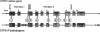Abstract
Genotyping for 10 mutations in the CYP21 gene was performed in 88 families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Southern blot analysis was used to detect CYP21 deletions or large gene conversions, and allele-specific hybridizations were performed with DNA amplified by the polymerase chain reaction to detect smaller mutations. Mutations were detected on 95% of chromosomes examined. The most common mutations were an A----G change in the second intron affecting pre-mRNA splicing (26%), large deletions (21%), Ile-172----Asn (16%), and Val-281----Leu (11%). Patients were classified into three mutation groups based on degree of predicted enzymatic compromise. Mutation groups were correlated with clinical diagnosis and specific measures of in vivo 21-hydroxylase activity, such as 17-hydroxyprogesterone, aldosterone, and sodium balance. Mutation group A (no enzymatic activity) consisted principally of salt-wasting (severely affected) patients, group B (2% activity) of simple virilizing patients, and group C (10-20% activity) of nonclassic (mildly affected) patients, but each group contained patients with phenotypes either more or less severe than predicted. These data suggest that most but not all of the phenotypic variability in 21-hydroxylase deficiency results from allelic variation in CYP21. Accurate prenatal diagnosis should be possible in most cases using the described strategy.
Full text
PDF

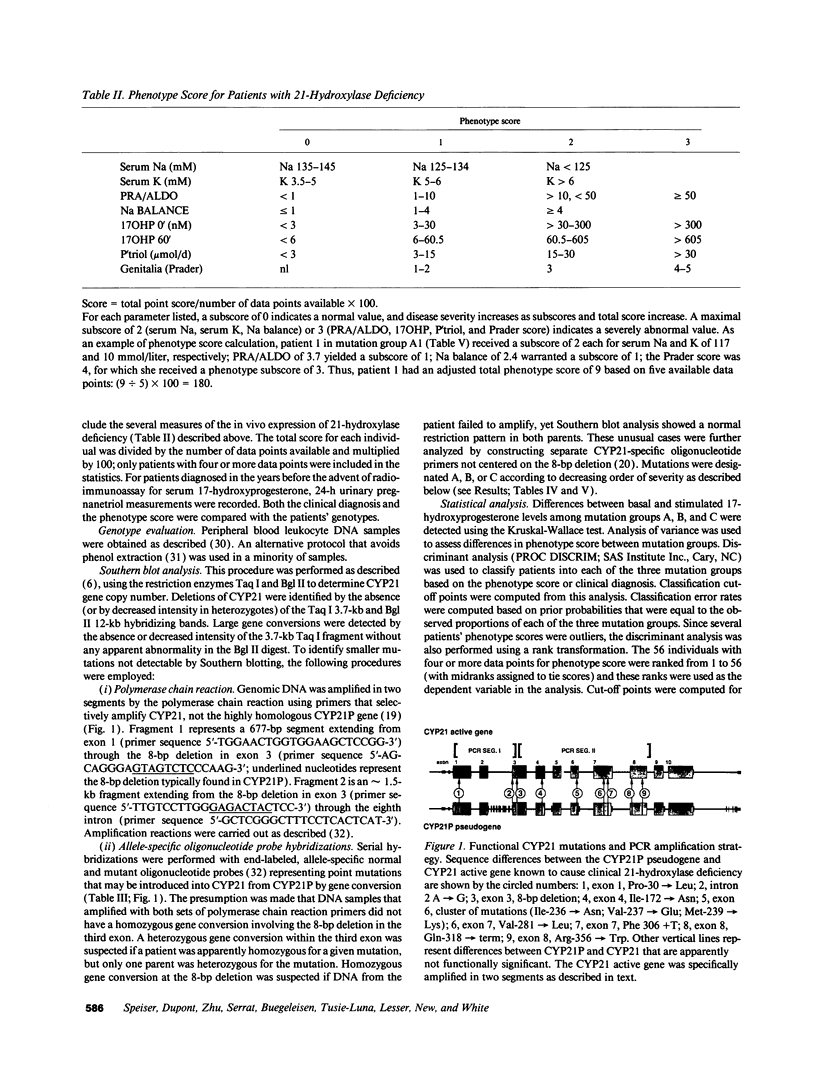
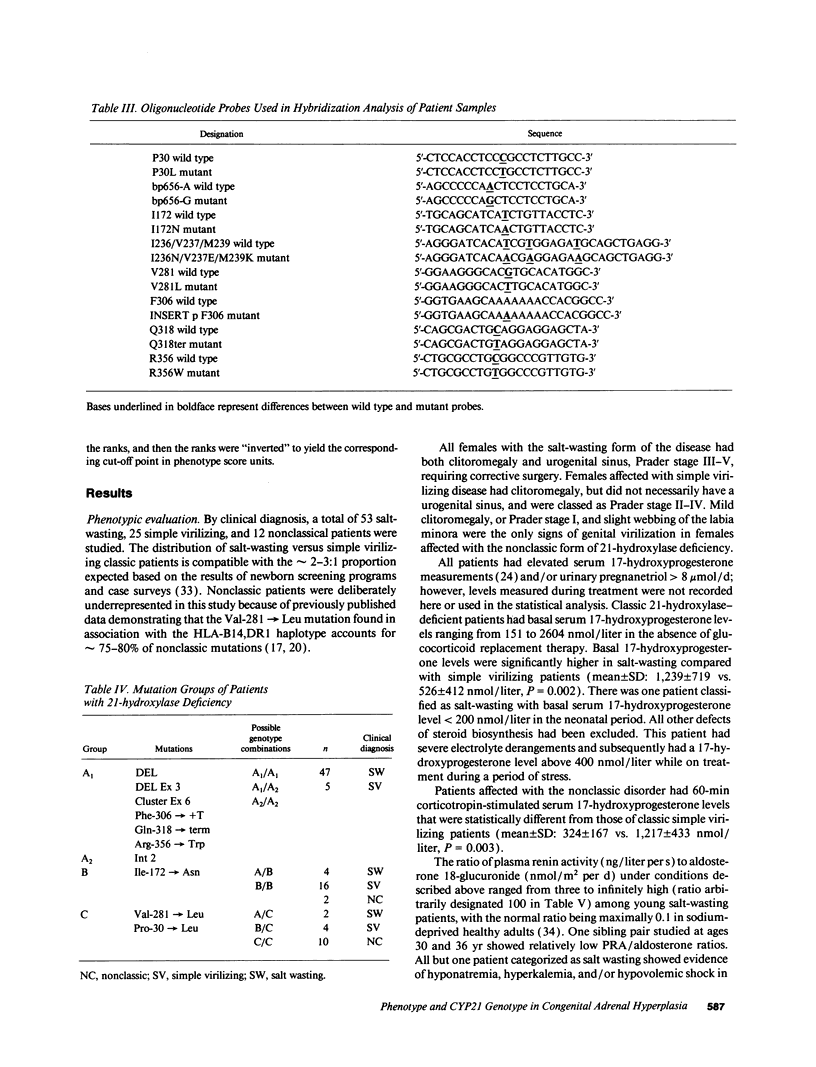
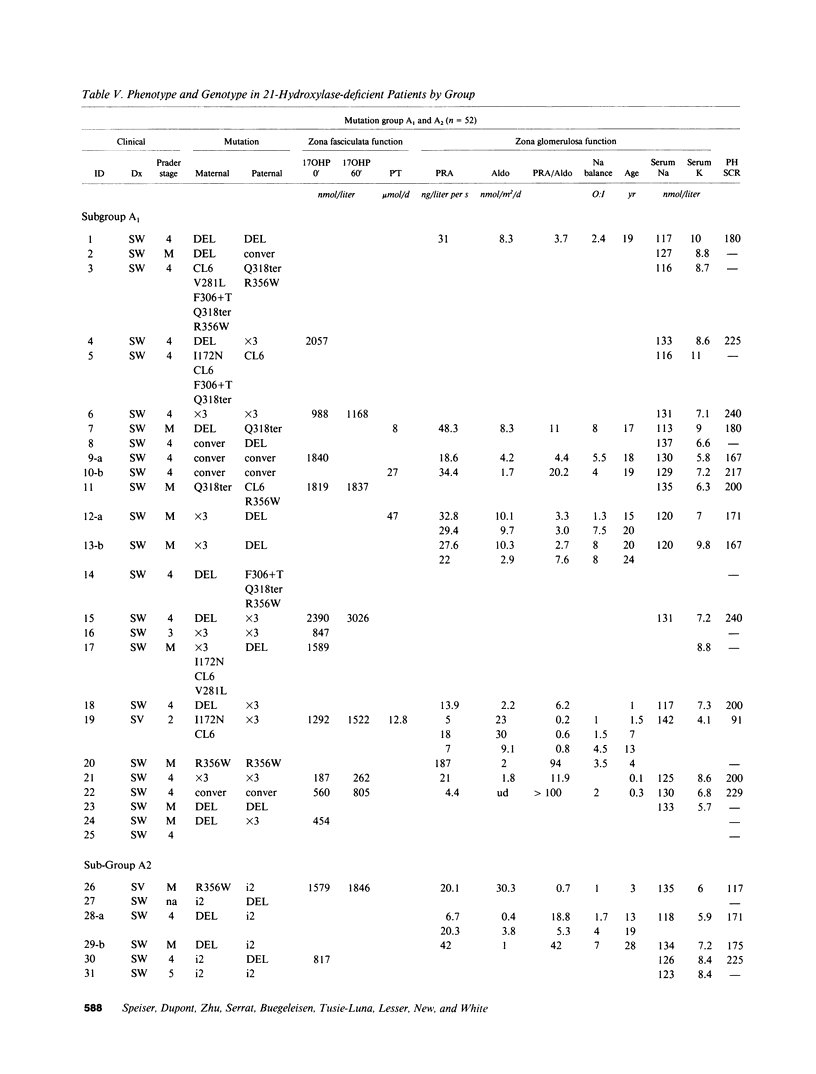



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor M., Parker K. L., Globerman H., New M. I., White P. C. Mutation in the CYP21B gene (Ile-172----Asn) causes steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1600–1604. doi: 10.1073/pnas.85.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Sinnott P. J., Dyer P. A., Price D. A., Harris R., Strachan T. Pulsed field gel electrophoresis identifies a high degree of variability in the number of tandem 21-hydroxylase and complement C4 gene repeats in 21-hydroxylase deficiency haplotypes. EMBO J. 1989 May;8(5):1393–1402. doi: 10.1002/j.1460-2075.1989.tb03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont B., Oberfield S. E., Smithwick E. M., Lee T. D., Levine L. S. Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet. 1977 Dec 24;2(8052-8053):1309–1312. doi: 10.1016/s0140-6736(77)90362-2. [DOI] [PubMed] [Google Scholar]
- Globerman H., Amor M., Parker K. L., New M. I., White P. C. Nonsense mutation causing steroid 21-hydroxylase deficiency. J Clin Invest. 1988 Jul;82(1):139–144. doi: 10.1172/JCI113562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G. How imprinting is relevant to human disease. Dev Suppl. 1990:141–148. [PubMed] [Google Scholar]
- Higashi Y., Hiromasa T., Tanae A., Miki T., Nakura J., Kondo T., Ohura T., Ogawa E., Nakayama K., Fujii-Kuriyama Y. Effects of individual mutations in the P-450(C21) pseudogene on the P-450(C21) activity and their distribution in the patient genomes of congenital steroid 21-hydroxylase deficiency. J Biochem. 1991 Apr;109(4):638–644. doi: 10.1093/oxfordjournals.jbchem.a123433. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Tanae A., Inoue H., Hiromasa T., Fujii-Kuriyama Y. Aberrant splicing and missense mutations cause steroid 21-hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7486–7490. doi: 10.1073/pnas.85.20.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M. B., Jr, Paulus-Thomas J. E. Purification of human genomic DNA from whole blood using sodium perchlorate in place of phenol. Anal Biochem. 1989 Aug 1;180(2):276–278. doi: 10.1016/0003-2697(89)90430-2. [DOI] [PubMed] [Google Scholar]
- Kohn B., Levine L. S., Pollack M. S., Pang S., Lorenzen F., Levy D., Lerner A. J., Rondanini G. F., Dupont B., New M. I. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1982 Nov;55(5):817–827. doi: 10.1210/jcem-55-5-817. [DOI] [PubMed] [Google Scholar]
- Korth-Schutz S., Virdis R., Saenger P., Chow D. M., Levine L. S., New M. I. Serum androgens as a continuing index of adequacy of treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1978 Mar;46(3):452–458. doi: 10.1210/jcem-46-3-452. [DOI] [PubMed] [Google Scholar]
- Kuhnle U., Chow D., Rapaport R., Pang S., Levine L. S., New M. I. The 21-hydroxylase activity in the glomerulosa and fasciculata of the adrenal cortex in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1981 Mar;52(3):534–544. doi: 10.1210/jcem-52-3-534. [DOI] [PubMed] [Google Scholar]
- LUETSCHER J. A., DOWDY A. J., CALLAGHAN A. M., COHN A. P. Studies of secretion and metabolism of aldosterone and cortisol. Trans Assoc Am Physicians. 1962;75:293–300. [PubMed] [Google Scholar]
- Levine L. S., Zachmann M., New M. I., Prader A., Pollack M. S., O'Neill G. J., Yang S. Y., Oberfield S. E., Dupont B. Genetic mapping of the 21-hydroxylase-deficiency gene within the HLA linkage group. N Engl J Med. 1978 Oct 26;299(17):911–915. doi: 10.1056/NEJM197810262991702. [DOI] [PubMed] [Google Scholar]
- Loukopoulos D. Thalassemia: genotypes and phenotypes. Ann Hematol. 1991 Apr;62(4):85–94. doi: 10.1007/BF01702920. [DOI] [PubMed] [Google Scholar]
- Morel Y., André J., Uring-Lambert B., Hauptmann G., Bétuel H., Tossi M., Forest M. G., David M., Bertrand J., Miller W. L. Rearrangements and point mutations of P450c21 genes are distinguished by five restriction endonuclease haplotypes identified by a new probing strategy in 57 families with congenital adrenal hyperplasia. J Clin Invest. 1989 Feb;83(2):527–536. doi: 10.1172/JCI113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet E., Crété P., Kuttenn F., Raux-Demay M. C., Boué J., White P. C., Boué A. Distribution of deletions and seven point mutations on CYP21B genes in three clinical forms of steroid 21-hydroxylase deficiency. Am J Hum Genet. 1991 Jan;48(1):79–88. [PMC free article] [PubMed] [Google Scholar]
- New M. I., Lorenzen F., Lerner A. J., Kohn B., Oberfield S. E., Pollack M. S., Dupont B., Stoner E., Levy D. J., Pang S. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983 Aug;57(2):320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- New M. I., Miller B., Peterson R. E. Aldosterone excretion in normal children and in children with adrenal hyperplasia. J Clin Invest. 1966 Mar;45(3):412–428. doi: 10.1172/JCI105356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano Y., Eisensmith R. C., Güttler F., Lichter-Konecki U., Konecki D. S., Trefz F. K., Dasovich M., Wang T., Henriksen K., Lou H. Molecular basis of phenotypic heterogeneity in phenylketonuria. N Engl J Med. 1991 May 2;324(18):1232–1238. doi: 10.1056/NEJM199105023241802. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Crawford Y. M., Draznin M. B. Direct analysis of CYP21B genes in 21-hydroxylase deficiency using polymerase chain reaction amplification. Mol Endocrinol. 1990 Jan;4(1):125–131. doi: 10.1210/mend-4-1-125. [DOI] [PubMed] [Google Scholar]
- PRADER A. Vollkommen männliche äussere Genitalentwicklung und Salzverlustsyndrom bei Madchen mit kongenitalem adrenogenitalem Syndrom. Helv Paediatr Acta. 1958 Feb;13(1):5–14. [PubMed] [Google Scholar]
- Pang S. Y., Wallace M. A., Hofman L., Thuline H. C., Dorche C., Lyon I. C., Dobbins R. H., Kling S., Fujieda K., Suwa S. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988 Jun;81(6):866–874. [PubMed] [Google Scholar]
- Pang S., Hotchkiss J., Drash A. L., Levine L. S., New M. I. Microfilter paper method for 17 alpha-hydroxyprogesterone radioimmunoassay: its application for rapid screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1977 Nov;45(5):1003–1008. doi: 10.1210/jcem-45-5-1003. [DOI] [PubMed] [Google Scholar]
- Partanen J., Koskimies S., Sipilä I., Lipsanen V. Major-histocompatibility-complex gene markers and restriction-fragment analysis of steroid 21-hydroxylase (CYP21) and complement C4 genes in classical congenital adrenal hyperplasia patients in a single population. Am J Hum Genet. 1989 May;44(5):660–670. [PMC free article] [PubMed] [Google Scholar]
- Rauh W., Levine L. S., Gottesdiener K., New M. I. Mineralocorticoids, salt balance and blood pressure after prolonged ACTH administration in juvenile hypertension. Klin Wochenschr. 1978;56 (Suppl 1):161–167. doi: 10.1007/BF01477468. [DOI] [PubMed] [Google Scholar]
- Speiser P. W., Agdere L., Ueshiba H., White P. C., New M. I. Aldosterone synthesis in salt-wasting congenital adrenal hyperplasia with complete absence of adrenal 21-hydroxylase. N Engl J Med. 1991 Jan 17;324(3):145–149. doi: 10.1056/NEJM199101173240302. [DOI] [PubMed] [Google Scholar]
- Speiser P. W., New M. I., White P. C. Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14,DR1. N Engl J Med. 1988 Jul 7;319(1):19–23. doi: 10.1056/NEJM198807073190104. [DOI] [PubMed] [Google Scholar]
- Stoner E., Dimartino-Nardi J., Kuhnle U., Levine L. S., Oberfield S. E., New M. I. Is salt-wasting in congenital adrenal hyperplasia due to the same gene as the fasciculata defect? Clin Endocrinol (Oxf) 1986 Jan;24(1):9–20. doi: 10.1111/j.1365-2265.1986.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Strachan T. Molecular pathology of congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 1990 Mar;32(3):373–393. doi: 10.1111/j.1365-2265.1990.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Tusie-Luna M. T., Speiser P. W., Dumic M., New M. I., White P. C. A mutation (Pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Mol Endocrinol. 1991 May;5(5):685–692. doi: 10.1210/mend-5-5-685. [DOI] [PubMed] [Google Scholar]
- Tusie-Luna M. T., Traktman P., White P. C. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990 Dec 5;265(34):20916–20922. [PubMed] [Google Scholar]
- White P. C. Analysis of mutations causing steroid 21-hydroxylase deficiency. Endocr Res. 1989;15(1-2):239–256. doi: 10.1080/07435808909039099. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., Vitek A., Dupont B., New M. I. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4436–4440. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Antonarakis S. E., Goff S. C., Orkin S. H., Forget B. G., Nathan D. G., Giardina P. J., Kazazian H. H., Jr Beta-thalassemia due to two novel nucleotide substitutions in consensus acceptor splice sequences of the beta-globin gene. Blood. 1989 Mar;73(4):914–918. [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]