Abstract
Purpose
To determine whether protein tyrosine phosphatase 1B (PTP1B) is expressed in rat retinal pigment epithelium (RPE) cells, to evaluate whether inhibition of PTP1B contributes to initiation of RPE cells into an active state, and to investigate the signaling pathways involved in this process.
Methods
Rat retinas were detached by trans-scleral injection of 1.4% sodium hyaluronate into the subretinal space. Immunocytochemistry evaluated the expression of PTP1B in RPE cells located at normal and detached retinas. From the cultured RPE cells treated with TCS-401, cell proliferation was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetracolium bromide assay, and the protein expression levels of cyclin A and cyclin D1 were determined. The effect of TCS-401 on cell differentiation was confirmed by immunostaining for α-smooth muscle actin and by western blot. Cell migration activity and PTP1B signaling mechanism were determined. Migration Assay was used to evaluate cell migration activity. PTP1B signaling mechanism was determined by use of PD98059 and LY294002.
Results
PTP1B was expressed in the RPE layer of the normal retina. After retinal detachment, weak immunolabeling of PTP1B was seen in the RPE cells. TCS-401 promoted the proliferation and expression of cyclin A and cyclin D1 in RPE cells. TCS-401 induced RPE cells to differentiate toward better contractility and motility. A migration assay proved that inhibiting PTP1B improved the migratory activity of RPE cells. TCS-401 activated extracellular signal-regulated kinase (Erk) and protein kinase B (Akt) phosphorylation. Pretreatment with PD98059 and LY294002 abolished TCS-401-induced activation of Erk, Akt, cell proliferation, and cell migration.
Conclusions
PTP1B may be involved in regulating the active state of RPE cells. The inhibition of PTP1B promoted the proliferation, myofibroblast differentiation, and migration of RPE cells, and MEK/Erk and PI3K/Akt signaling pathways played important roles in the proliferation and migration process.
Introduction
Several cellular components of proliferative vitreoretinopathy (PVR) membranes have been previously identified [1-3], leading much of the recent work to focus on understanding and modulating cellular activities involved [4-6]. It has been recognized that the epithelial-mesenchymal transition (EMT) of retinal pigment epithelial (RPE) cells contributes to the nascency of PVR [7]. RPE cells undergo EMT in PVR membranes, and as such are major contributors to the excessive deposition of the extracellular matrix in these membranes [8-10]. However, the mechanism of initiation of EMT is not well understood.
Protein tyrosine phosphatases (PTPs) comprise a diverse family of transmembrane and cytoplasmic enzymes. PTPs play an important role in regulating the proliferative activity of cells and the integrity of cell-cell and cell-matrix contacts [11-14]. Previous research in our laboratory indicated that sodium orthovanadate (SOV), a general inhibitor of PTPs, could accelerate the cell cycle of RPE cells, induce RPE cells to differentiate toward better motility, and improve their migration activity [15]. The inhibition of PTPs may be the main initiator of the EMT of RPE cells. However, it is not known which isoform plays a more important role in the activation of RPE cells.
Based on the distribution in cells, the classical PTPs can be divided into two types: non-receptor PTPs and receptor PTPs [16]. Protein tyrosine phosphatase 1B (PTP1B) is a non-receptor PTP frequently associated with the endoplasmic reticulum and vesicles subjacent to the plasma membrane [17]. A study has found that PTP1B associates with N-cadherin and may act as a regulatory switch controlling cadherin function by dephosphorylating β-catenin, thereby maintaining cells in an adhesion-competent state [18]. Previous research by our laboratory has indicated that the increased expression of N-cadherin in the RPE cells of the retina after retinal detachment may contribute to the migration of RPE cells and photoreceptor cell survival [19]. Therefore, the role played by PTP1B in the activation of RPE cells needs to be clarified.
This study was the first to investigate the expression of PTP1B in RPE cells and the role of PTP1B in regulating cell proliferation, differentiation, and migration using TCS-401, a selective inhibitor of PTP1B. The data may be useful for understanding the EMT of RPE cells in many pathological events, such as the formation and contraction of fibrous membranes.
Methods
Antibodies and reagents
Monoclonal rabbit-anti-human PTP1B was purchased from Abcam (Cambridge, UK). Monoclonal rabbit-anti-rat extracellular signal-regulated kinase (Erk)1/2, p-Erk1/2, protein kinase B (Akt; pan), and p-Akt were obtained from Cell Signaling Technology (Danvers, MA). Monoclonal mouse-anti-human α-smooth muscle actin (α-SMA), monoclonal rabbit-anti-human cyclin A and cyclin D1 antibodies, fluorescein isothiocyanate (FITC)-conjugated goat-ant-rabbit, and horseradish peroxidase-conjugated donkey-anti-rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit-anti-human β-actin was obtained from Biomedical Technologies (Stoughton, MA). TCS-401 was obtained from Tocris Bioscience (Tocris, Bristol, UK). PD98059 (an inhibitor of mitogen-activated protein kinase kinase/extracellular-signal-regulated kinase (MEK/Erk)), LY294002 (an inhibitor of phosphatidylinositol 3-kinase (PI3K)), and propidium iodide (PI) were purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Norcross, GA). BSA (BSA) was purchased from Fisher Scientific (Pittsburgh, PA).
Model of retinal detachment
Adult Sprague-Dawley (SD) rats of either gender (180–200 g; Vitalriver Laboratory Animal Equipment Co., Ltd., Beijing, China) were used in this study. Pupils were dilated with a topically applied mixture of 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P; Santen Pharmaceutical Co., Ltd., Osaka, Japan). Retinas were detached from the right eyes of SD rats by trans-scleral injection of 1.4% sodium hyaluronate (Healon GV; Pharmacia and Upjohn Co., Kalamazoo, MI) into the subretinal space (SRS) with a 30-gauge needle (BD Biosciences, Franklin Lakes, NJ) [19]. Care was taken not to make a break in the detached retina. Animals were excluded from the study if they developed intraocular hemorrhage. The left eyes of all animals served as normal control eyes. Animals were sacrificed 10 days after surgery. All procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the Medical Ethics Committee of the Affiliated Hospital, Qingdao University (Permit Number: 00,863). All surgeries were performed under pentobarbital sodium and a topical anesthetic (Oxyben; Santen Pharmaceutical Co., Ltd.). All efforts were made to minimize suffering.
Primary culture of rat RPE cells
Retinal tissues were obtained from adult male SD rats. Rat RPE cells were isolated by modifying the method developed by Edwards [20]. Briefly, enucleated eyes were soaked in phosphate-buffered saline (PBS) solution containing 0.1 mg/mL of streptomycin and 100 U/mL of penicillin. The anterior section of the eye was removed and the vitreous humor was aspirated. The posterior eyecup was incubated in a hyaluronidase (1%) solution for 2 min at room temperature. The remaining neural retina was removed. Exposed RPE cells were trypsinized (0.25% trypsin), incubating the eyecup at 37 °C for 20 min. RPE cells were isolated from Bruch’s membrane using a gentle water jet of buffered saline through a 1 ml pipette. Later, dissociated RPE cells were aspirated and seeded into cell culture flasks. The cells were cultured in Dulbecco’s modified eagle’s medium/nutrient mixture F-12 (DMEM/F12; Invitrogen, Carlsbad, CA) supplemented with 10% FBS. The culture medium was changed every two days. After approximately seven days, the RPE cells were grown to confluence. Primary cultured cells were then trypsinized, resuspended in a culture medium, and seeded into cell culture flasks at a density of 3.0×105 cells per flask. The cells were used between passages 3 and 5.
Immunofluorescence staining
The eyes in the model of retinal detachment were enucleated after cardiac perfusion of 4% paraformaldehyde. The anterior segment of the eye and the vitreous were removed. After neural retinas were removed by forceps, the eyecups were divided into four segments (retinal detachment area and non-detached area were labeled, respectively), and then spread on the slides. The stretched preparations were fixed with 4% paraformaldehyde for 20 min at room temperature. After washing three times with PBS, stretched preparations were pre-incubated for 20 min in 5% BSA to block nonspecific binding, and then incubated with a primary antibody at room temperature for 3 h. The concentration of monoclonal rabbit-anti-human PTP1B was 1:50. To assess the specificity of the staining, the negative control group was processed without the primary antibody. Following incubation of the primary antibody, stretched preparations were washed with PBS, and then incubated for 1 h with a 1:200 dilution of the appropriate FITC-conjugated secondary antibody at 37 °C. After another wash with PBS, slides were coverslipped with glycerol. Positive staining was visualized using a confocal laser scanning microscope (LSM-510; Carl Zeiss, Jena, Germany).
For immunofluorescent analysis of cultured cells, cells were seeded onto chamber slides at a density of 1×105 cells per chamber. After washing three times with PBS, cells were fixed in 95% ethanol at room temperature for 10 min, followed by a further wash with PBS. The fixed cells were then permeabilized with 1% Triton X-100 in PBS for 10 min. Later cells were blocked, incubated with the primary antibody overnight at 4 °C, and washed in PBS. The concentration of anti-α-SMA was 1:100. The FITC-conjugated secondary antibody was applied for 1 h with a 1:200 dilution at 37 °C. Cells were later washed with PBS and mounted in a medium containing PI for visualizing the nuclei. Positive staining was visualized using the confocal laser scanning microscope.
MTT assay
Cell proliferation was examined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetracolium bromide (MTT; Roche Molecular Biochemicals, Mannheim, Germany) assay. The 5×103 cells grown in a 96-well plate for 24 h were partially starved in DMEM/F12 supplemented with 1% FBS for 12 h, and then stimulated with various concentrations of TCS-401 for an additional 24 h. MTT was added to the culture medium, and the cells were incubated for an additional 4 h. The formazan crystals formed were then dissolved by adding dimethyl sulfoxide (100 μL per well). Absorbance at 490 nm was measured using a microplate reader (Model 550; Bio-Rad, Tokyo, Japan).
Protein extraction and immunoblotting
Protein preparation and western blot analysis were performed as described previously [15]. Cultured cells grown in culture flasks were trypsinized; suspended in a lysis buffer containing 1% Triton X-100, 250 mM sodium chloride, 2 mM EDTA, 50 mM tris (hydroxymethyl) aminomethane (Tris)-HCl, 10 μg/ml leupeptin, and 1 mm phenylmethylsulfonyl fluoride (all from Sigma-Aldrich); and homogenized. Equal amounts of extracted protein were loaded on 4%–12% polyacrylamide gels (Invitrogen) for sodium dodecyl sulfate PAGE. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Millipore Corp, Bedford, MA). Nonspecific binding was blocked by overnight incubation at 4 °C with 5% nonfat dry milk in PBS. The membranes were then incubated at room temperature for 2 h with anti-cyclin A, anti-cyclin D1, anti-α-SMA, anti-Erk, anti-p-Erk, anti-Akt, or anti-p-Akt antibodies at a dilution of 1:500. Anti-β-actin was used as an internal control for the immunoblot. The membranes were washed three times with PBS with Tween-20 and then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody at a final dilution of 1:1000. After final washes with 0.1% Triton X-100 in PBS, signals were detected by enhanced chemiluminescence following the manufacturer’s instructions (Pierce, Rockford, IL) and exposed to autoradiographic film.
Migration assay
Cell migration was determined using a transwell assay: 1×104 cells were placed in the upper chamber (Costar, Cambridge, MA) with a volume of 200 μl serum-free medium with various concentrations of TCS-401. Next, DMEM/F12 with 10% FBS was placed in the bottom chamber, with a volume of 600 μl per well. After 12 h incubation, the cells were fixed in 95% ethanol for 10 min, stained with hematoxylin for 5 min, and washed in Dulbecco’s calcium and magnesium free PBS (Gibco®; Invitrogen). The remaining cells on the upper surface of the filter were removed by wiping with a cotton swab. Then the filters were cut off, dehydrated using graded ethanol, hyalinized by dimethylbenzene, and fixed by neutral resins. Cell migration was quantified by the number of cells that migrated across the filter toward the lower surface in five random fields per filter under microscope. All migration assays were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS version 15.0 (SPSS, Chicago, IL). Data are expressed as mean±standard deviation. Statistical analysis was performed using one-way ANOVA and the student’s t test. p<0.05 was considered statistically significant.
Results
Detection of PTP1B in rat RPE cells in vivo
At present, many PTP isoforms have been identified in various cells, but not all tissues express all isoforms. There was no report on the expression of any PTPs in RPE cells until now. In this study, ICC was used to determine the relative expression of PTP1B in RPE stretched preparations (normal and retinal detachment areas). As shown in Figure 1A, retinal (one-half to one-third) detachments were created in the right eyes of the rats. In vivo, intense staining of PTP1B was observed in the cytoplasm of RPE cells (Figure 1B). However, the expression of PTP1B in RPE cells located at the retinal detachment area (Figure 1C) was significantly lower than that in RPE cells located at the non-detachment area (Figure 1B).
Figure 1.
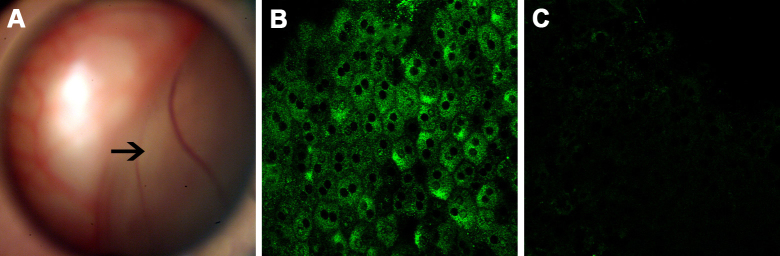
Immunolocalization of PTP1B in RPE cells of a rat model of retinal detachment. A: Fundus photograph shows half-side retinal detachment (arrow) without retinal breaks. B, C: Immunolocalization of PTP1B in stretched preparations of rat RPE (B: non-retinal detachment area, C: retinal detachment area). The data are representative of at least three independent experiments. Original magnification=400X. PTP1B, protein tyrosine phosphatase 1B; RPE, retinal pigment epithelium.
Inhibition of PTP1B increases proliferation of RPE cells in vitro
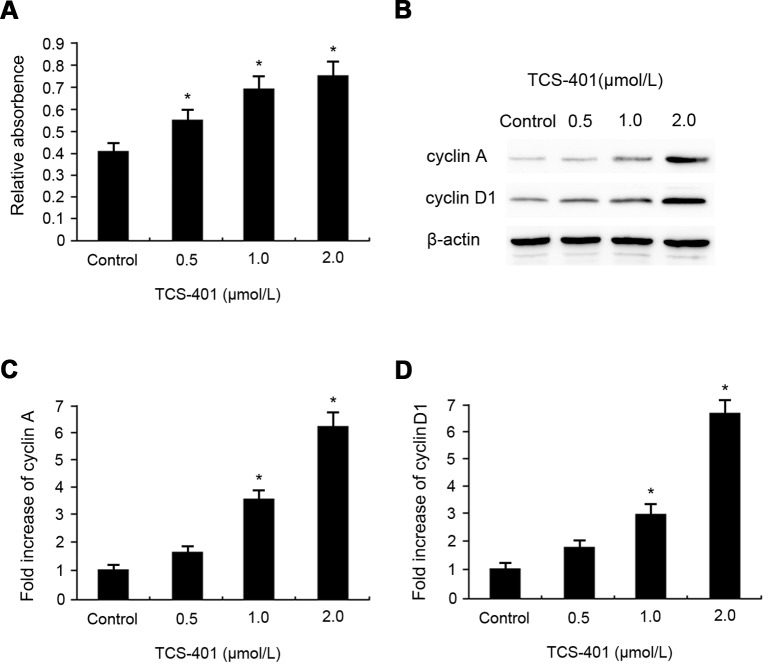
Experiments were performed to evaluate whether TCS-401 had any effect on the proliferation of RPE cells with MTT. Cells were incubated with TCS-401 at concentrations of 0.5, 1, and 2 μM for 24 h. Among the various concentrations of TCS-401 tested, TCS-401 at a concentration of 0.5, 1, or 2 μM was observed to significantly increase the proliferation of RPE cells compared to the control group (Figure 2A).
Figure 2.
Promotion effects of TCS-401 on the proliferation of rat RPE cells in vitro. A: Proliferation of RPE cells was determined with MTT after 24 h incubation with concentrations of 0.5, 1, and 2 μM TCS-401. The 0.5, 1, and 2 μM TCS-401 treatment increased the proliferation of RPE cells. *p<0.05, compared to the control group. B-D: Contact-inhibited RPE cells were incubated with 0.5, 1, and 2 μM TCS-401 for 24 h, and levels of cyclin A and cyclin D1 were determined with western blot analysis, respectively. The 1 and 2 μM TCS-401 treatment promoted cell cycle progression. The data represent the mean±standard deviation of three independent experiments. *p<0.05, compared to the control group. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetracolium bromide; RPE, retinal pigment epithelium.
The effects of TCS-401 on protein expression levels of cyclin A and cyclin D1 in RPE cells were examined. Contact-inhibited RPE cells were stimulated with fresh growth medium with various concentrations of TCS-401 (0.5, 1, and 2 μM) and incubated for 24 h. The results demonstrate that the basal levels of cyclin A and cyclin D1 were minimal. TCS-401 significantly increased the expression of cyclin A and cyclin D1 at the concentrations of 1 and 2 μM in a concentration-dependent manner (Figure 2B-D).
Inhibition of PTP1B regulates differentiation of RPE cells
The alteration in the cell phenotype was demonstrated by the expression of α-SMA, which is characteristic of the mesenchymal phenotype. Immunofluorescence and western blot evaluated α-SMA in rat RPE cells treated with TCS-401 at concentrations of 0.5, 1, and 2 μM for 24 h. The results demonstrate that the normal RPE cells exhibited little immunoreactivity and protein expression for α-SMA. However, the immunopositive reaction (Figure 3A) and protein expression (Figure 3B) were strengthened along with the increased concentration of TCS-401. Furthermore, Figure 3A-C and Figure 3A-D show that, like the effect of SOV studied in our previous research [15], the treatment of confluent cultures with TCS-401 also induced a concentration-dependent release of cell-cell contacts, resulting in the appearance of gaps between cells and a gradual loss of monolayer integrity.
Figure 3.
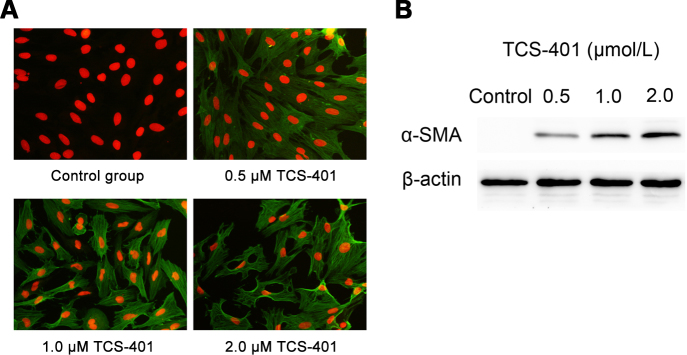
Promotion effects of TCS-401 on the expression of α-SMA in rat RPE cells in vitro. A: Immunolocalization of α-SMA in cultured rat RPE cells. RPE cells were stimulated with medium at different concentrations of TCS-401. ICC studies confirmed the TCS-401 concentration-dependent expression of α-SMA (green) and change in morphological characteristics of cells. PI staining (red) indicated nuclei. Original magnification=200X. B: Western blots showed levels of α-SMA proteins in rat RPE cells treated with TCS-401 at concentrations of 0.5, 1, and 2 μM for 24 h. The level of α-SMA protein rose along with higher concentrations. The data are representative of at least three independent experiments. α-SMA, α-smooth muscle actin; ICC, immunocytochemistry; PI, propidium iodide; RPE, retinal pigment epithelium.
Inhibition of phosphatase improves migration activity of RPE cells
Results of an in vitro migration assay are shown in Figure 4. Cells were measured in a transwell chamber in which RPE cells migrated through a porous membrane. The mean number of migrated cells in the TCS-401-treated RPE cells was significantly higher than the mean number of migrated control cells (Figure 4). The mean number increased along with the increased concentration of TCS-401 (0.5, 1, and 2 μM).
Figure 4.
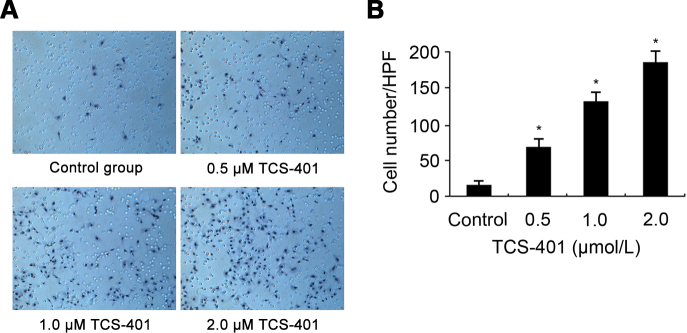
Migration of RPE cells in response to TCS-401 treatment. A: Migration assay confirmed that TCS-401 increased the number of migrated RPE cells. Original magnification=100X. B: The number of migrated cells per HPF is shown. The data represent the mean±standard deviation of three independent experiments. *p<0.05, compared to the control group. HPF, high-power field; RPE, retinal pigment epithelium.
Inhibition of PTP1B activates MEK/Erk and PI3K/Akt signaling pathways in RPE cells
To investigate whether a PTP1B blockade promotes Erk and Akt phosphorylation in RPE cells, various concentrations (0.5, 1, and 2 μM) of TCS-401 were added to these cells. It was found that TCS-401 at concentrations of 0.5, 1, and 2 μM significantly increased phosphorylation of Erk and Akt compared to the control group (Figure 5A-B). The activation of Erk and Akt by TCS-401 was blocked by pretreatment with PD98059 and LY294002, respectively (Figure 5C-F).
Figure 5.
Phosphorylation of Erk and Akt induced by TCS-401. A, B: RPE cells were incubated with 0.5, 1, and 2 μM TCS-401 for 30 min for an assay of Erk and Akt phosphorylation, and levels of phosphorylated and total Erk and Akt were determined with western blot analysis, respectively. C, D: RPE cells were pretreated with 20 μM PD98059 for 30 min and then incubated with 2 μM TCS-401 for 30 min for an assay of Erk phosphorylation using western blot. E, F: RPE cells were pretreated with 10 μM LY294002 for 30 min and then incubated with 2 μM TCS-401 for 30 min for an assay of Akt phosphorylation using western blot. The data represent the mean±standard deviation of three independent experiments. *, #p<0.05, compared to the control group; &p<0.05, compared to treatment with only TCS-401. Akt, protein kinase B; Erk, extracellular signal-regulated kinase; RPE, retinal pigment epithelium.
MEK/Erk and PI3K/Akt signaling pathways mediate the effect of inhibition of PTP1B on proliferation and migration of RPE cells
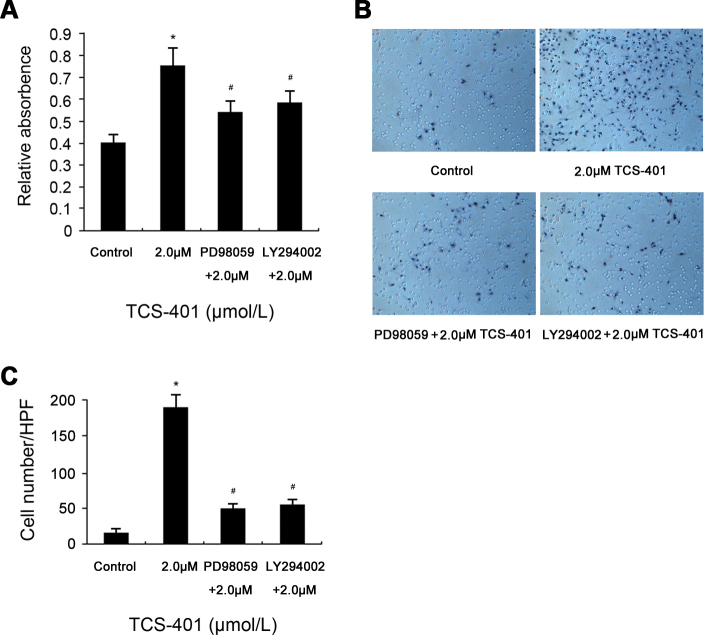
Having found that TCS-401 treatment activated the MEK/Erk and PI3K/Akt signaling pathways and induced proliferation, differentiation, and migration in RPE cells, it was examined whether the activation of the MEK/Erk and PI3K/Akt signaling pathways played a vital role in TCS-401-induced activation of RPE cells. MTT and migration assay were used to evaluate the role of MEK/Erk and PI3K/Akt signaling pathways on the proliferation and migration of RPE cells. As shown in Figure 6A, pretreatment of RPE cells with PD98059 or LY294002 significantly inhibited TCS-401-induced proliferation at a concentration of 2 μM. In addition, fewer migrated cells were observed in the pretreatment groups (treated with PD98059 or LY294002) than non-pretreatment group when the cells were treated with TCS-401 at a concentration of 2 μM (Figure 6B-C).
Figure 6.
The inhibitory effect of PD98059 and LY294002 on TCS-401-induced proliferation and migration in RPE cells. A: Inhibition of TCS-401-induced proliferation by pretreatment with 10 μM LY294002 and 20 μM PD98059 for 30 min, as measured with MTT after 24 h incubation. B: Inhibition of TCS-401-induced migration by pretreatment with 10 μM LY294002 and 20 μM PD98059 for 30 min, as measured with a transwell assay after 12 h incubation. Original magnification=100X. C: The number of migrated cells per HPF was shown. The data represent the mean±standard deviation of three independent experiments. *p<0.05, compared to the control group. #p<0.05, compared to the treatment with only TCS-401. HPF, high-power field; RPE, retinal pigment epithelium.
Discussion
Retinal detachment followed by blood-retinal barrier breakdown and EMT of RPE cells contribute to the formation of fibrous membranes [21,22]. In this process, RPE cells lose typical epithelial features and acquire mesenchymal features that promote migratory capacity, invasiveness, and elevated resistance to apoptosis [23,24]. It was proved that the inhibition of PTPs enhanced physiologic functions such as growth, proliferation, differentiation, and motility in RPE cells [15]. Here, it was demonstrated that the deficiency of PTP1B is a strong inducer of EMT in RPE cells.
It was found that trans-scleral injection of sodium hyaluronate into the SRS (mimicking retinal detachment) downregulated the expression of PTP1B in RPE cells in vivo. Animals that developed choroidal hemorrhage were excluded because the serum of the extravasated blood contained chemoattractants to RPE cells [25]. Retinal breaks were not made in this experimental model because this investigation focused on the induced changes in the expression of PTP1B in RPE cells, and any additional interference deriving from the vitreous was avoided. These results revealed that normal rat RPE cells expressed PTP1B abundantly. However, the level of expression of PTP1B in the detachment areas may not be detected by this immunoreactive technique. The prominent difference in the expression of PTP1B between them suggests that retinal detachment may promote substrate phosphorylation related to EMT in RPE cells. The downregulation of PTP1B in retinal detachment areas may contribute to phosphorylation and the activation of several plasma membrane-associated receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR), which seems to be an important feature of the pathogenesis of PVR [26-28].
To verify the role of depletion of PTP1B on EMT, the biologic behaviors of RPE cells in vitro were observed, using TCS-401 (a selected inhibitor of PTP1B). First, it was found that, when RPE cells were incubated with various concentrations of TCS-401, cell proliferation was significantly increased at concentrations of 0.5, 1 and 2 μM, as determined with MTT. Exposure of confluent cells to TCS-401 promoted cell cycle progression, as seen by the results of protein expression levels of cell cycle regulatory factors, including cyclin A and cyclin D1. This finding suggests that the activity of PTP1B must help mediate the suppression of cell cycle entry in the RPE monolayer. Second, EMT is also associated with the enrichment of mesenchymal proteins, such as α-SMA, a highly conserved protein and a major component of microfilaments that control cell morphology and motility [29-31]. This study showed that the TCS-401-induced inhibition of PTP1B enhanced the expression of α-SMA in RPE cells at concentrations of 0.5, 1, and 2 μM. The associated upregulation of α-SMA contributes to fibrous membrane contractility [3]. Finally, the effect of TCS-401 on the migration of RPE cells was determined using a transwell assay. Treatment with TCS-401 significantly increased the migration of RPE cells at concentrations of 0.5, 1, and 2 μM. Hence, PTP1B may be involved in the regulation of proliferation, differentiation, the migration of RPE cells, and the re-entry of contact-inhibited rat RPE cells into the cell cycle.
Phosphorylation of Erk and Akt is involved in proliferation, angiogenesis, migration, and vascular remodeling [32-34]. Furthermore, phosphorylation of some plasma membrane-associated receptor tyrosine kinases promotes the proliferation and survival of RPE cells, signaling through the MEK/Erk and PI3K/Akt pathways [35]. However, the signaling mechanism of PTP1B in RPE cells is unclear. To gain further insight into the molecular mechanisms by which inhibition of PTP1B induces proliferation, the expression of α-SMA, and migration in RPE cells, intracellular signaling pathways were examined. It was found that treatment of RPE cells with various concentrations of TCS-401 significantly increased phosphorylation of Erk and Akt. Treatment with the MEK/Erk inhibitor PD98059 and PI3K inhibitor LY294002 blocked the phosphorylation of Erk and Akt in RPE cells, indicating that the activation of Erk and Akt depend on PI3K and MEK. Next, the functional involvement of the MEK/Erk and PI3K/Akt signaling pathways in TCS-401-induced proliferation and migration of RPE cells was examined. It was found that treatment with PD98059 and LY294002 partially blocked TCS-401-induced proliferation and migration in RPE cells.
To our knowledge, we are the first to explore PTP1B expression in RPE. In this study, PTP1B played a role in proliferation, myofibroblast differentiation, and migration of the RPE cells. PTP1B-induced protein tyrosine phosphorylation and dephosphorylation regulate the activity of RPE cells and may be important physiologic mechanisms of initiation of EMT.
Acknowledgments
The authors thank Dr. Jing Lin, Nan Jiang, Qian Wang, Qiang Xu, and Sheng Qiu for their expert assistance. This study was supported by Shandong Natural Science Foundation (No. ZR2012HQ004), the Research Fund for Fundamental Research Project of Qingdao (No. 1314180jch), the Scientific Research Fund of Huangdao District of Qingdao City (No. 2014174) and the Young People Scientific Research Fund of Affiliated Hospital, Qingdao University (No.QDFY134). The authors report no declarations of interest.
References
- 1.Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, Sassa Y, Arita R, Miyazaki M, Enaida H, Oshima Y, Murakami N, Niiro H, Ono J, Matsuda A, Goto Y, Akashi K, Izuhara K, Kudo A, Kono T, Hafezi-Moghadam A, Ishibashi T. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J. 2014;28:131–42. doi: 10.1096/fj.13-229740. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–41. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- 3.Pastor JC, de la Rúa ER, Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002;21:127–44. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 4.Feist RM, Jr, King JL, Morris R, Witherspoon CD, Guidry C. Myofibroblast and extracellular matrix origins in proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2014;252:347–57. doi: 10.1007/s00417-013-2531-0. [DOI] [PubMed] [Google Scholar]
- 5.Hou Q, Tang J, Wang Z, Wang C, Chen X, Hou L, Dong XD, Tu L. Inhibitory effect of microRNA-34a on retinal pigment epithelial cell proliferation and migration. Invest Ophthalmol Vis Sci. 2013;54:6481–8. doi: 10.1167/iovs.13-11873. [DOI] [PubMed] [Google Scholar]
- 6.Parrales A, López E, Lee-Rivera I, López-Colomé AM. ERK1/2-dependent activation of mTOR/mTORC1/p70S6K regulates thrombin-induced RPE cell proliferation. Cell Signal. 2013;25:829–38. doi: 10.1016/j.cellsig.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Bastiaans J, van Meurs JC, van Holten-Neelen C, Nagtzaam NM, van Hagen PM, Chambers RC, Hooijkaas H, Dik WA. Thrombin induces epithelial-mesenchymal transition and collagen production by retinal pigment epithelial cells via autocrine PDGF-receptor signaling. Invest Ophthalmol Vis Sci. 2013;54:8306–14. doi: 10.1167/iovs.13-12383. [DOI] [PubMed] [Google Scholar]
- 8.Casaroli-Marano RP, Pagan R, Vilaró S. Epithelial-mesenchymal transition in proliferative vitreoretinopathy: intermediate filament protein expression in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:2062–72. [PubMed] [Google Scholar]
- 9.Mony S, Lee SJ, Harper JF, Barwe SP, Langhans SA. Regulation of Na,K-ATPase β1-subunit in TGF-β2-mediated epithelial-to-mesenchymal transition in human retinal pigmented epithelial cells. Exp Eye Res. 2013;115:113–22. doi: 10.1016/j.exer.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HC, Zhu YT, Chen SY, Tseng SC. Wnt signaling induces epithelial-mesenchymal transition with proliferation in ARPE-19 cells upon loss of contact inhibition. Lab Invest. 2012;92:676–87. doi: 10.1038/labinvest.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Ubreva FJ, Cariaga-Martinez AE, Cortés MA, Romero-De Pablos M, Ropero S, López-Ruiz P, Colás B. Knockdown of protein tyrosine phosphatase SHP-1 inhibits G1/S progression in prostate cancer cells through the regulation of components of the cell-cycle machinery. Oncogene. 2010;29:345–55. doi: 10.1038/onc.2009.329. [DOI] [PubMed] [Google Scholar]
- 12.Medgyesi D, Hobeika E, Biesen R, Kollert F, Taddeo A, Voll RE, Hiepe F, Reth M. The protein tyrosine phosphatase PTP1B is a negative regulator of CD40 and BAFF-R signaling and controls B cell autoimmunity. J Exp Med. 2014;211:427–40. doi: 10.1084/jem.20131196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonks NK. PTP1B: from the sidelines to the front lines! FEBS Lett. 2003;546:140–8. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen WL, Lin CT, Lo HF, Lee JW, Tu IH, Hu FR. The role of protein tyrosine phosphorylation in the cell-cell interactions, junctional permeability and cell cycle control in post-confluent bovine corneal endothelial cells. Exp Eye Res. 2007;85:259–69. doi: 10.1016/j.exer.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Du ZD, Hu LT, Hu YT, Ma ZZ. The effects of sodium orthovanadate-induced phosphatase inhibition on rat retinal pigment epithelium cell activity. Cutan Ocul Toxicol. 2010;29:261–8. doi: 10.3109/15569527.2010.509851. [DOI] [PubMed] [Google Scholar]
- 16.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–11. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- 18.Xu G, Arregui C, Lilien J, Balsamo J. PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J Biol Chem. 2002;277:49989–97. doi: 10.1074/jbc.M206454200. [DOI] [PubMed] [Google Scholar]
- 19.Chen HJ. Ma ZZ. N-cadherin expression in a rat model of retinal detachment and reattachment. Invest Ophthalmol Vis Sci. 2007;48:1832–8. doi: 10.1167/iovs.06-0928. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RB. Culture of mammalian retinal pigment epithelium and neural retina. Methods Enzymol. 1982;81:39–43. doi: 10.1016/s0076-6879(82)81008-2. [DOI] [PubMed] [Google Scholar]
- 21.Ricker LJ, Dieri RA, Beckers GJ, Pels E, Liem AT, Hendrikse F, Kijlstra A, Hemker HC, La Heij EC. High subretinal fluid procoagulant activity in rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2010;51:5234–9. doi: 10.1167/iovs.10-5354. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Wang H, Wang F, Gu Q, Xu X. Snail involves in the transforming growth factor β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS ONE. 2011;6:e23322. doi: 10.1371/journal.pone.0023322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campochiaro PA, Jerdan J, Glaser BM. Serum contains chemoattractants for human retinal pigment epithelial cells. Arch Ophthalmol. 1984;102:1830–3. doi: 10.1001/archopht.1984.01040031488029. [DOI] [PubMed] [Google Scholar]
- 26.Ishino Y, Zhu C, Harris DL, Joyce NC. Protein tyrosine phosphatase-1B (PTP1B) helps regulate EGF-induced stimulation of S-phase entry in human corneal endothelial cells. Mol Vis. 2008;14:61–70. [PMC free article] [PubMed] [Google Scholar]
- 27.Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–72. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- 28.Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Chien MW, Chen JT. Glucosamine inhibits epidermal growth factor-induced proliferation and cell-cycle progression in retinal pigment epithelial cells. Mol Vis. 2010;16:2559–71. [PMC free article] [PubMed] [Google Scholar]
- 29.Bi WR, Jin CX, Xu GT, Yang CQ. Effect of alendronate sodium on the expression of mesenchymal-epithelial transition markers in mice with liver fibrosis. Exp Ther Med. 2013;5:247–52. doi: 10.3892/etm.2012.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin AK, Cain SA, Lennon R, Godwin A, Merry CL, Kielty CM. Epithelial-mesenchymal status influences how cells deposit fibrillin microfibrils. J Cell Sci. 2014;127:158–71. doi: 10.1242/jcs.134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Si Y, Wang J, Guan J, Han Q, Hui Y. Platelet-derived growth factor induced alpha-smooth muscle actin expression by human retinal pigment epithelium cell. J Ocul Pharmacol Ther. 2013;29:310–8. doi: 10.1089/jop.2012.0137. [DOI] [PubMed] [Google Scholar]
- 32.Chung BH, Kim JD, Kim CK, Kim JH, Won MH, Lee HS, Dong MS, Ha KS, Kwon YG, Kim YM. Icariin stimulates angiogenesis by activating the MEK/ERK-and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem Biophys Res Commun. 2008;376:404–8. doi: 10.1016/j.bbrc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Qin D, Zheng XX, Jiang YR. Apelin-13 induces proliferation, migration, and collagen I mRNA expression in human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol Vis. 2013;19:2227–36. [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Qiu T, Zhang P, Wang X, Yin Y, Li S. IKVAV regulates ERK1/2 and Akt signalling pathways in BMMSC population growth and proliferation. Cell Prolif. 2014;47:133–45. doi: 10.1111/cpr.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan F, Hui YN, Li YJ, Guo CM, Meng H. Epidermal growth factor receptor in cultured human retinal pigment epithelial cells. Ophthalmologica. 2007;221:244–50. doi: 10.1159/000101926. [DOI] [PubMed] [Google Scholar]