Abstract
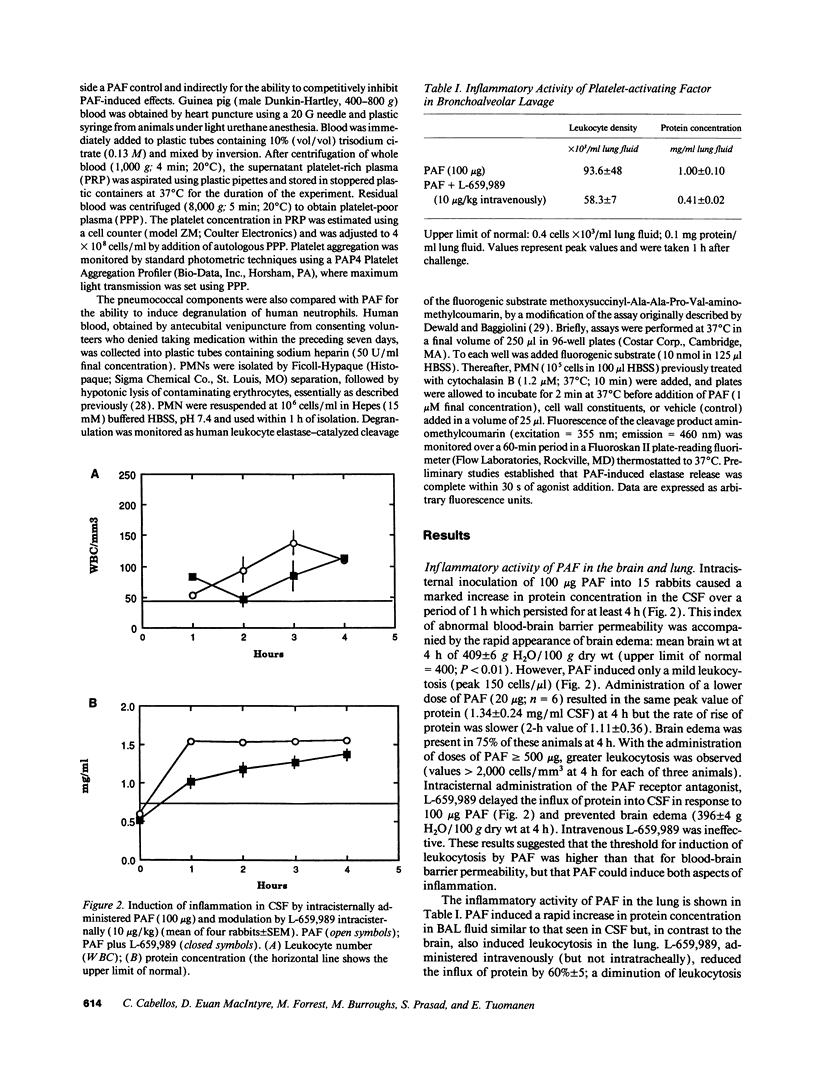
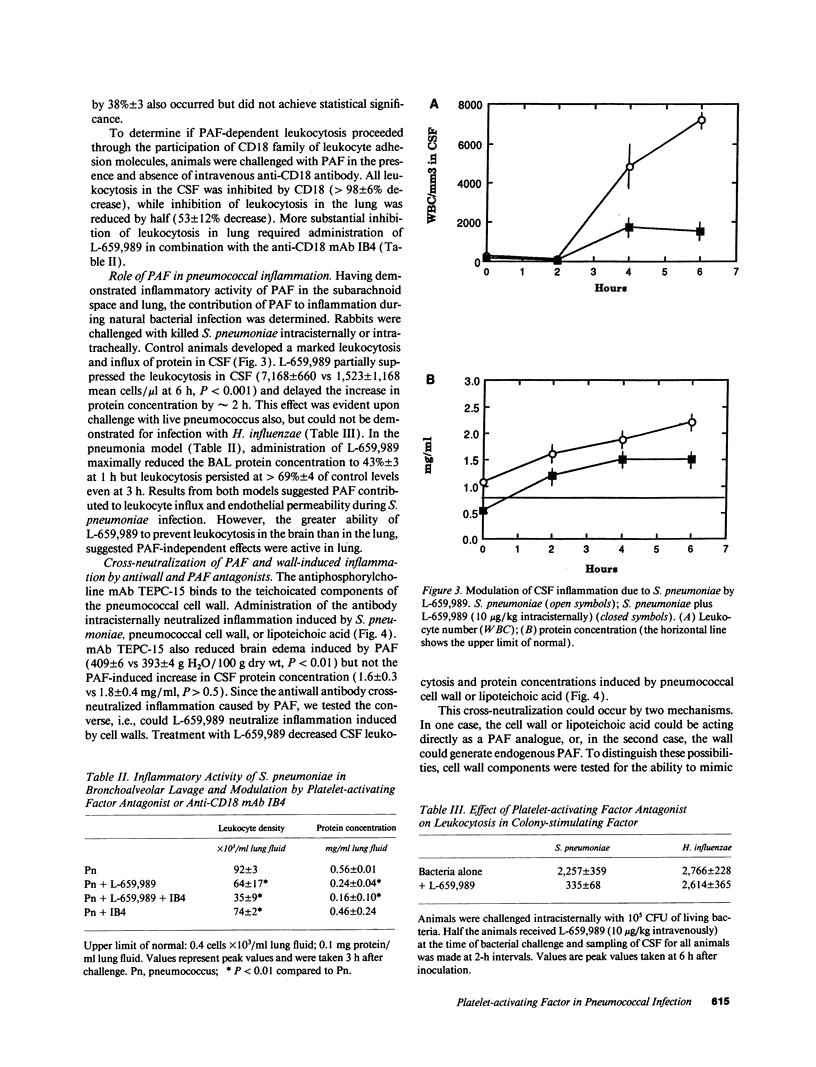
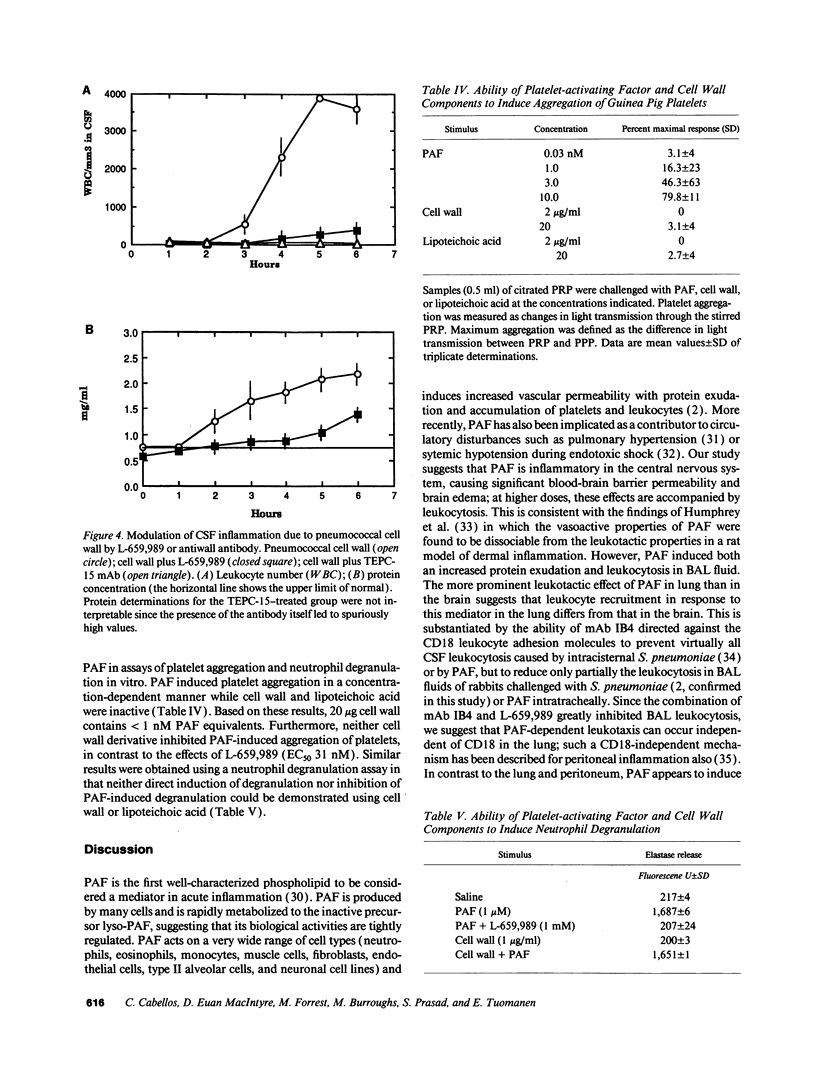
Although well-characterized in the lung, the role of platelet-activating factor (PAF) in inflammation in the central nervous system is undefined. Using rabbit models of meningitis and pneumonia, PAF was found to induce significant blood-brain barrier permeability and brain edema at doses five times lower than those required to generate leukocyte recruitment to the subarachnoid space. Both leukocytosis and increased vascular permeability occurred in response to PAF in the lung. Antibody to the CD-18 family of leukocyte adhesion molecules inhibited leukocyte recruitment in response to PAF in the brain (greater than 80%); a similar level of inhibition in the lung required treatment with a combination of a PAF receptor antagonist (L-659,989) and anti-CD18 antibody. Treatment with L-659,989 decreased abnormal cerebrospinal fluid cytochemical values induced by intracisternal challenge with pneumococci but not Haemophilus influenzae, indicating a special role for PAF in pneumococcal disease. Antibodies directed at phosphorylcholine, a unique, shared determinant of bioactivity of PAF and pneumococcal cell wall, obviated the inflammatory potential of both agents. However, no evidence for a direct PAF-like activity of pneumococcal cell wall components was detected in vitro by bioassay using platelets or neutrophils. It is concluded that PAF can induce inflammation in the subarachnoid space. In brain, PAF effects appear to be mediated through CD-18-dependent events, while in lung, PAF effects independent of CD-18 are also evident. At both sites, PAF is of particular clinical importance during inflammation induced by pneumococci apparently due to a unique proinflammatory relationship between the pneumococcal cell wall teichoic acid and PAF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditi M., Manogue K. R., Caplan M., Yogev R. Cerebrospinal fluid cachectin/tumor necrosis factor-alpha and platelet-activating factor concentrations and severity of bacterial meningitis in children. J Infect Dis. 1990 Jul;162(1):139–147. doi: 10.1093/infdis/162.1.139. [DOI] [PubMed] [Google Scholar]
- Baughman R. P., Bosken C. H., Loudon R. G., Hurtubise P., Wesseler T. Quantitation of bronchoalveolar lavage with methylene blue. Am Rev Respir Dis. 1983 Aug;128(2):266–270. doi: 10.1164/arrd.1983.128.2.266. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M. Evaluation of PAF antagonists using human neutrophils in a microtiter plate assay. Biochem Pharmacol. 1987 Aug 1;36(15):2505–2510. doi: 10.1016/0006-2952(87)90523-5. [DOI] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M., Winn R. K., Coxson H. O., Harlan J. M. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990 Mar 15;144(6):2327–2333. [PubMed] [Google Scholar]
- Ferguson-Chanowitz K. M., Katocs A. S., Jr, Pickett W. C., Kaplan J. B., Sass P. M., Oronsky A. L., Kerwar S. S. Platelet-activating factor or a platelet-activating factor antagonist decreases tumor necrosis factor-alpha in the plasma of mice treated with endotoxin. J Infect Dis. 1990 Nov;162(5):1081–1086. doi: 10.1093/infdis/162.5.1081. [DOI] [PubMed] [Google Scholar]
- Hamasaki Y., Mojarad M., Saga T., Tai H. H., Said S. I. Platelet-activating factor raises airway and vascular pressures and induces edema in lungs perfused with platelet-free solution. Am Rev Respir Dis. 1984 May;129(5):742–746. doi: 10.1164/arrd.1984.129.5.742. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991 Jan 24;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Satouchi K., Hanahan D. J., Pinckard R. N. Vasoactive properties of acetyl glyceryl ether phosphorylcholine and analogues. Lab Invest. 1982 Apr;46(4):422–427. [PubMed] [Google Scholar]
- Hwang S. B., Lam M. H., Alberts A. W., Bugianesi R. L., Chabala J. C., Ponpipom M. M. Biochemical and pharmacological characterization of L-659,989: an extremely potent, selective and competitive receptor antagonist of platelet-activating factor. J Pharmacol Exp Ther. 1988 Aug;246(2):534–541. [PubMed] [Google Scholar]
- Issekutz A. C., Szpejda M. Evidence that platelet activating factor may mediate some acute inflammatory responses. Studies with the platelet-activating factor antagonist, CV3988. Lab Invest. 1986 Mar;54(3):275–281. [PubMed] [Google Scholar]
- Kohayakawa M., Inoue K. Augmentation of 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (PAF)-induced human platelet activation by C-reactive protein. Thromb Res. 1986 Mar 1;41(5):649–657. doi: 10.1016/0049-3848(86)90361-0. [DOI] [PubMed] [Google Scholar]
- Meurer R., MacIntyre D. E. Lack of effect of pertussis toxin on TNF-alpha-induced formation of reactive oxygen intermediates by human neutrophils. Biochem Biophys Res Commun. 1989 Mar 15;159(2):763–769. doi: 10.1016/0006-291x(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Mileski W., Harlan J., Rice C., Winn R. Streptococcus pneumoniae-stimulated macrophages induce neutrophils to emigrate by a CD18-independent mechanism of adherence. Circ Shock. 1990 Jul;31(3):259–267. [PubMed] [Google Scholar]
- Mold C., Nakayama S., Holzer T. J., Gewurz H., Du Clos T. W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981 Nov 1;154(5):1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Ponpipom M. M., Hwang S. B., Doebber T. W., Acton J. J., Alberts A. W., Biftu T., Brooker D. R., Bugianesi R. L., Chabala J. C., Gamble N. L. (+/-)-trans-2-(3-Methoxy-5-methylsulfonyl-4-propoxyphenyl)-5-(3,4,5- trimethoxyphenyl)tetrahydrofuran (L-659,989), a novel, potent PAF receptor antagonist. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1213–1220. doi: 10.1016/0006-291x(88)90758-9. [DOI] [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrogiannopoulos G. A., Olsen K. D., Reisch J. S., McCracken G. H., Jr Dexamethasone in the treatment of experimental Haemophilus influenzae type b meningitis. J Infect Dis. 1987 Feb;155(2):213–219. doi: 10.1093/infdis/155.2.213. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Saukkonen K. The nature of cell wall-derived inflammatory components of pneumococci. Pediatr Infect Dis J. 1989 Dec;8(12):902–903. doi: 10.1097/00006454-198912000-00034. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Surface components of Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):190–211. doi: 10.1093/clinids/3.2.190. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. I., Saukkonen K., Sande S., Cioffe C., Wright S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989 Sep 1;170(3):959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Hill R. Quantitation of bronchoalveolar lavage with methylene blue. Am Rev Respir Dis. 1984 Jul;130(1):146–147. doi: 10.1164/arrd.1984.130.1.146. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Rich R., Zak O. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis. 1987 Apr;135(4):869–874. doi: 10.1164/arrd.1987.135.4.869. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Tomasz A., Hengstler B., Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985 Mar;151(3):535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Khayam-Bashi H., Sande M. A. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J Infect Dis. 1985 Mar;151(3):528–534. doi: 10.1093/infdis/151.3.528. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]
- Wissner A., Schaub R. E., Sum P. E., Kohler C. A., Goldstein B. M. Analogues of platelet activating factor. 4. Some modifications of the phosphocholine moiety. J Med Chem. 1986 Mar;29(3):328–333. doi: 10.1021/jm00153a005. [DOI] [PubMed] [Google Scholar]



