Abstract
Background: The Rosemary extract (RE) possesses various antioxidant, cytoprotective and cognition- improving bioactivities. In this study, we postulated which doses of RE have a more effect on the hippocampus of middle-aged rats.
Methods: In this experimental study, thirty-two middle-aged male Wistar rats were fed by different doses (50,100 and 200 mg/kg/day) of RE (containing 40% carnosic acid) or distilled water for 12 weeks. The effects of different RE doses on learning and spatial memory scores, hippocampal neuronal survival, antioxidant enzymes and lipid peroxidation amount were evaluated by one and two way analysis of variance (ANOVA).
Results: It seemed that RE (100mg/kg) could recover the spatial memory retrieval score (p< 0.05). The amount of activity of SOD, GPx and CAT enzymes in the hippocampus of animals of the RE (100mg/kg) group showed a significant increase compared to the normal group (p< 0.01), (p< 0.01) and (p< 0.05), respectively. Also, the amount of activity of GPx in the RE (50 mg/kg) group of animals showed a significant increase compared to the normal group (p< 0.05). No significant difference was found between the groups in the MDA level.
Conclusion: The results revealed that rosemary extract (40% carnosic acid) may improve the memory score and oxidative stress activity in middle aged rats in a dose dependent manner, especially in 100mg/kg.
Keywords: Rosemary Extract, Morris Water Maze, Antioxidant Enzyme, Hippocampus, Rat
Introduction
In recent decades, traditional medicine has become more popular. A main reason of this popularity is that herbs are rich in natural antioxidants (1). Natural antioxidants are not usually toxic compared to synthetic antioxidants. Antioxidants have an important role in the prevention of many diseases and aging. These materials are capable of blocking initiation or propagation of oxidizing chain reactions (2).
The Lamiaceae family seems to be a rich source of large amounts of phenolic acids, so it is revealed to be a potential source of natural antioxidant (3). Rosemary (Rosmarinus officinalis L. family Lamiaceae) is a spice and medicinal herb which is widely used around the world (4).
Several studies have found some beneficial effects for rosemary leaves such as anti-inflammatory, anti-viral, cytoprotective and anti-tumoral effects (5). Rosemary contains numerous compounds including diterpenes, triterpenes, flavones and steroids. The main compounds, which cause RE efficacy, are two phenolic diterpenes: carnosic acid (CA) and carnosol (about 90% of antioxidant activity) (6).
CA is a lipophilic antioxidant that removes the most free radicals. Free radicals atoms and molecules that have an unpaired electron, including superoxide radical anion, hydroxyl radicals and lipid peroxyl radicals may produce oxidative damage directly to critical biological molecules. Free radicals are generally the by-products of various endogenous processes that can have a noxious effect on cell macromolecules, such as proteins, lipoproteins, carbohydrates, DNA and RNA (7).
Researches showed that CA protects cortical neurons by activating the Keap1/Nrf2 pathway (8). On the other hand, Satoh et al. suggested that the neuroprotective effects of CA critically require both free carboxylic acid and catechol hydroxyl moieties. Thus, neuroprotective effects of CA may be due to its hydrophilicity (9).
Oxidative damage is particularly detrimental to the brain, because brain weighs about 2% of the body mass, but it utilizes 20% of the total oxygen consumption. Meanwhile, except for some limited areas of the brain, the neuronal cells are largely post-mitotic and tend to accumulate oxidative damage. The brain is enriched by polyunsaturated fatty acids, which are peroxidizable. In addition, the brain contains large amounts of ascorbate and iron, which are important catalysts for lipid peroxidation. Also, It has moderate amounts of endogenous antioxidant enzymes, superoxide dismutase and glutathione peroxidase and a very low level of catalase activity (10).
Hippocampus is a part of the brain which plays an important role in cognition, mood regulation, response to stress, learning and memory (11). The Hippocampus is one of the most vulnerable brain regions to oxidative stress (12).
Numerous enzymatic and non-enzymatic antioxidant defense systems exist in cells to protect them from the damaging effects of free radical reactions (13). Since the endogenous antioxidant protection systems are not 100% effective, we assume that nutritional antioxidants could have beneficial effects on the memory, neurogenesis and activities of enzymatic oxidative in the brain.
The main goal of this study was to determine which RE dose has the best potency to improve the memory and antioxidant status of the hippocampus in middle-aged rats. We focused on the effect of different RE doses ( 50mg, 100mg and 200mg) on learning and spatial memory in rats by Morris Water Maze test, pyramidal cells survival of CA1 area in hippocampus, activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and lipid peroxidation (MDA).
Methods
Data
Male Wistar rats (n= 32) weighing 250-330g (middle-aged: 9 months) were obtained from the Animal Care Center of Iran University of Medical Sciences. These rats were housed (3/cage) under controlled environmental conditions 12:12-h light–dark. The room temperature was maintained at 21–23 ºC; and they were fed by standard pellet rat chow and had free access to tap water. All experiments were conducted according to the policy of Ethics Committee of the Iran University of Medical Sciences.
The rats were randomly divided into four groups (n= 8 per group): one normal and three RE groups of rats. For the RE groups, rosemary extract was dissolved in distilled water in different doses (50,100 and, 200 mg/kg) and was administered (1mL) orally as a single daily dose for twelve weeks. The normal group received 1 mL of distilled water every day using the same protocol.
Materials
The rosemary extract (RE) containing 40% carnosic acid was purchased from Hunan Geneham Biomedical Technological Company of China, (RAP20-110401). Cresyl violet acetate was purchased from Sigma Chemical Co. Saint Louis, Missouri USA. SOD, GPx, and, CATA kits were obtained from Randox, UK.
Behavioral Assessment
Morris water maze (MWM) test was performed for all the rats after 12-weeks and after they completed the treatment period. This test is used to evaluate learning and spatial memory in rodents (14,15). Behavioral tests were done two hours after RE gavage.
Morris Water Maze Test: This test was carried out in a quiet room containing multiple external cues (e.g., a white door and a colorful poster on the wall). The water maze was made up from a stainless steel black circular tank with a diameter of 210 cm and 50 cm in height. The tank was filled with 25 cm deep water (20±1ºC). The maze was divided geographically into four quadrants: Northeast, Northwest, Southeast and Southwest. A hidden circular black platform with a diameter of 10cm was located in the N.W quadrant center, 20 cm away from the sidewall and submerged 1 cm below the water surface. A video camera was mounted directly above the water maze to record the rats’ swim path.
Acquisition Testing (Learning Phase): This process was previously described by Jacobson (16). The starting position was randomly changed and the rats received four trials each day for five consecutive days. During each trial, each rat was placed in the water with its head pointed toward the sidewall and was given 60s to find the hidden platform. If the rat found the platform, it was allowed to remain on it for 20s. If the rat was unable to locate the platform within 60 s, it was guided to the platform. During the acquisition phase, the platform position remained unchanged. The traveled distance, escape latency and swimming speed of each rat were calculated (Figs. 1, 2).
Fig. 1 .
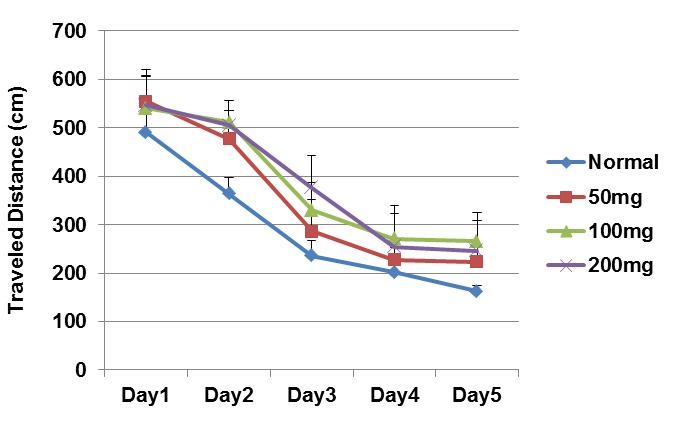
Traveled Distance in Different Days of the Learning Phase in the Treatment Groups (mean ± SEM).
Fig. 2 .
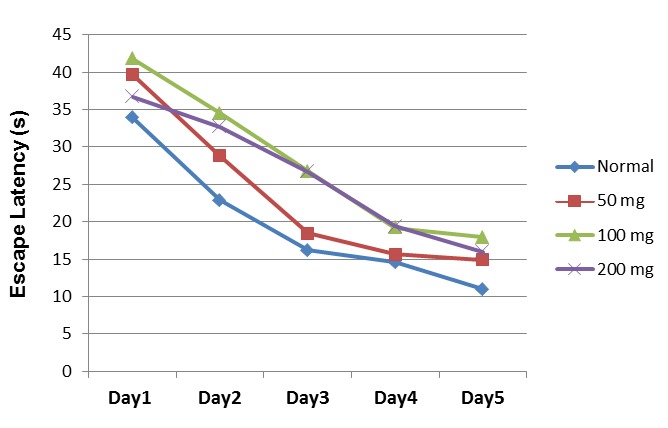
Escape Latency in Different Days of the Learning Phase in the Treatment Groups (mean ±SEM).
Spatial Probe Test (Memory Retrieval): On the 6th day of the experiment, the platform was removed and during 60 seconds, the time spent in this zone was recorded and analyzed for retention of spatial reference memory (Fig. 3).
Fig. 3 .
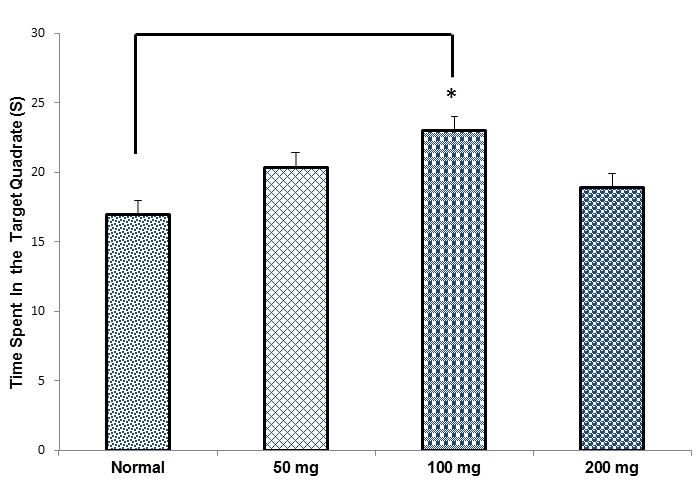
Comparing The Mean Time Spent in the Target Quadrant in the Treatment Groups in the Probe Test (Mean ± SEM). *p< 0.05.
Histological Study
After the behavioral tests, the animals (n= 3 in each group) were perfused transcardially, with 4% paraformaldehyde. Then the brains were placed in the post-fix solution overnight. The samples were processed and embedded in the paraffin. Coronal sections (4µ thickness) were prepared by a microtome rotary (LEICA RM 2235) and were stained with Nissl staining (cresyl violet acetate: 0/01%) (17). The pyramidal neurons in the CA1 region of the hippocampus were counted in 3 sections (which found between -2.8 to -3.1mm from bregma) for each animal (Fig. 4).
Fig. 4 .
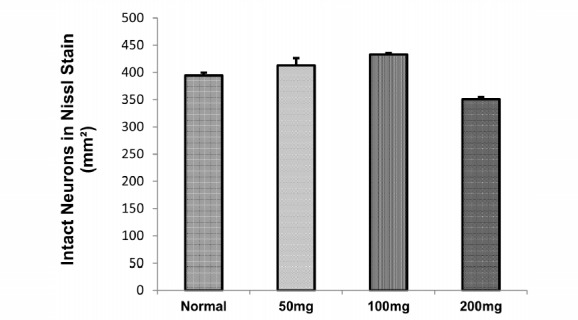
Density of Neurons in CA1 Area of the Hippocampus
Antioxidant Enzymes Detect
After the behavior tests, the animals (n= 4 in each group) were anesthetized with ketamine (150mg/kg) and xylazine (15mg/kg), then were decapitated (by animal guillotine). The brains were immediately removed, and the hippocampus was isolated; all samples were washed in an ice-cold phosphate buffer saline (PBS). The washed samples were immediately immersed in liquid nitrogen and stored at −80º C until biochemical analysis. On the day of use, the frozen tissue samples were quickly weighed and homogenized 1:10 (W/V) in ice-cold tris-Hcl centrifuged at 12000 × g for 20 min at 4º C. The supernatants were separated and used for enzyme activity assay and lipid peroxidation level.
Superoxide Dismutase (SOD) Activity
SOD activity was determined by method of Misra and Fridovich (18). In this process, an aliquot of 0.25 ml ice cold chloroform was added to 0.1 ml of supernatant followed by adding 0.15 ml ice cold ethanol. The mixture was centrifuged at 3000 rpm for 10 minutes at 4 °C. Then 0.2 ml of supernatant was taken and 1.5 ml carbonate buffer, 0.5CC EDTA, and 0.8 ml distilled water was added. Reaction was started by adding 0.4 ml epinephrine. Change in absorbance ΔOD/min at 480 nm was read for 3 minutes. The results were expressed in terms of nmol/min/mg protein.
Glutathione Peroxidase (GPx) Activity
GPx activity was determined spectrophotometrically based on Wheeler method (19) and using Randox kit recommendations. The reaction was initiated by adding 0.2mM H2O2, 1.0mM GSH, 0.14U of glutathione reductase GR, 1.5mM NADPH, 1.0mM sodium azide, 0.1M phosphate buffer (pH= 7.4), and 1 mg/ml of supernatant hippocampus at room temperature. Enzyme activity was calculated as the nmol of oxidized nicotinamide adenin dinucleotide phosphate. Absorbance changes were recorded at 340 nm.
Catalase (CAT) Activity
CAT activity was measured based on enzyme ability to break down H2O2. The method was performed according to modified method of Aebi (20). For this purpose, the tissues were homogenized in isotonic buffer (pH= 7.4) and were centrifuged at 1,000 g for 10 minutes. A volume of 20µl 100-fold diluted supernatant was added to 980 µl assay mixture, containing 10mmol/l of H2O2 (900µl), Tris-HCl buffer (50µl, pH= 8), and distilled water (30µl). The rate of H2O2 decomposition was monitored spectrophotometrically at 240nm.
Lipid Peroxidation (LP) Level
One of the products of LP is malondialdehyde (MDA). Lipid peroxidation level was determined by Satoh method (21). This method was used to measure, spectrophotometrically, the color produced by TBA and MDA reaction. The supernatant of hippocampus was precipitated by trichloroacetic acid (TCA), and the mixture was heated with thioburbituric acid in 2M sodium sulphate in a boiling water bath for 30 minutes. The resulting chromogenic color which was extracted by n-butyl alcohol and the sample absorbance was measured at 530 nm. MDA levels were expressed as nm/mg protein.
Statistical Analysis
Data were evaluated by one way analysis of variance (ANOVA) followed by the Tukey's post hoc test. Repeated ANOVA were run for MWM traveled distance and escape latency data. Analyses were performed using SPSS. Significance level was set at p< 0.05. Values were expressed as mean ± SEM.
Results
Behavioral Test (MWM)
The predescribed test had two stages, acquisition test (learning) and probe test (retention of spatial memory). In the first stage, we measured traveled distance, escape latency and swimming speed. In the latter stage, time spent in the target quadrant was calculated.
Traveled Distance
Traveled distance to find the platform is one of the parameters evaluated in MWM, which describes the learning process. The results, analyzed by repeated measures ANOVA, were F(3,141)= 4.017, (p=0.008); F(4,970)= 27.49, (p= 0.001); and F(12,45)= 1.3, (p= 0.221) for groups, days and groups-days, respectively.
No significant difference was observed between the normal and treatment groups during the five-day learning period with respect to the traveled distance scores (one way ANOVA) (Fig. 1).
Escape Latency
Escape latency is the time required by a rat to find the target platform. The results, analyzed by repeated measures ANOVA, were F(3,94)= 6.83, (p= 0.001); F(4,37)= 26.85, (p= 0.001(; F(12,71)= 0.517 (p= 902) for groups, days and groups-days, respectively.
No considerable difference was found between the normal and treatment groups during the five-day learning process with respect to escape latency (one way ANOVA) (Fig. 2).
Swimming Speed
The mean±SEM of swimming speed scores in the normal, RE (50mg/kg), RE (100mg/kg), and RE (200mg/kg) groups were 12.64±0.31, 13.91±0.34, 12.99±0.39, and 13.04±0.46, respectively. No significant difference was observed between all groups with respect to the swimming speed.
Time Spent in the Target Quadrant
On the 6th day of the MWM test (probe test), or on the retention day, the time spent in the target quadrant was tested. The time spent (mean±SEM) in the normal, RE (50mg/kg), RE (100mg/kg) and RE (200mg/kg) groups were 16.98±1.09, 20.34±1.68, 22.97±1.4 and 18.87±1.8, respectively.
The animals in the RE (100mg/kg) group spent a significantly (by one way analysis of variance: ANOVA) more time in the target quadrant compared to the normal group (*p< 0.05), (Fig. 3).
Density of Neurons in Nissl Staining
Figure 5 demonstrates the nissle staining in different groups. Analysis of variance of our results revealed no significant difference in the number of pyramidal cells in the hippocampus CA1 in different groups (Fig. 4).
Fig. 5 .
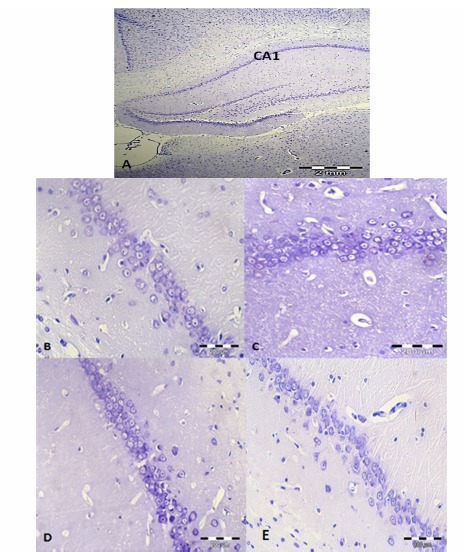
Nissl Staining in the Hippocampus in the Treatment Groups. A: CA1 Region in the Hippocampus (40× magnification), B, C, D & E: CA1 Region of Hippocampus in Normal, RE (50mg/kg), RE (100mg/kg) and RE (200mg/kg) Groups Respectively (400× Magnification).
Figure 5 presents the Nissl staining in CA1 area of the hippocampus in all groups.
Antioxidant Enzymes
The SOD activity (mean±SEM) in the normal, RE (50mg/kg), RE (100mg/kg), and RE (200mg/kg) groups were 24.6±0.8, 27±1.7, 31.76±0.7, and 22.6±1.2, respectively. One way analysis of variance (ANOVA) level of SOD activity showed a significant increase in 100mg between the treatment and normal groups compared to the normal group (p< 0.01) (Fig. 6A).
Fig. 6 .
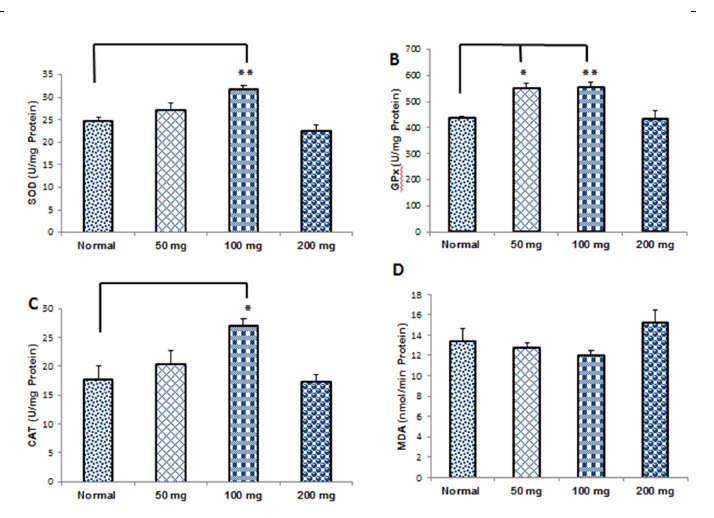
Comparison of Oxidative Enzymes Activities and Malondialdehyde Level in the Treatment Groups (Mean±SEM). The Superoxide Dismutase (SOD): (A), Glutathione Peroxidase (GPx): (B), Catalase (CAT): (C) and the Levels of Malondialdehyde (MDA): (D) ,* p< 0. 05, **p< 0. 01.
The mean±SEM of GPx activity in the normal, RE (50mg/kg), RE (100mg/kg) and RE (200mg/kg) groups were 436.6±7.7, 549.6±18.27, 556±18, and 432±3146, respectively. The results indicated that GPx activity was increased significantly in RE (50mg/kg) and RE (100mg/kg) groups compared to the normal group (p< 0.05), (p< 0.01), respectively (Fig. 6B).
The mean±SEM of CAT activity in the normal, RE (50mg/kg), RE (100mg/kg), and RE (200mg/kg) groups were 17.8±2.26, 20.33±2.48, 27.16±1.16, and 17.45±1.12, respectively. The increase of CAT activity was significant in the RE (100mg/kg) compared to the normal group (p< 0.05) (Fig. 6C).
The mean±SEM of lipid peroxidation level (MDA) in the normal, RE (50mg/kg), RE (mg/kg) and RE (200mg/kg) groups were 13.45±1.24, 12.83±0.38, 12.06±0.38 and 15.23±1.25, respectively. The level of lipid peroxidation (MDA) showed no significant difference between the treatment and normal and RE groups (Fig. 6D).
Discussion
The aim of this study was to examine the effect of administration of different doses (50, 100 and 200mg/kg) of RE on spatial memory and learning scores, density of neurons in the CA1 area of hippocampus, the oxidative stress status and level of lipid peroxidation in hippocampus in middle-aged rat.
The diet supplement with natural antioxidant properties was shown to have beneficial effects (De la Fuente and Victor, 2000). Antioxidants can inhibit the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance by an oxidizing agent. Oxidation reactions can produce free radicals. Antioxidants are various substances that show remarkable radical scavenging effects even at low concentrations (22).
One natural compound that possesses high levels of antioxidants is found in the Rosemary plant. This plant has been widely used as a preservative in the food industry (23).
The most important components in RE are phenolic diterpenes such as carnosic acid (CA), carnosol and other phenolic acids (24). CA is a lipophilic antioxidant with the ability to prevent lipid peroxidation, and it avoids the disruption of the biological membrane by scavenging free radicals (25).
It is believed that hippocampus play an essential role in spatial memory formation and is highly vulnerable to oxidative damage. The maintenance of a normal redox state in the hippocampus is important for the prevention of cognitive functions decline in aging (26).
Rasoolijazi et al. suggested that CA (10mg/kg) may improve the memory and learning scores which were decreased by the toxicity of β-amyloid in the rat hippocampus (27).
Memory is often considered to be a process that has several stages including acquisition, consolidation and retrieval (28). Performance of MWM test is a key technique to investigate the hippocampal circuitry, and also it has been linked to long-term potentiation (LTP) and N-methyl-D-aspartate (NMDA) receptor function (15).
This study found that animals in all groups traveled longer distances and spent lesser time to find the platform during the five-day learning phase. However, no significant difference was found in this score between the groups. On the other hand, in the probe test stage, animals in the RE group (100mg/kg) spent more time in the target quadrant compared to the normal group (p< 0.05).
Recent studies have suggested that changes in diet can have positive consequences on neurogenesis, learning and memory as well as the cognitive function. Polyphenolic compounds are phytochemicals which can decrease the reactive oxygen species (ROS) production known for their biological antioxidant capacity and neuro-protective effects (29).
Zanella et al. found that treatment with 150 and 300 mg/kg of rosemary extract (hydro-alcoholic) improves new social short-term memory. Also, they suggested that treatment with 150 mg/kg of rosemary extract improves long-term memory when ordered in the consolidation phase of learning (30).
There are two neurogenic areas in the brains of adults: the sub-ventricular zone and the sub-granular layer (in dentate gyrus of hippocampus). Thus, the adult brain tissue has more ability for plasticity at the cellular level than it was thought in the past. It is believed that newly formed neurons can play a role in learning and memory (31).
In this study, the results from the probe test stage showed that animals in the RE (100mg/kg) group spent significantly more time in the target quadrant compared to the normal group. Therefore, it seems that at least a part of this memory improvement is due to the induction of neurogenesis by RE in the hippocampus. Drapeau et al. found a quantitative relationship between neurogenesis and memory capability in rodents (32).
Other researchers suggested that i.p injection of rosemary extract for 30 days (100mg/kg) can have neuroprotective effects against acrylamide induced neurotoxicity injury (33).
Our results showed that although treatment for 12 weeks with different doses of RE had no significant effect on the density of neurons in the CA1 region of the hippocampus between the groups, we observed a 14% reduction of neuron density in the RE group (200mg/kg) compared to the normal group. A research conducted in 2012, revealed a clear morphological evidence of dose dependent (250mg and 500mg) antifertility potential of rosemary in male albino rats (34). Therefore, using RE in high doses may have destructive effects on tissues.
Antioxidant enzymes like superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) have a noticeable role in the management of oxidative stress within the cell. Analysis of these antioxidant enzymes as well as the contents of lipid peroxidation may yield a snapshot of oxidative stress (2).
Based on the results of this study, SOD and CAT activities significantly increased in the animals in the RE (100mg/kg) group compared to the normal group (p< 0.05 & P<0.01 respectively). Also, GPx activity increased significantly in RE (50 and 100mg/kg) groups compared to the normal group (p< 0.05 & p< 0.01, respectively).
Another research found that the rosemary extract which is added to the diet of quails may alter the antioxidant activity in the liver and spleen tissues (35). Also, Posadas et al. indicated that supplementing the diet with RE (CA rich) reduces oxidative stress in the brain tissue of the aged rats (6).
On the other hand, our results in MDA level showed 13.2 % increase in the animals in the ER (200mg) group compared to the normal group.
Devi and et al. showed that vitamin E as an antioxidant, not only increased the antioxidant enzymes activities such as SOD, GPx and CAT, but also reduced MDA content in the hippocampus of the middle-aged rat (36).
Conclusion
Our results demonstrated that administration of rosemary extract (100mg/kg) for 12 weeks in middle-aged rats caused an increase in the score of spatial memory in MWM test and also an increase in the activity of SOD and CAT antioxidant enzymes. In addition, two doses of rosemary extract (50 and 100mg/kg) could increase the GPx activity.
This study suggests that consumption of a specified dose of rosemary extract may have useful effects on the memorial functions and the antioxidant capacity of the hippocampus in adolescents.
Conflict of interests
The authors declare no conflict of interest concerning the publication of this paper.
Cite this article as: Rasoolijazi H, Mehdizadeh M, Soleimani M, Nikbakhte F, Eslami Farsani M, Ababzadeh Sh. The effect of rosemary extract on spatial memory, learning and antioxidant enzymes activities in the hippocampus of middle-aged rats. Med J Islam Repub Iran 2015 (9 March). Vol. 29:187.
References
- 1.Abu-Al-Basal Mariam A. Healing potential of Rosmarinus officinalis L on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. Journal of ethnopharmacology. 2010;131(2):443–450. doi: 10.1016/j.jep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology. 2010;278(1):55–67. doi: 10.1016/j.tox.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Couladius M, Tzakou O, Verykokidou E, Harvala C. Screening of some Greek aromatic plants for antioxidant activity. Phytotherapy Research. 2003;17(2):194–195. doi: 10.1002/ptr.1261. [DOI] [PubMed] [Google Scholar]
- 4.Peng C H, Su J D, Chtau C C, Sung T Y, Ho S S, Peng C C. et al. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Bioscience, biotechnology, and biochemistry. 2007;(0):0707310512. doi: 10.1271/bbb.70199. [DOI] [PubMed] [Google Scholar]
- 5.Ojeda-sana A M, Van baren C M, Elechosa M A, Juarez M A, Morenos S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013;31(1):189–195. [Google Scholar]
- 6.Posadas S, Caz V, Largo C, De La Gandara B, Matallans B, Reglero G. et al. Protective effect of supercritical fluid rosemary extract, Rosmarinus officinalis, on antioxidants of major organs of aged rats. Experimental gerontology. 2009;44(6):383–389. doi: 10.1016/j.exger.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Arts M J, Haenen G R, Voss H P, Bast A. Masking of antioxidant capacity by the interaction of flavonoids with protein. Food and Chemical Toxicology. 2001;39(8):787–791. doi: 10.1016/s0278-6915(01)00020-5. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y. et al. Carnosic acid, a catechol‐type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S‐alkylation of targeted cysteines on Keap1. Journal of neurochemistry. 2008;104(4):1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka Kosaka. et al. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid-and catechol hydroxyl moieties-dependent manners. Neuroscience letters. 2008;434(3):260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 10.Lau F C, Shukitt-Hale B, Joseph J A. The beneficial effects of fruit polyphenols on brain aging. Neurobiology of Aging. 2005;26(1, Supplement):128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Balu D T, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neuroscience & Biobehavioral Reviews. 2009;33(3):232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candelario-Jalil E, Mhadu N H, Al-Dalain S M, Martinez G, Leon O S. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neuroscience research. 2001;41(3):233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 13.Evans WJ. Vitamin E, vitamin C, and exercise. The American journal of clinical nutrition. 2000;72(2):647s–652s. doi: 10.1093/ajcn/72.2.647S. [DOI] [PubMed] [Google Scholar]
- 14.Morris R, Garrud P, Rawlins J, O'keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 15.Vorhees C V, Williams M T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson L, Zhang R, Elliffe D, Chen K F, Mathai S, Mccarthy D. et al. Correlation of cellular changes and spatial memory during aging in rats. Experimental gerontology. 2008;43(10):929–938. doi: 10.1016/j.exger.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17. Paxinos G, Watson C. The rat brain in stereotaxic coordinates: hard cover edition. 2006: Access Online via Elsevier.
- 18.Misra H P, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 19.Wheeler C R, Salzman J A, Elsayed N M, Omaye S T, Korte Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Analytical biochemistry. 1990;184(2):193–199. doi: 10.1016/0003-2697(90)90668-y. [DOI] [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica Chimica Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 22.Sies H. Oxidative stress: oxidants and antioxidants. Experimental physiology. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 23.Angioni A, Barra A, Cereti E, Barile D, CoÏsson J D, Arlorio M. et al. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. Journal of Agricultural and Food Chemistry. 2004;52(11):3530–3535. doi: 10.1021/jf049913t. [DOI] [PubMed] [Google Scholar]
- 24.Abd El-Ghany M A, Motawee M M, El-Kewawy H E M. Biological effects of yoghurt with rosemary on injured liver rats. Australian Journal of Basic and Applied Sciences. 2012;6(3):525–532. [Google Scholar]
- 25.Munné-Bosch S, Alegre L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant physiology. 2001;125(2):1094–1102. doi: 10.1104/pp.125.2.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marosi K, Bori Z, Hart N, Sarga L, Koltal E, Raddak Z. et al. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Rasoolijazi H, Azad N, Joghataie M, Kerdari M, Nikbakht F, Solimani M. The Protective Role of Carnosic Acid against Beta-Amyloid Toxicity in Rats. The Scientific World Journal. 2013 doi: 10.1155/2013/917082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abel T, Lattal K M. Molecular mechanisms of memory acquisition, consolidation and retrieval. Current opinion in neurobiology. 2001;11(2):180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 29. Dias G.P, Cavegn N, Nix A, Do Nascimento Bevilaqua M.C, Stangl D, Zainuddin M.S.A, et al. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxidative medicine and cellular longevity, 2012. 2012. [DOI] [PMC free article] [PubMed]
- 30.Zanella C.A, C A, Treichel H, Cansian R.L, Roman S. The effects of acute administration of the hydroalcoholic extract of rosemary (Rosmarinus officinalis L)(Lamiaceae) in animal models of memory. Brazilian Journal of Pharmaceutical Sciences. 2012;48(3):389–397. [Google Scholar]
- 31.Lazarov O, Mattson M P, Peterson D A, Pimplikar S W, Van praag H. When neurogenesis encounters aging and disease. Trends in neurosciences. 2010;33(12):569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza P V, Abrous D N. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proceedings of the National Academy of Sciences. 2003;100(24):14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waggas A M, Balawi A E. Neurophysiological Study on Possible Protective Effect of Rosemary (Rosemarinus officinalis) Leaves Extract in Male Albino Rats Treated with Acrylamide. American-Eurasian Journal of Scientific Research. 2008;3(2):163–171. [Google Scholar]
- 34.Salah El-Din R A, El-Shahat A El, Ahmed Elmansy R. An Electron Microscopic Study of the Antifertility Potential of Rosemary (Rosmarinus officinalis L) in Male Albino Rats. Int J Morphol. 2012;30(2):666–672. [Google Scholar]
- 35.Bulbul A, Bulbul T, Biricik H, Yesilbag D, Gezen S S. Effects of various levels of rosemary and oregano volatile oil mixture on oxidative stress parameters in quails. African Journal of Biotechnology. 2012;11(7):1800–1805. [Google Scholar]
- 36.Devi S A, Kiran T R. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiology of aging. 2004;25(4):501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]


