cohort study | 1859 (45) >50 years |
Structured questionnaire Nappers versus non-nappers |
9–11 | Death certificate ICD-9 codes 390–458 | Total death 405 1.36 (0.93–1.97) men 0.91 (0.63–1.31) women CVD(NA) 1.53 (0.84–2.77) men 0.79 (0.45–1.38) women |
Age; smoking; BMI; SBP; self-appraised health; homocysteine, glucose and albumin; creatinine; history of CHD, CHF, DB and stroke; and night sleep duration (For women + origin of country, education and smoking) |
| Bursztyn et al. [6] 2005 | Israel | Prospective cohort study | 455 (NR) >70 years |
2-part questionnaire After nap versus no nap |
12 | Israeli National Population Register | Total death 147 1. 6 (1.0–2.6) |
Sex, dependence in activities of daily living, physical activity, self-rated health, DB, hypertension, IHD, malignancy, renal dysfunction, nocturnal sleep satisfaction, smoking, cholesterol, BMI, working status |
| Lan et al. [12] 2007 | Taiwan | Prospective cohort study | 3079 (56.8) ≥60 years |
Home-based interview | 8.4 | National death registry at the Department of Health. CVD: ICD-9 codes 390–459 | Total death 1338 0.68 (0.37–1.25) <60 min + men 1.06 (0.72–1.55) 60–89 min + men 0.98 (0.60–1.61) 90–119 min + men 1.23 (0.72–2.10) ≥120 min + men 1.24 (0.51–3.04) <60 min + women 0.76 (0.39–1.50) 60–89 min + women 0.47 (0.16–1.41) 90–119 min + women 1.04 (0.46–2.37) ≥120 min + women |
Age, marital status, monthly income, cigarettes smoking, alcohol consumption, BMI, exercise, disease history (heart disease, stroke, and cancer), and depression |
| Stone et al. [7] 2009 | USA | Prospective cohort study | 8,101 (0) ≥65 years |
Self-administered questionnaire or interview. Nappers versus non-nappers |
6.9 (mean) | Death certificate ICD-9 codes 401–404, 410–414, 425, 428, 429.2, 430–438, 440–444, and 798 | Total death 1922 1.67 (1.26–2.20)* CVD 723 1.96 (1.26–3.06)* |
Age, BMI, history of medical condition, including DB, PD, dementia, COPD, non-skin cancer, and osteoarthritis, history of hypertension, walks for exercise, alcohol use, smoking status, depression, cognitive impairment, estrogen use, and benzodiazepine use |
| Tanabe et al. [8] 2010 | Japan | Prospective study | 67,129 (41.3) 40–79 years |
Self-administered questionnaire. Nappers versus non-nappers |
14.3 | The Minister for Internal Affairs and Communications | Total death 9643 1.19(1.14–1.24) CVD 2852 1.31 (1.22–1.42) |
Age, sex, sleeping duration, treated hypertension, history of DB, any disease under medical treatment, smoking, BMI, weight loss from age 20 years, BP, perceived mental stress, depressive symptoms, working status, educational status and time for walking |
| Cohen-Mansfield et al. [10] 2012 | Israel | Prospective cohort study | 1,166 (54.5) 75–94 years |
interview | 20 | Israeli National Population Register | Total death 1108 0.82 (0.67–1.00) <7 night 1.385 (1.07–1.79) >9 night |
Age, sex, country of origin, education, financial status, and having children, demographics, health, and function variables. |
| Leng et al. [9] 2014 | UK | Prospective population-based cohort study | 16,374 (43.7) 40–79 years |
Post questionnaires | 13 | Death certificate CVD: ICD-9 codes 401–448 or ICD-10 codes I10–I79 | CVD 1034 1.00 (0.81–1.22) <60 min 0.92 (0.57–1.48) ≥60 min Total death 3521 1.14(1.02–1.27) <60 min 1.19(1.14–1.24) ≥60 min |
Age, sex, social class, education, marital status, employment status, BMI, physical activity, smoking, alcohol, depression, self-reported health, hypnotic drug use, antidepressant use, COPD drug use, time spent in bed at night, self-reported preexisting DB and underlying sleep apnea |
NR – not report; BMI – body mass index; HR – hazard risk; RR – risk ratio; BP – blood pressure; SBP – systolic blood pressure; DBP – diastolic blood pressure; DB – diabetes mellitus; CHF – chronic heart failure; CHD – coronary heart disease; COPD – chronic obstructive pulmonary disease; PD – Parkinson’s disease. * Results from the disease-free cohort.
All-cause mortality
All the included studies reported data on all-cause mortality. As shown in Figure 2, daytime napping was associated with a greater risk of all-cause mortality (RR 1.15; 95% CI 1.07–1.24) comparing with non-nappers in a random-effects model. The heterogeneity was statistically significant (I2=52.1%; P=0.007). No evidence of publication bias was found by the Begg’s rank correlation test (P=0.284) and Egger’s linear regression test (P=0.290).
Figure 2.
RR and 95% CI of all-cause mortality comparing daytime nappers to non-nappers in a random-effects model.
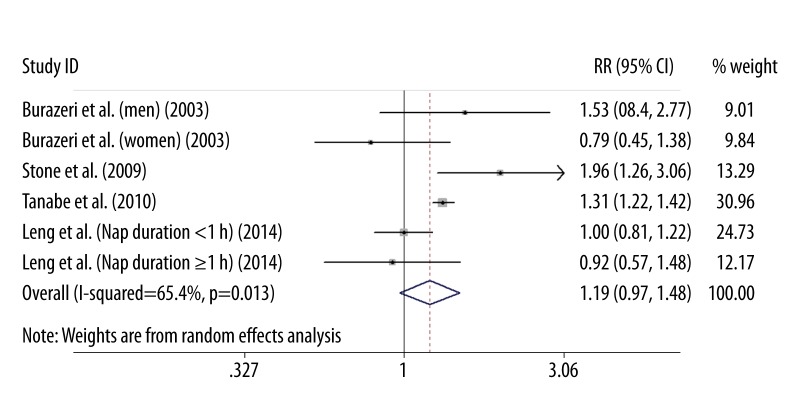
Cardiovascular mortality
Four studies [7–9,11] reported cardiovascular mortality involving 93,463 subjects. As shown in Figure 3, the pooled estimate of multivariate RR was 1.19 (95% CI 0.97–1.48) comparing daytime napping to non-nappers in a random effect model. Substantial heterogeneity was observed (I2=65.4%; P=0.013). No evidence of publication bias was found using by the Begg’s rank correlation test (P=0.707) and Egger’s linear regression test (P=0.586).
Figure 3.
RR and 95% CI of cardiovascular mortality comparing daytime nappers to non-nappers in a random-effects model.
Subgroup analyses
The detailed results of subgroup analyses are presented in Supplemental Table 2. Daytime napping was associated with a greater risk of all-cause mortality among women (RR 1.21; 95% CI 1.00–1.47), but not men (RR 1.09; 95% CI 0.89–1.34). Sensitivity analyses were performed based on all-cause mortality. In the sensitivity analysis, there was little influence in the quantitative pooled measure of RR or 95% CI when excluding one study at a time (Data not shown).
Discussion
This meta-analysis provides evidence that self-reported daytime napping is a mild but statistically significant risk predictor for all-cause mortality, but not for cardiovascular mortality. Participants with daytime napping had 15% greater risk of all-cause mortality. However, there is likely substantial heterogeneity in the duration of daytime napping. Many of the included studies did not adjust overall sleep duration; therefore, the excess risk of all-cause mortality might be explained by the impact of excessive daytime sleepiness rather than daytime napping itself.
In the current study, a daytime napping duration over 60 min was significantly and independently associated with all-cause mortality in the general population. Subjects with daytime napping showed some trend to increase cardiovascular mortality, in which this association was not statistically significant (RR 1.19; 95% CI 0.97–1.48). A case-control study [17] reported an elevated risk of acute myocardial infarction associated with long-duration daytime napping. In a prospective study [18] conducted in Germany, midday napping was associated with 2.12 times the risk of nonfatal myocardial infarction and sudden cardiac death. Contrary to these findings, 2 studies [19,20] conducted in younger healthy persons in Greece suggested a protective effect of afternoon siesta against coronary mortality. Naps longer than 30 min can cause sleep inertia and are usually not recommended [21]. These results indicated that daytime napping might have different impact on younger and elderly persons. However, we could not conduct subgroup analysis by age due to most of the subjects were elderly persons.
A more recently published study [22] suggested that daytime napping with duration >30 min was significantly and independently associated with all-cause mortality in men but not in women. However, our subgroup analysis based on gender did not found gender-specific association between daytime napping and mortality. Therefore, the gender-specific association between daytime napping and mortality needs further study.
Although the biological mechanisms by which daytime napping results in a higher mortality risk are not completely elucidated, a possible explanation is that abrupt increase in blood pressure and heart rate upon awakening from a nap results in sympathetic nervous activation [23]. The morning has the highest risk for cardiovascular events. Another explanation is that the prothrombotic effects after daytime napping may trigger thrombotic cardiovascular events [24]. Moreover, napping was also linked to depression [25,26] and obstructive sleep apnea (OSA) [27], cancer [28], Parkinson’s disease [29], and type 2 diabetes [30,31], all of which may increase risk of mortality. In addition, daytime napping might be a consequence of nighttime sleep disturbance, which was associated with greater risk of mortality [32].
Several potential limitations of this study should be addressed. First, a major limitation is the reliance on self-reported daytime napping. Data relating to daytime rest without sleep were unavailable. All the included studies measured napping by using subjective questionnaires, which are more prone to misclassification. However, self-reports are the primary methods for evaluating napping in our daily life. Second, duration of napping, working status, culture, educational background, climate, and age are the common confounding factors accounting for napping and mortality risk. However, the study did not adjust confounding variables in a consistent way. Lack of adjustment or over-adjustment for these variables might lead to a slight overestimation or underestimation of the real risk estimate. Third, most of the subjects were older persons (age over 40 years), which limited generalizability to young populations. Moreover, a tendency to underreport napping habits was found in older persons [33], and this may result in underestimation of the relationship. Fourth, the information on nighttime sleep duration, as well as napping duration or times, is not sufficient. Long or short sleep duration are likely to increase the risk of mortality [34], and most of the included studies did not consider these potential factors.
Afternoon napping is common in many countries and is considered a healthy activity. A short nap during the day can aid the return of normal cellular immune function [35]. However, the protective effect of afternoon nap is yet to be proven in different populations. Daytime napping may reflect a low quality of nighttime sleep and might be a marker of undiagnosed health problems. Given the close link between OSA and mortality [36], daytime napping might be used as a surrogate measure of OSA. Therefore, objective measures of sleep are needed to determine whether sleep apnea contributes to these relationships. Further detection of the possibility of OSA is warranted in these populations.
Conclusions
Self-reported daytime napping is a mild but statistically significant risk predictor for all-cause mortality but not for cardiovascular mortality. However, whether the risk is attributable to excessive daytime sleepiness or daytime napping alone remains unclear. More well-designed prospective studies based on younger populations are required to determine whether daytime napping is an independent predictor for mortality.
Supplementary materials
Supplemental Table 1.
Quality assessment of studies included in meta-analysis.
| Study/year | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome was not present at study start | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Enough follow-up periods (≥11 years) | Adequacy of follow-up of cohorts | Overall NOS scores |
|---|---|---|---|---|---|---|---|---|---|
| Burazeri et al. [11] 2003 | * | * | * | * | * | * | 6 | ||
| Bursztyn et al. [6] 2005 | * | * | * | * | * | * | * | 7 | |
| Lan et al. [12] 2007 | * | * | * | * | * | * | 6 | ||
| Stone et al. [7] 2009 | * | * | * | * | * | * | 6 | ||
| Tanabe et al. [8] 2010 | * | * | * | * | * | * | * | 7 | |
| Cohen-Mansfield et al. [10] 2012 | * | * | * | * | * | * | * | 7 | |
| Leng et al. [9] 2014 | * | * | * | * | * | * | * | 7 |
Supplemental Table 2.
Subgroup analyses of self-reported daytime napping and risk of all-cause mortality.
| Group | Number of studies | Event/total number | Pooled risk ratio | 95% CI |
|---|---|---|---|---|
| Region | ||||
| Asia | 2 | 10,981/70,208 | 1.12 | 1.01–1.26 |
| No Asia | 5 | 4,616/27,955 | 1.19 | 1.05–1.34 |
|
| ||||
| Follow-up duration | ||||
| ≤11 years | 3 | 3,665/13,039 | 1.08 | 0.89–1.32 |
| >11 years | 4 | 12,746/85,124 | 1.16 | 1.08–1.25 |
|
| ||||
| Sample size | ||||
| <5000 | 4 | 3,812/6,559 | 1.05 | 0.89–1.24 |
| >5000 | 3 | 12,599/91,604 | 1.19 | 1.14–1.26 |
|
| ||||
| Nap duration | ||||
| <60 min | 3 | 2,777/21,312 | 1.10 | 0.92–1.32 |
| >60 min | 3 | 2,777/21,312 | 1.15 | 1.04–1.27 |
|
| ||||
| Gender | ||||
| Men | 3 | 6,676/30,347 | 1.15 | 1.09–1.21 |
| Women | 4 | 6,632/49,821 | 1.15 | 0.92–1.43 |
Footnotes
Conflict of interest
None declared.
Source of support: Self financing
References
- 1.Picarsic JL, Glynn NW, Taylor CA, et al. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2008;56:1674–80. doi: 10.1111/j.1532-5415.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 3.Fang W, Li Z, Wu L, et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng-Tongji cohort of retired workers. Sleep Med. 2013;14:950–54. doi: 10.1016/j.sleep.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Lovato N, Lack L. The effects of napping on cognitive functioning. Prog Brain Res. 2010;185:155–66. doi: 10.1016/B978-0-444-53702-7.00009-9. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M, Arito H. Maintenance of alertness and performance by a brief nap after lunch under prior sleep deficit. Sleep. 2000;23:813–19. [PubMed] [Google Scholar]
- 6.Bursztyn M, Stessman J. The siesta and mortality: twelve years of prospective observations in 70-year-olds. Sleep. 2005;28:345–47. [PubMed] [Google Scholar]
- 7.Stone KL, Ewing SK, Ancoli-Israel S, et al. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57:604–11. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanabe N, Iso H, Seki N, et al. Daytime napping and mortality, with a special reference to cardiovascular disease: the JACC study. Int J Epidemiol. 2010;39:233–43. doi: 10.1093/ije/dyp327. [DOI] [PubMed] [Google Scholar]
- 9.Leng Y, Wainwright NW, Cappuccio FP, et al. Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol. 2014;179:1115–24. doi: 10.1093/aje/kwu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen-Mansfield J, Perach R. Sleep duration, nap habits, and mortality in older persons. Sleep. 2012;35:1003–9. doi: 10.5665/sleep.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep. 2003;26:578–84. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 12.Lan TY, Lan TH, Wen CP, et al. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30:1105–10. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. [accessed May 6, 2014]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. Int J Epidemiol. 2000;29:429–37. [PubMed] [Google Scholar]
- 18.Stang A, Dragano N, Moebus S, et al. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study. Sleep. 2012;35:1705–12. doi: 10.5665/sleep.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trichopoulos D, Tzonou A, Christopoulos C, et al. Does a siesta protect from coronary heart disease? Lancet. 1987;2:269–70. doi: 10.1016/s0140-6736(87)90848-8. [DOI] [PubMed] [Google Scholar]
- 20.Naska A, Oikonomou E, Trichopoulou A, et al. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 21.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4:341–53. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 22.Jung KI, Song CH, Ancoli-Israel S, et al. Gender differences in nighttime sleep and daytime napping as predictors of mortality in older adults: the Rancho Bernardo study. Sleep Med. 2013;14:12–19. doi: 10.1016/j.sleep.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bursztyn M, Mekler J, Ben-Ishay D. The siesta and ambulatory blood pressure: is waking up the same in the morning and afternoon? J Hum Hypertens. 1996;10:287–92. [PubMed] [Google Scholar]
- 24.Thrall G, Lane D, Carroll D, et al. A systematic review of the prothrombotic effects of an acute change in posture: a possible mechanism underlying the morning excess in cardiovascular events? Chest. 2007;132:1337–47. doi: 10.1378/chest.06-2978. [DOI] [PubMed] [Google Scholar]
- 25.Stang A, Dragano N, Poole C, et al. Daily siesta, cardiovascular risk factors, and measures of subclinical atherosclerosis: results of the Heinz Nixdorf Recall Study. Sleep. 2007;30:1111–19. doi: 10.1093/sleep/30.9.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gump BB, Matthews KA, Eberly LE, et al. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 27.Masa JF, Rubio M, Perez P, et al. Association between habitual naps and sleep apnea. Sleep. 2006;29:1463–68. doi: 10.1093/sleep/29.11.1463. [DOI] [PubMed] [Google Scholar]
- 28.Cairns BJ, Travis RC, Wang XS, et al. A short-term increase in cancer risk associated with daytime napping is likely to reflect pre-clinical disease: prospective cohort study. Br J Cancer. 2012;107:527–30. doi: 10.1038/bjc.2012.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J, Huang X, Park Y, et al. Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol. 2011;173:1032–38. doi: 10.1093/aje/kwq478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam KB, Jiang CQ, Thomas GN, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. 2010;33:402–7. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Q, Song Y, Hollenbeck A, et al. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173:300–9. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dautovich ND, McCrae CS, Rowe M. Subjective and objective napping and sleep in older adults: are evening naps “bad” for nighttime sleep? J Am Geriatr Soc. 2008;56:1681–86. doi: 10.1111/j.1532-5415.2008.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cappuccio FP, D’Elia L, Strazzullo P, et al. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Ouyang Y, Wang Z, et al. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169:207–14. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1.
Quality assessment of studies included in meta-analysis.
| Study/year | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome was not present at study start | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Enough follow-up periods (≥11 years) | Adequacy of follow-up of cohorts | Overall NOS scores |
|---|---|---|---|---|---|---|---|---|---|
| Burazeri et al. [11] 2003 | * | * | * | * | * | * | 6 | ||
| Bursztyn et al. [6] 2005 | * | * | * | * | * | * | * | 7 | |
| Lan et al. [12] 2007 | * | * | * | * | * | * | 6 | ||
| Stone et al. [7] 2009 | * | * | * | * | * | * | 6 | ||
| Tanabe et al. [8] 2010 | * | * | * | * | * | * | * | 7 | |
| Cohen-Mansfield et al. [10] 2012 | * | * | * | * | * | * | * | 7 | |
| Leng et al. [9] 2014 | * | * | * | * | * | * | * | 7 |
Supplemental Table 2.
Subgroup analyses of self-reported daytime napping and risk of all-cause mortality.
| Group | Number of studies | Event/total number | Pooled risk ratio | 95% CI |
|---|---|---|---|---|
| Region | ||||
| Asia | 2 | 10,981/70,208 | 1.12 | 1.01–1.26 |
| No Asia | 5 | 4,616/27,955 | 1.19 | 1.05–1.34 |
|
| ||||
| Follow-up duration | ||||
| ≤11 years | 3 | 3,665/13,039 | 1.08 | 0.89–1.32 |
| >11 years | 4 | 12,746/85,124 | 1.16 | 1.08–1.25 |
|
| ||||
| Sample size | ||||
| <5000 | 4 | 3,812/6,559 | 1.05 | 0.89–1.24 |
| >5000 | 3 | 12,599/91,604 | 1.19 | 1.14–1.26 |
|
| ||||
| Nap duration | ||||
| <60 min | 3 | 2,777/21,312 | 1.10 | 0.92–1.32 |
| >60 min | 3 | 2,777/21,312 | 1.15 | 1.04–1.27 |
|
| ||||
| Gender | ||||
| Men | 3 | 6,676/30,347 | 1.15 | 1.09–1.21 |
| Women | 4 | 6,632/49,821 | 1.15 | 0.92–1.43 |