Abstract
Zinc plays an integral role in numerous cellular processes including regulation of gene expression. This randomized placebo-controlled trial in adult women evaluated the effects of 20 mg Zn for 23 days. The mRNA abundance of zinc transporters (ZnT1/ZIP3/ZIP4/ZIP8) and metallothionein (MT1) from peripheral blood mononuclear cells was determined by real-time quantitative polymerase chain reaction. In paired samples (n = 6–9), the ZIP4 (P = 0.036) and ZIP8 (P = 0.038) mRNA abundance decreased following zinc supplementation. ZnT1, ZIP3, and MT1 mRNA abundance did not change significantly. The mean ± standard deviation plasma zinc concentration (by inductively coupled plasma mass spectrometry) at baseline was 680 ± 110 μg/L for the zinc group (n = 24) and 741 ± 92 μg/L for the placebo group (n = 23). At endpoint, plasma zinc in the zinc group increased to 735 ± 80 μg/L (P < 0.01) while in the placebo group (717 ± 100 μg/L) it did not change significantly from baseline. The change in mRNA abundance highlights the importance of further investigating ZIP4 and ZIP8 mRNA abundance as potential zinc status biomarkers.
Keywords: zinc supplementation, zinc, ZIP4, ZIP8
Introduction
Zinc is an essential trace element with indispensable roles in numerous processes in the human body.1,2 It has various functions including structural, catalytic, and regulatory roles. A wide range of fundamental physiological processes require zinc including protein synthesis, cellular signaling, and modulation of transporters as well as regulation of enzymes, apoptosis, and transcription factors. Proteomic analysis reveals that approximately 4%–10% of proteins have zinc-binding motifs.3 Suboptimal levels of zinc in physiological systems will likely have several negative biological and clinical effects; thus, zinc has widespread public health importance.
Zinc uptake and excretion is homeostatically regulated by transport of zinc ions actively across biological membranes.2 Zinc transporters contribute to the regulation of zinc homeostasis by coordinating extracellular or organellar zinc influx and cellular zinc efflux or its sequestration into intracellular organelles. Two families of mammalian zinc transporters exist: ZnT (solute-linked carrier 30 [SLC30]) and Zrt-Irt-like protein (ZIP or solute-linked carrier 39 [SLC39]). ZnTs reduce intracellular zinc concentration by transporting zinc out of the cytoplasm or into vesicles.4–6 In contrast to the function of ZnTs, ZIP transporters increase intracellular zinc concentration by facilitating zinc transport into cytosol either from extracellular space or from the lumen of intracellular compartments.7 Similarly, metallothionein (MT), a ubiquitous cellular zinc storage protein, regulates intracellular zinc by releasing/binding ions through the reduction of the thiol group in the MT protein.5,8
Zinc has regulatory roles in gene expression leading to the suggestion that specific genomic changes and functional outcomes may be used to assess zinc status. However, this suggestion is largely unexplored and is a promising area to investigate for potential biomarkers to assess zinc status.9 Some studies showed significant change in the mRNA abundance of selected zinc transporters and MT from peripheral blood mononuclear cells (PBMCs) and from leukocyte subsets in cell culture studies and zinc-supplemented humans.10–13
An investigation of mRNA for eight zinc transporters in PBMCs of healthy men and women (n = 40) with an adequate zinc intake revealed considerable variation in the relative expression of zinc transporter mRNA, with ZnT1, ZnT7, and ZIP1 being the most abundantly expressed.14 Similarly, a comparison of the relative expression of nine zinc transporter genes in human monocytes, T lymphocytes, and granulocytes from blood samples (n = 3) indicated major differences in zinc transporter transcript abundance.12 A comparison study of the expression of ZnT1–8 and ZIP1–8 in visceral and subcutaneous adipose fat from lean and obese individuals revealed different expression levels, but all tested zinc transporter mRNAs were detectable.15
The search to identify specific biomarkers of zinc status has been ongoing and to date largely unsuccessful.12,16 However, assessing the molecular aspects of the cellular biology of zinc using mRNA abundance of zinc transporter proteins and MT in response to zinc supplementation has been suggested as a possible technique for the investigation of zinc status.8,17
Real-time quantitative polymerase chain reaction (qPCR) is a highly sensitive and reproducible method that can be useful for detecting changes in gene expression, even from samples collected during interventional trials or field studies wherein biological samples may be limiting.10,12 Collection of PBMCs is relatively simple, and these cells may represent a readily available tissue source for the exploration of human zinc transporter mRNA expression.14
Despite an appreciation for the prevalence of zinc deficiency in humans, relatively few zinc depletion–repletion studies or studies in populations with inadequate zinc intakes have been conducted to examine the effectiveness of zinc supplementation on increasing zinc status in zinc-deficient individuals.14,18 Thus, based on previous human study outcomes,12,14,15 the objectives of the current study were to evaluate the effect of zinc supplementation on the abundance of mRNA encoding the zinc transporters ZnT1, ZIP3, ZIP4, and ZIP8 and the zinc-responsive MT protein encoded by MT1 mRNA as well as plasma zinc concentrations in apparently healthy adult women. Women were selected because their limited diets and frequent child bearing make them particularly vulnerable to micronutrient deficiencies.19,20 We hypothesized that there will be significant differences in the mRNA abundance of MT and zinc transporters ZnT1, ZIP3, ZIP 4, ZIP8 as well as plasma zinc due to zinc supplementation for 23 days.
Experimental Section
Participants
This study was conducted from June to July 2012 in a rural community (Finchawa) in Sidama zone, southern Ethiopia. The diet in rural Sidama is composed of cereals (mainly maize), kocho, a fermented product of the plant Enset ventricosum, kale and kidney beans with very low intake of animal source foods. Our previous studies have shown these food items had low concentrations of zinc and high phytate to zinc molar ratios.21 Several prior studies in rural Sidama indicated the presence of zinc deficiency. A zinc assessment study found that all the pregnant women (n = 99) in the study had inadequate zinc intake, with a very low median zinc intake of 5.0 mg Zn/d.20,22 Also, a cross-sectional study of pregnant women (n = 700) in 2011 confirmed the presence of zinc deficiency in the same region.23 To our knowledge, there has been no zinc intervention program in the study area.
A list of eligible adult women in the community (n = 1349) was obtained through the Community Health Center. The sample size was calculated with the inputs of 95% confidence level and 85% power for repeated measures using G-Power version 3.1.24 Forty-eight adult women were selected randomly using SPSS software version 20.0 (IBM-SPSS, Armonk, NY, USA) from the provided list. Women who were pregnant, breast-feeding, under- or overweight (body mass index [BMI] <18.5 or >24.9), or who self-reported illness within 1 week prior to the start of the study were excluded. To determine each participant’s BMI for screening, anthropometric measurements were taken in duplicate using a standardized protocol and calibrated equipment.25 Seventeen women who were below 18.5 BMI and one woman above 24.9 BMI were excluded and replaced by a random selection from the original list. All eligible participants gave informed consent to participate in the study. The research procedure complied with the principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the Oklahoma State University. In Ethiopia, Hawassa University Ethical Committee, the Ministry of Science and Technology National Research Ethics Review Committee, and the Food and Medicine Administration and Control Authority approved the study.
Study design
The study was a double-blind placebo-controlled zinc supplementation trial. Forty-eight adult women were randomly assigned either to placebo or zinc supplementation groups using SPSS software version 20.0 (IBM-SPSS). The principal investigator and all research assistants as well as the participants were blinded to the treatment. Baseline data were collected, and then for 23 days each participant consumed a zinc supplement capsule (20 mg of Zn as ZnSO4) or a placebo capsule (lactose monohydrate) (Perry Pharmacy, Perry, OK, USA). The duration of the study and the interval for sample collection were comparable to similar zinc supplementation studies.12,26 The zinc supplement was well below the upper level of 40 mg/d of zinc.27,28 Supplement or placebo was consumed daily between the hours of 8:00 and 10:00 am under the direct supervision of the PI and compliance was 100%. No adverse effects were reported.
Questionnaire administration
At baseline, demographic, socioeconomic, and dietary data were collected using a structured questionnaire. Dietary diversity (DD) was assessed for the 24 h prior to interview by asking participants if they had taken any food from 12 predefined food categories (cereals, roots and tubers, vegetables, fruits, meat, eggs, fish and seafood, pulses [legumes/nuts], milk and milk products, oil and fats, sweets and spices, condiments, and beverages).
Blood and urine collection
Blood was collected in accordance with the standard protocol suggested by the International Zinc Nutrition Consultative Group.28 Whole-blood samples were collected from participants (n = 48) in seated position at baseline and endpoint in a trace mineral–free tube containing ethylene diamine tetraacetic acid (EDTA) (Sarstedt Inc., Newton, NC, USA) by venipuncture using butterfly needles (Sarstedt, Inc.) after an overnight fast of at least 10 h. On the same morning as blood collection, urine samples were collected in plastic cups, transferred to plastic tubes and stored at −20°C (Falcon, Becton Dickinson Labware, NJ, USA).
Total RNA preparation
Whole blood was immediately processed for the isolation of PBMCs. PBMCs were isolated using a density gradient (NycoPrep™ 1.007; Axis-Shield, Norton, MA, USA) in 15-mL conical tubes. Anticoagulated whole blood (3 mL) was diluted with 3 mL of 0.9% NaCl. The 6 mL of diluted blood was carefully poured on 3 mL of the separation gradient in a 15-mL conical tube. The tube was centrifuged at 800× g for 30 min at ambient temperature. The PBMC layer formed at the interface between plasma and the separation medium was transferred to a 1.5-mL microfuge tube. The PBMCs from individual samples were washed twice with Dulbecco’s phosphate-buffered saline (PBS) (Ca and Mg free) (Sigma-Aldrich Inc, St. Louis, MO, USA), suspended in RNAlater (Life Technologies, Carlsbad, CA, USA), and stored at −20°C prior to being shipped frozen to Oklahoma State University.
Total RNA was isolated from PBMCs using STAT-60 according to the manufacturer’s instructions (Tel-Test, Inc., Friendswood, TX, USA). The RNA concentration and integrity were determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Middletown, VA, USA) and agarose gel electrophoresis, respectively. Samples generating A260:A280 ratios within the range of 1.8–2.2 were used to synthesize cDNA. Prior to cDNA synthesis, total RNA (1 μg) was treated with DNase I (Roche Diagnostics, Indianapolis, IN, USA), reverse-transcribed using SuperScript II (Invitrogen, Grand Island, NY, USA), and brought to a final volume of 100 μL.
Gene expression analysis by real-time quantitative polymerase chain reaction
For gene expression analyses by qPCR using SYBR green chemistry on an ABI 7900HT system (Applied Biosystems, Grand Island, NY), cDNA was prepared as described above. Primers for qPCR were designed using Primer Express v 3.0 (Applied Biosystems) and validated if they met the following criteria: (1) single peak on dissociation curve and (2) amplification efficiency slope of approximately −3.3 using a titrated standard curve. Additionally, whenever possible, primers were designed such that the amplicon spanned at least one intron. Relative quantitation of ZnT1, ZIP3, ZIP4, ZIP8, and MT1 mRNA abundance was determined using the 2−ΔΔCt method (Applied Biosystems User Bulletin #2) with Cyclophilin B (Cyclo) as the invariant control. Oligonucleotide primers were obtained from Integrated DNA Technologies (IDT, Inc. Coralville, IO, USA) and their nucleotide sequences are presented in Tables 1 and 2.
Table 1.
Reference gene symbols and accession numbers.
Table 2.
Primer sequences for reference gene analyses by qPCR.
| GENE SYMBOL | FORWARD PRIMER | REVERSE PRIMER |
|---|---|---|
| ZNT1 | 5′ TCACCACTTCTGGGGTTTTC | 5′ ACCAGGAGGAGACCAACACC |
| ZIP3 | 5′ TGTGAGGGAAAAGCTCCAGAAG | 5′ TTCGGCCAGCGGGTAGT |
| ZIP4 | 5′ ATGTCAGGAGCGGGTCTTGC | 5′ GCTGCTGTGCTGCTGGAAC |
| ZIP8 | 5′ AATAGGGACGATTGCCTGGAT | 5′ GCCAGGCCATCGATGAAAT |
| MT1G | 5′ GCACCTCCTGCAAGAAGAGCT | 5′ GCAGCCTTGGGCACACTT |
| PPIB | 5′ TGCCATCGCCAAGGAGTAG | 5′ TGCACAGACGGTCACTCAAA |
Plasma and urine zinc and CRP determinations
Whole blood collected in a tube containing anticoagulant (EDTA) was centrifuged for 10 min within 2 h at ~1000× g at ambient temperature to obtain plasma. Plasma was transferred to trace mineral–free microtubes (Sarstedt, Inc.), frozen at −20°C and shipped to Oklahoma State University for analysis. Plasma and urine zinc were analyzed using inductively coupled plasma mass spectrometry (ICP-MS, Elan 9000, Perkin Elmer Life and Analytical Sciences, Norwalk, CT, USA). For ICP-MS analyses, 200 μL plasma was mixed with 3.8 mL of 0.1% nitric acid (GFS Chemicals, Powell, OH, USA) containing 0.01% Triton X-100 (octyl phenoxy polyethoxyethanol, Sigma-Aldrich, Inc). For urine zinc analyses, 500 μL urine was mixed with 4.5-mL 0.1% nitric acid. For plasma as well as urine, internal standard (Gallium, Perkin Elmer Life and Analytical Sciences, Shelton, CT, USA) and external serum standards were used for quality control (Utak Laboratories Inc., Valencia, CA, USA). The reference cut-off for fasting (morning) plasma zinc for a population of adult women was used with >700 μg/L considered to be normal.26 Plasma high-sensitivity CRP (hsCRP) was assessed by enzyme-linked immunosorbent assay (Helica Biosystems, Inc., Fullerton, CA, USA). The optical density was measured at 450 nm using a plate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT, USA). Plasma CRP concentrations of <3.8 μg/mL were considered normal.
Statistics
SPSS software version 20.0 (IBM-SPSS) was used for statistical analyses. All data including participant characteristics are described using mean ± standard deviation (SD) unless otherwise indicated. Analysis of variance was used to compare values between groups, and paired t tests were used for within-group comparisons between baseline and endpoint after zinc supplementation as well as intraindividual variation in zinc transporter mRNA abundance over time. Bivariate Pearson correlation coefficients were used to describe correlations between zinc transporter mRNA abundance and plasma zinc. Linear regression models were constructed to explore associations between plasma zinc concentrations and zinc transporter gene expression. Statistical significance was set at P < 0.05.
Results
Baseline characteristics of the participants
There were no significant differences at baseline in participant characteristics analyzed by group (Table 3). The average age of the participants was 33 ± 5 years, and there were 6.9 ± 2.6 members in each household. Nearly half (44.9%) of the participating women and 24.5% of their spouses had no formal education. The maximum level of education attained by a woman was sixth grade. The mean DD score was 4.5 ± 1.0 with a consumption range of two to seven of the 12 food categories. Very few participants reported consuming beef (8%) or mutton (2%) as frequently as once per week. None of the participants had consumed meat in the 24 h prior to blood collection (data not shown). Neither zinc nor iron supplements were taken by the participants prior to the study.
Table 3.
Baseline characteristics of the participant women in rural Sidama, southern Ethiopia, in June 2012.
| VARIABLE | UNIT | PLACEBO | ZINC SUPPLEMENT |
|---|---|---|---|
| Age | Years | 33 ± 5 | 33 ± 5 |
| Height | m | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Weight | kg | 53 ± 6 | 51 ± 5 |
| BMI | kg/m2 | 21 ± 2 | 20 ± 2 |
| Number of children | 5 ± 2 | 4 ± 2 |
Note: Data are expressed as mean ± SD; n = 24 in each group.
Change in zinc transporter and MT mRNA abundance
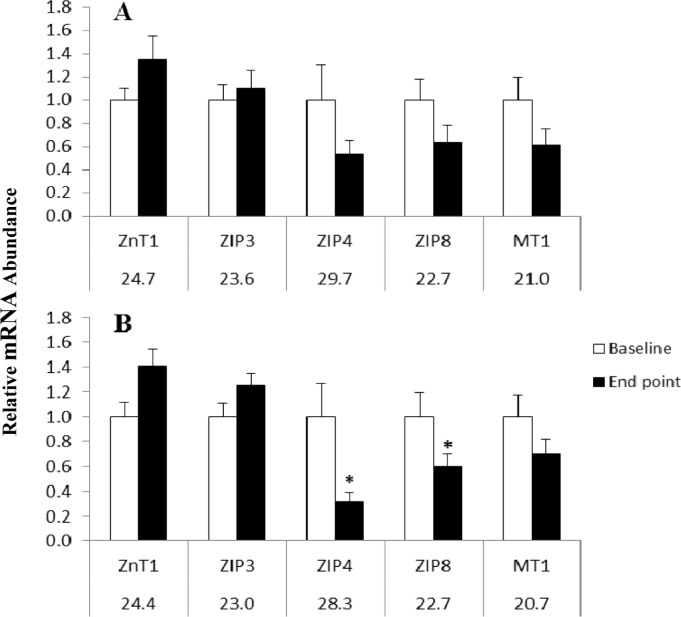
Although biological samples were collected from each participant, not all RNA isolated from these samples met minimum standards for analysis by qPCR; thus, data were analyzed using only matching pairs of baseline and endpoint samples from each treatment group that met minimum criteria (n = 6–9). For these matching pairs from baseline and endpoint, mRNA abundance for zinc transporters ZnT1, ZIP3, ZIP4, and ZIP8 and for MT1 was determined by qPCR. For both groups, the abundance of zinc transporter mRNA did not significantly differ at baseline. After 23 days of zinc supplementation, there was a significant decrease in mean mRNA abundance of ZIP4 (P = 0.036) and ZIP8 (P = 0.038) (Fig. 1). However, the mean expression of ZnT1, ZIP3, and MT1 mRNAs did not change significantly between the two time points, with P-values ranging from 0.10 for ZnT1 to 0.41 for ZIP3. In the placebo group, there was no difference (between baseline and endpoint) in mRNA abundance for any zinc transporter or for MT1 (Fig. 1). Likewise, there was no significant correlation (P = 0.18–0.97) between plasma zinc and the mRNA abundance of any of the individual zinc transporters or MT1 after zinc supplementation.
Figure 1.
Comparison of zinc transporters and MT1 mRNA abundance from PBMCs at baseline and following 23 days of 20 mg Zn/d or placebo to adult women in rural Sidama southern Ethiopia (paired samples n = 6–9). (A) Placebo group (Cyclophilin B, Mean Cq = 21.4). (B) Zinc-supplemented group (Cyclophilin B, Mean Cq = 21.0). The relative abundance of mRNA was determined using the CT method with Cyclophilin B serving as the invariant control. Numbers beneath gene names indicates Cq (quantitative cycle) obtained for baseline measurement. Asterisk indicates statistical significance between pre- and post-zinc measurement (P < 0.05). Error bars show standard error of the mean.
Plasma and urine zinc and CRP concentrations
Plasma zinc was analyzed at baseline and endpoint (Table 4). At baseline, for all participants, plasma zinc concentration ranged from 538 to 928 μg/L (n = 47). From these, 46.8% (54.2% in the zinc group [n = 24] and 39.0% in the placebo group [n = 23]) exhibited plasma zinc concentrations below 700 μg/L, the suggested lower cut-off for assessing adequacy of zinc status.26 Despite the random assignment of participants to each treatment group, plasma (P = 0.038) and urinary (P = 0.02) zinc concentrations were higher in the placebo group at baseline. There was not a significant difference in the placebo group between baseline and the end of the 23-day supplementation for plasma (P = 0.31) or urine (P = 0.07). However, within the zinc-supplemented group, there was a significant increase in both plasma (P < 0.014) and urinary (P = 0.013) zinc concentrations from baseline to the end of treatment. After zinc supplementation, the proportion of women in the zinc group with plasma zinc above 700 μg/L increased significantly from 45.8% to 62.5%.
Table 4.
Plasma and urinary zinc concentrations of adult women in rural Sidama, southern Ethiopia after 23 days of placebo or zinc supplementation.
| n | PLASMA ZINC (μg/L) | BASELINE TO ENDPOINT | ||
|---|---|---|---|---|
| BASELINE | ENDPIONT | |||
| Placebo | 23 | 741 ± 92a | 717 ± 100 | P = 0.31 |
| (543–928) | (521–931) | |||
| Zn supplemented | 24 | 680 ± 110b | 735 ± 80* | P = 0.014 |
| (538–897) | (597–893) | |||
| URINARY ZINC (μg/L) | ||||
| Placebo | 24 | 280 ± 135a | 233 ± 131 | P = 072 |
| (105–615) | (34–530) | |||
| Zinc supplemented | 24 | 201 ± 118b | 289 ± 184* | P = 0.013 |
| (80–609) | (46–782) | |||
Notes: Data are expressed as mean ± SD (range). Different letters within a column indicate difference between groups, P < 0.05.
Indicates significant difference between baseline and endpoint.
Concentrations of plasma hsCRP between and within groups were not significantly different at either baseline or endpoint. Fewer than 9% of woman at either time point had slightly elevated hsCRP concentrations (4–9.5 mg/L) but omitting these women had no effect on plasma and urinary zinc.
Discussion
Zinc has been recognized as an important micronutrient for many different functions in the human body. However, more than 50 years after recognition of the importance of zinc for humans, a sensitive and specific biomarker to assess zinc status has not been identified.29,30 Indeed, there are a variety of measures currently used by investigators to assess an individual’s zinc status.31
Zinc transporter proteins are thought to play critical roles in the regulation of intracellular zinc concentrations in mammalian cells through the control of transport across the plasma membrane or from intracellular organelles/compartments. The ZnT family of zinc transporters decreases cytoplasmic zinc concentrations by increasing the secretion, sequestration, or efflux of zinc between extracellular and intracellular compartments. The ZIP family of zinc transporters increases intracellular zinc level by influx or release of stored zinc from extracellular space into the cytoplasm.4 Therefore, it has been hypothesized that the expression of the ZnT mRNA would be increased, whereas the ZIP mRNA would be decreased when dietary zinc intake increases.29,32
In the present study, PBMCs were stored and frozen in RNAlater prior to being shipped from Ethiopia to the Nutritional Sciences Laboratories at the Oklahoma State University. Although not all samples yielded high-quality RNA, there was sufficient RNA from matched samples to compare zinc transporter mRNA abundance for ZnT1, ZIP3, ZIP4, and ZIP8 and for MT1 by qPCR. Others have shown recovery of mRNA from both cell and tissue samples stored in RNAlater solution to be of similar quality compared to frozen samples.33,34
ZIP4 is among the most studied zinc transporters for understanding its key role in maintaining systemic zinc homeostasis in humans. Consistent with its role in modulating zinc uptake, tissues involved in the acquisition of zinc from the diet (ie, by the absorptive intestinal epithelial cell) or from the extracellular space, have relatively abundant ZIP4 expression. Despite some understanding of the role of ZIP4 in modulating zinc transport, the detailed mechanisms of the regulation and expression of ZIP4 among different tissues or cell types is not fully characterized.7 In our study, ZIP4 mRNA abundance from PBMCs decreased significantly after the 23-day zinc supplementation. Other investigators have demonstrated that the expression of ZIP4 mRNA and protein is downregulated at transcriptional and posttranslational levels in response to zinc availability.35–37
Similarly, ZIP8 mRNA abundance decreased after the 23-day zinc supplementation. In rat H4IIE cells (a differentiated rat liver cell line) treated with the zinc chelating agent, TPEN (N,N,N’,N’-tetrakis (2-pyridylmethyl) ethylenediamine), zinc deficiency alone did not increase ZIP8 levels, whereas treatment with iron increased the level of ZIP8. As a result of the observation that ZIP8 expression increases in response to iron loading but not zinc depletion, investigators have suggested that the effect might be mediated through a secondary zinc deficiency induced by the high-iron treatment.38 In a 24-day zinc depletion–repletion (acclamation 10.4 mg/d for 7 days; depletion <0.5 mg/d for 10 days, and repletion 15 mg/d for 7 days with habitual diet) study of healthy young men (n = 9), the mRNA expression of zinc transporters ZnT1 and ZIP8 in erythrocytes did not change significantly due to dietary zinc depletion. Also, in contrast to our finding, ZIP8 measured in only three subjects was not changed at the end of zinc depletion.39
Gene abundance profiles were assessed for zinc transporters ZnT1, ZIP3, ZIP4, ZIP8, and MT1 quantified from PBMC samples in the present study. However, in stepwise regression analyses, plasma zinc variability was not explained by mRNA abundance of these zinc transporters or MT1. Similarly, in a study of 40 healthy adult women and men, aged 21–65 years, with adequate zinc intakes, Foster and colleagues, reported the absence of correlation between dietary or plasma zinc with the abundance of any of the measured individual zinc transporters (ZnT1, ZnT5, ZnT7, ZnT8, ZIP1, ZIP3, ZIP7, and ZIP10).14 Plasma zinc has a high fractional turnover rate,40 and information on the factors that trigger influx and efflux of zinc from cellular compartments, as well as the pattern by which intracellular zinc is preferentially maintained in different tissues, is limited.14 Thus, adaptations of mRNA expression patterns and plasma zinc concentrations in response to zinc intake may vary in different tissues.
Human studies have demonstrated a decrease in abundance of ZnT1 mRNA in association with high zinc intake. The increase in ZnT1 mRNA abundance is consistent with the roles of ZnT1 in regulating zinc efflux from cells.12 Although we did not observe a significant increase in mRNA abundance for ZnT1 in response to zinc supplementation, ZnT1 mRNA abundance tended to increase at endpoint. Using monocytes, T lymphocytes, and granulocytes, Aydemir and colleagues,12 found that compared to placebo, supplementation with 15 mg/d zinc for 10 days to young male subjects consuming adequate dietary zinc produced an increase in ZnT1 and MT1 and a decrease in ZIP3 mRNA abundance for these leukocyte subsets. However, in an investigation of the abundance of ZnT1 and ZIP6 in PBMCs from pulmonary tuberculosis patients (mean serum zinc 56.8 ± 17.1 μg/dL) and healthy controls (97.7 ± 15.9 μg/dL), ZnT1 did not differ between the two groups41 regardless of the significant difference in the serum zinc concentration. In a short-term study of zinc effects on transporters from PBMCs from healthy adults (n = 9), ZIP3 remained unchanged, whereas ZnT1 and MT1 mRNA levels significantly decreased with 0.3 mg/d zinc supplementation for 10 days.9
Sample handling environment, storage time, or sample type may partially explain the differences in our study and some reports in the literature. For instance, differences in transporter gene expression were observed when whole blood and isolated leukocytes were compared.42 Different outcomes may also be due to variation in RNA stabilization and isolation technique for the evaluation of gene abundance in these types of samples.43 In a previous study that found significant positive correlations between a number of zinc transporters, the PBMCs were obtained from 40 individuals with an adequate dietary zinc intake and were processed immediately.14 Also zinc transporter mRNA levels from leukocyte subsets of PBMCs analyzed immediately from zinc-supplemented (15 mg/d for 10 days) young men demonstrated an increase in expression pattern of ZnT1 and MT1 and a decrease in ZIP3.12 In the present study, the PBMCs were stored in RNAlater for ~3 months at −20°C, which might contribute to an overall decrease in RNA concentration.
Biological differences between study populations also might be a significant factor between studies evaluating effects of acute and chronic zinc deficiency. Lack of significant change in MT1 and zinc transporter ZnT1, ZIP3, and mRNA expression over the study period might suggest interrelationships between the plasma membrane and intracellular membrane zinc transporter mRNA expression patterns that maintain cellular zinc homeostasis, possible adaptations in response to conditions of chronic zinc deficiency.14 Typically, the mean dietary zinc intake for pregnant women in the study area was 5 mg/d,20 and our participants consumed a similar plant-based diet with very limited amounts of animal source foods.
Plasma zinc is a small proportion of the total body zinc pool which is regulated homeostatically within a narrow range and adapts to a range of zinc intakes resulting in its lack of sensitivity and specificity as a biomarker.13,40,44 Despite this challenge, plasma zinc remains the most widely used biomarker of zinc status.13,44,45 Hair and urine have not been considered reliable indicators of zinc status, and zinc metal-loenzyme activities (alkaline phosphatase, 5-nucleotidase, and erythrocyte and plasma superoxide dismutase) are limited in their utility to function as indicators of zinc status.13,44
At the end of the zinc supplementation in the present study, the percentage of women who remained in the low-zinc category was reduced from 54.2% to 37.5% in the zinc-supplemented group, whereas the proportion (39.1%) of women remaining below 700 μg/L did not change in the placebo group. The decrease in proportion of women below the cut-off level in the zinc-supplemented group might indicate a transient increase in plasma zinc due to supplementation after prolonged inadequate dietary zinc intake; however, these women all had BMIs greater than 18.5. Unlike the current study, zinc supplementation of adult women with a BMI range of 16.5–24.1 for 17 days with 20 mg zinc (as ZnSO4) in rural Sidama, Ethiopia, did not result in significant change in plasma zinc concentration.46
Our calculated urinary zinc after zinc supplementation was comparable to the mean (SD) reference of 301.5 μg/L (253.9 μg/L) reported in a previous study, which examined representative ranges of reference values for trace minerals in urine.47 However, other studies have found both higher and lower urinary zinc excretion values.48,49
Strengths of the current study are that mRNA abundance may represent a biomarker of zinc status and that we were able to collect and utilize samples from women living in a remote area with widespread chronic zinc depletion. Although the number of samples available for analysis of zinc transporters was less than those for plasma zinc, the data provide important information for future studies. Improved cell separation, storage, and transport conditions may improve preservation of samples.
Conclusion
In conclusion, an ideal biomarker for zinc status has not yet been determined. In our study of women with chronically low zinc intake, the significant decrease in ZIP4 and ZIP8 mRNA abundance with zinc supplementation was biologically plausible. Our results, as well as indications in the literature that particular zinc transporters are regulated by zinc intake support the need for further research to determine mRNA abundance for specific zinc transporters in order to identify potential zinc biomarkers in humans.29,50 This study provides a basis to further investigate zinc transporter mRNA abundance to assess chronic zinc deficiency, reducing the challenges associated with depletion–repletion studies conducted in a laboratory environment.
Acknowledgments
The authors wish to thank the participants for volunteering to be in the study.
Footnotes
ACADEMIC EDITOR: Joseph Zhou, Editor in Chief
FUNDING: We acknowledge funding from the Oklahoma Agricultural Experiment Station Regional Project, W-3002. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties.
Author Contributions
Conceived and designed the research: AB, BJS, SLC, and KMH. Conducted the research laboratory analysis: AB, BJS, SLC, and JF. Analyzed statistical data: AB, BJS, and SLC. First draft of the manuscript: AB, SLC, and BJS. Joint revisions: AB, BJS, SLC, JF, and KMH. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Hambidge M. Human zinc deficiency. J Nutr. 2000;130(5S suppl):1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 2.Martin L, Lodemann U, Bondzio A, et al. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J Nutr. 2013;143(8):1205–1210. doi: 10.3945/jn.113.177881. [DOI] [PubMed] [Google Scholar]
- 3.Kambe T. Methods to evaluate zinc transport into and out of the secretory and endosomal-lysosomal compartments in DT40 cells. Methods Enzymol. 2014;534:77–92. doi: 10.1016/B978-0-12-397926-1.00005-6. [DOI] [PubMed] [Google Scholar]
- 4.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 5.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281(34):24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 6.Fukada T, Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3(7):662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Chen Y, Wang Y, et al. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 2013;15(8):1008–1016. doi: 10.1093/neuonc/not042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.dos Santos Rocha PB, de Castro Amorim A, de Sousa AF, et al. Expression of the zinc transporters genes and metallothionein in obese women. Biol Trace Elem Res. 2011;143(2):603–611. doi: 10.1007/s12011-010-8887-7. [DOI] [PubMed] [Google Scholar]
- 9.Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci. 2011;108(52):20970–20975. doi: 10.1073/pnas.1117207108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousins RJ, Blanchard RK, Popp MP, et al. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci. 2003;100(12):6952–6957. doi: 10.1073/pnas.0732111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase H, Mazzatti DJ, White A, et al. Differential gene expression after zinc supplementation and deprivation in human leukocyte subsets. Mol Med. 2007;13(7–8):362–370. doi: 10.2119/2007-00049.Haase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci. 2006;103(6):1699–1704. doi: 10.1073/pnas.0510407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao J, Cousins RJ. Metallothionein mRNA in monocytes and peripheral blood mononuclear cells and in cells from dried blood spots increases after zinc supplementation of men. J Nutr. 2000;130(9):2180–2187. doi: 10.1093/jn/130.9.2180. [DOI] [PubMed] [Google Scholar]
- 14.Foster M, Hancock D, Petocz P, Samman S. Zinc transporter genes are coordinately expressed in men and women independently of dietary or plasma zinc. J Nutr. 2011;141(6):1195–1201. doi: 10.3945/jn.111.140053. [DOI] [PubMed] [Google Scholar]
- 15.Smidt K, Pedersen SB, Brock B, et al. Zinc-transporter genes in human visceral and subcutaneous adipocytes: lean versus obese. Mol Cell Endocrinol. 2007;264(1–2):68–73. doi: 10.1016/j.mce.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 16.King J, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th. Baltimore: Lippincott Williams and Wilkins; 2005. pp. 271–285. [Google Scholar]
- 17.Menon R, Garg G, Gasser RB, Ranganathan S. TranSeqAnnotator: large-scale analysis of transcriptomic data. BMC Bioinformatics. 2012;13(suppl 17):S24. doi: 10.1186/1471-2105-13-S17-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood RJ. Assessment of marginal zinc status in humans. J Nutr. 2000;130(5S suppl):1350S–1354S. doi: 10.1093/jn/130.5.1350S. [DOI] [PubMed] [Google Scholar]
- 19.Pathak P, Kapil U, Kapoor SK, et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr. 2004;71(11):1007–1014. doi: 10.1007/BF02828117. [DOI] [PubMed] [Google Scholar]
- 20.Abebe Y, Bogale A, Hambidge KM, et al. Inadequate intakes of dietary zinc among pregnant women from subsistence households in Sidama, Southern Ethiopia. Public Health Nutr. 2008;11(4):379–386. doi: 10.1017/S1368980007000389. [DOI] [PubMed] [Google Scholar]
- 21.Abebe Y, Bogale A, Hambidge KM, Stoecker BJ, Bailey K, Gibson RS. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, Southern Ethiopia, and implications for bioavailability. J Food Comp Anal. 2007;20(3–4):161–168. [Google Scholar]
- 22.Stoecker BJ, Abebe Y, Hubbs-Tait L, et al. Zinc status and cognitive function of pregnant women in Southern Ethiopia. Eur J Clin Nutr. 2009;63(7):916–918. doi: 10.1038/ejcn.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebremedhin S, Enquselassie F, Umeta M. Independent and joint effects of prenatal zinc and vitamin A deficiencies on birthweight in rural Sidama, Southern Ethiopia: prospective cohort study. PLoS One. 2012;7(12):e50213. doi: 10.1371/journal.pone.0050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 25.Gibson RS. Principles of Nutritional Assessment. 2nd. New York: Oxford University Press; 2005. [Google Scholar]
- 26.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007;28(3 suppl):S403–S429. doi: 10.1177/15648265070283S303. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine . Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington DC: National Academy Press; 2001. [Google Scholar]
- 28.Brown KH, Rivera J, Bhutta Z, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25(1 suppl 2):S99–S203. [PubMed] [Google Scholar]
- 29.Andree KB, Kim J, Kirschke CP, et al. Investigation of lymphocyte gene expression for use as biomarkers for zinc status in humans. J Nutr. 2004;134(7):1716–1723. doi: 10.1093/jn/134.7.1716. [DOI] [PubMed] [Google Scholar]
- 30.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR, Darby WJ. Biochemical studies on dwarfism, hypogonadism, and anemia. Arch Intern Med. 1963;111(4):407–428. doi: 10.1001/archinte.1963.03620280007003. [DOI] [PubMed] [Google Scholar]
- 31.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr. 2008;99(suppl 3):S14–S23. doi: 10.1017/S0007114508006818. [DOI] [PubMed] [Google Scholar]
- 32.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763(7):711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Mutter GL, Zahrieh D, Liu C, et al. Comparison of frozen and RNAlater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004:5–88. doi: 10.1186/1471-2164-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros M, Sharma VK, Ding R, et al. Optimization of RNA yield, purity and mRNA copy number by treatment of urine cell pellets with RNAlater. J Immunol Methods. 2003;279(1–2):135–142. doi: 10.1016/s0022-1759(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 35.Huang L, Kirschke CP, Zhang Y, Yu YY. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem. 2005;280(15):15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 36.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279(6):4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 37.Kambe T, Weaver BP, Andrews GK. The genetics of essential metal homeostasis during development. Genesis. 2008;46(4):214–228. doi: 10.1002/dvg.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang CY, Jenkitkasemwong S, Duarte S, et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem. 2012;287(41):34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryu MS, Guthrie GJ, Maki AB, Aydemir TB, Cousins RJ. Proteomic analysis shows the upregulation of erythrocyte dematin in zinc-restricted human subjects. Am J Clin Nutr. 2012;95(5):1096–1102. doi: 10.3945/ajcn.111.032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011;94(2):679s–684s. doi: 10.3945/ajcn.110.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao YT, Huang Q, Jiang YL, et al. Up-regulation of Slc39A2(Zip2) mRNA in peripheral blood mononuclear cells from patients with pulmonary tuberculosis. Mol Biol Rep. 2013;40(8):4979–4984. doi: 10.1007/s11033-013-2598-z. [DOI] [PubMed] [Google Scholar]
- 42.Cobb JP, Mindrinos MN, Miller-Graziano C, et al. Inflammation and Host Response to Injury Large-Scale Collaborative Research Program Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci. 2005;102(13):4801–4806. doi: 10.1073/pnas.0409768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feezor RJ, Baker HV, Mindrinos M, et al. Inflammation and Host Response to Injury, Large-Scale Collaborative Research Program Whole blood and leukocyte RNA isolation for gene expression analyses. Physiol Genomics. 2004;19(3):247–254. doi: 10.1152/physiolgenomics.00020.2004. [DOI] [PubMed] [Google Scholar]
- 44.Hambidge M. Biomarkers of trace mineral intake and status. J Nutr. 2003;133(suppl 3):948S–955S. doi: 10.1093/jn/133.3.948S. [DOI] [PubMed] [Google Scholar]
- 45.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr. 2009;89(6):2040S–2051S. doi: 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 46.Joray ML, Yu TW, Ho E, et al. Zinc supplementation reduced DNA breaks in Ethiopian women. Nutr Res. 2015;35(1):49–55. doi: 10.1016/j.nutres.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komaromy-Hiller G, Ash KO, Costa R, Howerton K. Comparison of representative ranges based on U.S. patient population and literature reference intervals for urinary trace elements. Clin Chim Acta. 2000;296(1–2):71–90. doi: 10.1016/s0009-8981(00)00205-9. [DOI] [PubMed] [Google Scholar]
- 48.Pfrimer K, Micheletto RF, Marchini JS, Padovan GJ, Moriguti JC, Ferriolli E. Impact of aging on urinary excretion of iron and zinc. Nutr Metab Insights. 2014;7:47–50. doi: 10.4137/NMI.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez E, Diaz C. Iron, copper and zinc levels in urine: relationship to various individual factors. J Trace Elem Med Biol. 1995;9(4):200–209. doi: 10.1016/s0946-672x(11)80025-8. [DOI] [PubMed] [Google Scholar]
- 50.Ryu MS, Lichten LA, Liuzzi JP, Cousins RJ. Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr. 2008;138(11):2076–2083. doi: 10.3945/jn.108.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]