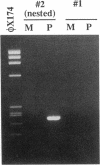
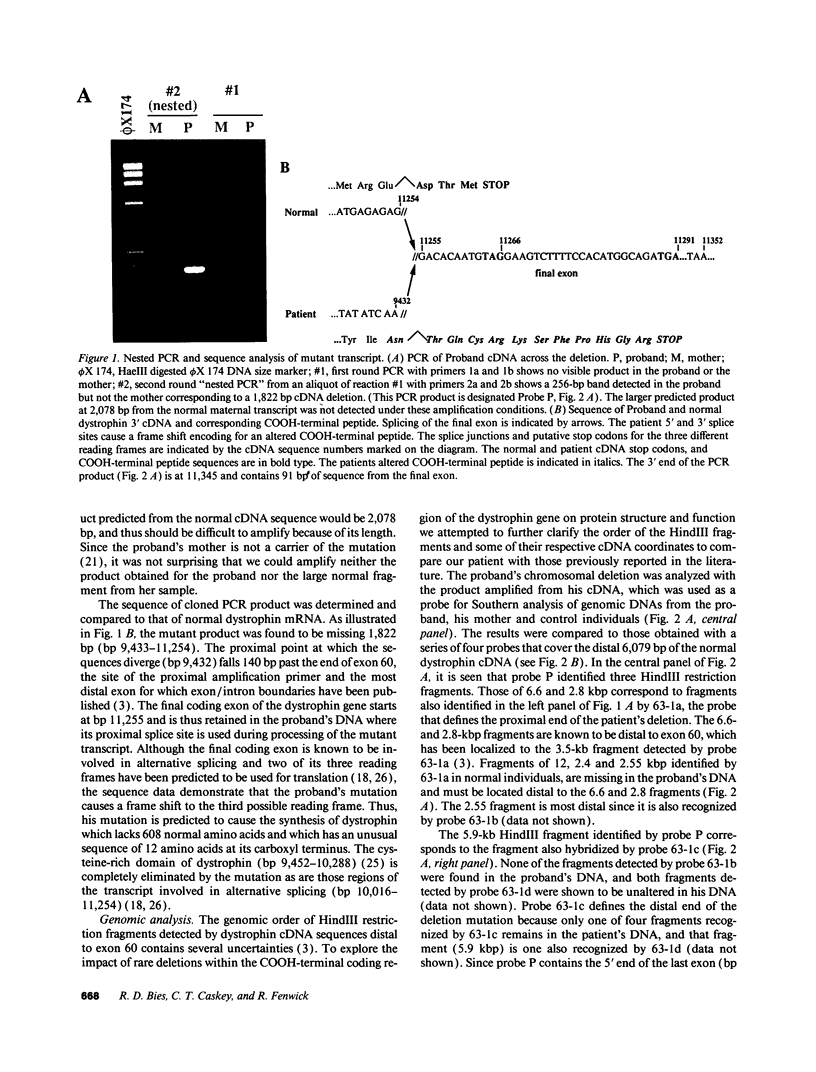
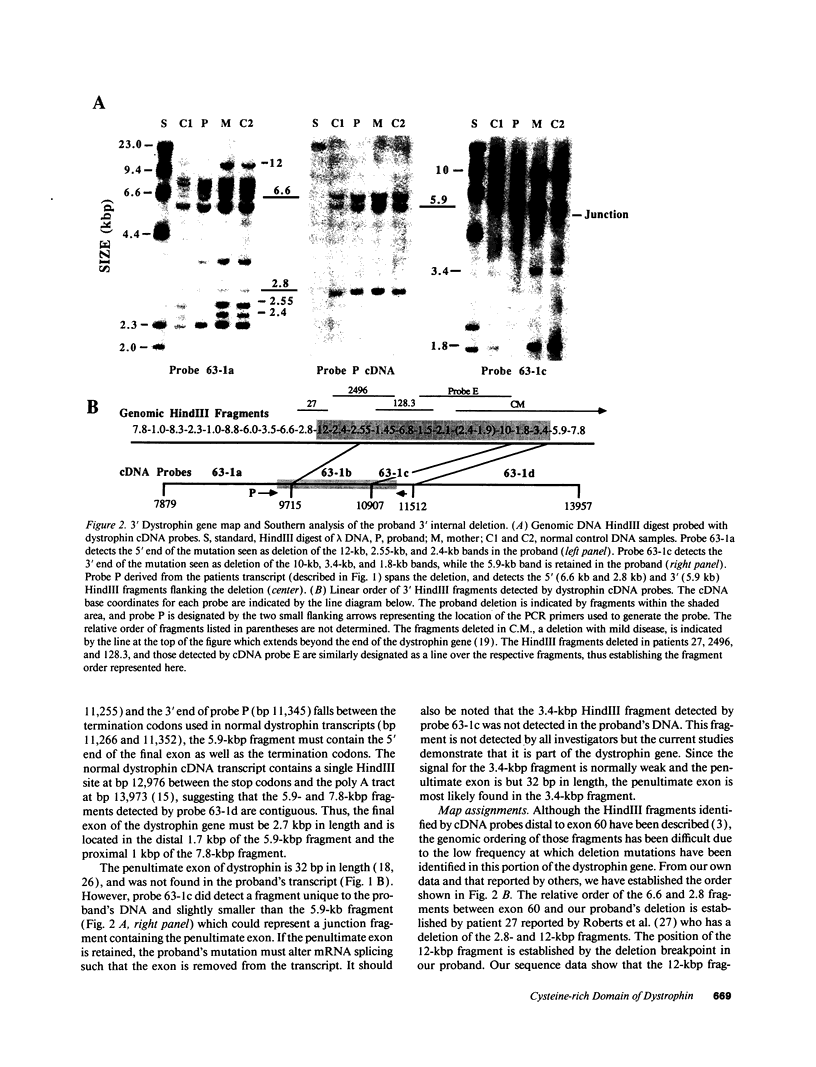
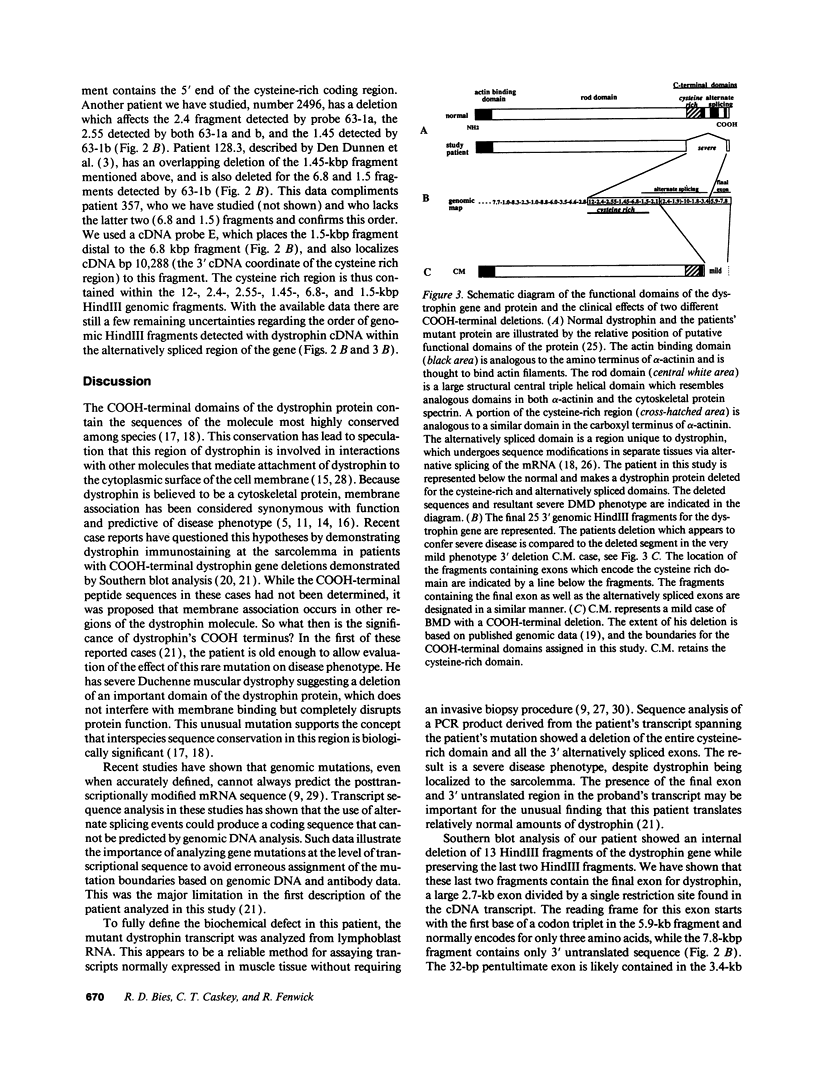
Abstract
The carboxyl terminus of dystrophin is encoded by a highly conserved, alternatively spliced region of the gene. The few rare mutations reported in this region are of interest in unraveling the function of the dystrophin molecule. An unusual case of infantile onset Duchenne muscular dystrophy (DMD) with an internal 3' genomic deletion, and a membrane localized non-functional dystrophin protein, was used to explore the functional activity of this region. The patient's cDNA sequence showed an intragenic 1824-bp deletion precisely excising the cysteine rich and alternatively spliced COOH-terminal domains of dystrophin. The unaltered final 2.7 kb of the patients transcript was defined as a single exon localized to two genomic fragments, with the 5.9 kb HindIII fragment containing the stop codon. To understand the significance of deletions in this important region of the dystrophin gene, we mapped the order and cDNA coordinates for the 3' genomic HindIII fragments encoding the cysteine rich and alternative splicing domains. This 3' gene map was used to compare the clinical phenotype of the other reported COOH-terminal deletions in the literature. Our analysis concludes that the cysteine-rich domain confers an important function for the dystrophin protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acsadi G., Dickson G., Love D. R., Jani A., Walsh F. S., Gurusinghe A., Wolff J. A., Davies K. E. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991 Aug 29;352(6338):815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Arahata K., Beggs A. H., Honda H., Ito S., Ishiura S., Tsukahara T., Ishiguro T., Eguchi C., Orimo S., Arikawa E. Preservation of the C-terminus of dystrophin molecule in the skeletal muscle from Becker muscular dystrophy. J Neurol Sci. 1991 Feb;101(2):148–156. doi: 10.1016/0022-510x(91)90039-a. [DOI] [PubMed] [Google Scholar]
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Baron M. D., Davison M. D., Jones P., Critchley D. R. The sequence of chick alpha-actinin reveals homologies to spectrin and calmodulin. J Biol Chem. 1987 Dec 25;262(36):17623–17629. [PubMed] [Google Scholar]
- Baumbach L. L., Chamberlain J. S., Ward P. A., Farwell N. J., Caskey C. T. Molecular and clinical correlations of deletions leading to Duchenne and Becker muscular dystrophies. Neurology. 1989 Apr;39(4):465–474. doi: 10.1212/wnl.39.4.465. [DOI] [PubMed] [Google Scholar]
- Bies R. D., Friedman D., Roberts R., Perryman M. B., Caskey C. T. Expression and localization of dystrophin in human cardiac Purkinje fibers. Circulation. 1992 Jul;86(1):147–153. doi: 10.1161/01.cir.86.1.147. [DOI] [PubMed] [Google Scholar]
- Bies R. D., Phelps S. F., Cortez M. D., Roberts R., Caskey C. T., Chamberlain J. S. Human and murine dystrophin mRNA transcripts are differentially expressed during skeletal muscle, heart, and brain development. Nucleic Acids Res. 1992 Apr 11;20(7):1725–1731. doi: 10.1093/nar/20.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989 Oct;109(4 Pt 1):1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darras B. T., Francke U. Myopathy in complex glycerol kinase deficiency patients is due to 3' deletions of the dystrophin gene. Am J Hum Genet. 1988 Aug;43(2):126–130. [PMC free article] [PubMed] [Google Scholar]
- Den Dunnen J. T., Grootscholten P. M., Bakker E., Blonden L. A., Ginjaar H. B., Wapenaar M. C., van Paassen H. M., van Broeckhoven C., Pearson P. L., van Ommen G. J. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989 Dec;45(6):835–847. [PMC free article] [PubMed] [Google Scholar]
- England S. B., Nicholson L. V., Johnson M. A., Forrest S. M., Love D. R., Zubrzycka-Gaarn E. E., Bulman D. E., Harris J. B., Davies K. E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990 Jan 11;343(6254):180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Fong P. Y., Turner P. R., Denetclaw W. F., Steinhardt R. A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990 Nov 2;250(4981):673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Franco A., Jr, Lansman J. B. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990 Apr 12;344(6267):670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Ginjaar I. B., Bakker E., den Dunnen J. T., van Paassen M. M., van Ommen G. J., Zubrzycka-Gaarn E., Kloosterman M. D., Wessels A., Moorman A. F. Immunological study of dystrophin in Duchenne fetus. Lancet. 1989 Nov 18;2(8673):1212–1213. doi: 10.1016/s0140-6736(89)91813-8. [DOI] [PubMed] [Google Scholar]
- Gospe S. M., Jr, Lazaro R. P., Lava N. S., Grootscholten P. M., Scott M. O., Fischbeck K. H. Familial X-linked myalgia and cramps: a nonprogressive myopathy associated with a deletion in the dystrophin gene. Neurology. 1989 Oct;39(10):1277–1280. doi: 10.1212/wnl.39.10.1277. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Garcia C. A., Chamberlain J. S., Angelini C., Lupski J. R., Fenwick R. Is the carboxyl-terminus of dystrophin required for membrane association? A novel, severe case of Duchenne muscular dystrophy. Ann Neurol. 1991 Oct;30(4):605–610. doi: 10.1002/ana.410300414. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M., Angelini C., Clarke A., Johnson M., Harris J. B. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology. 1989 Aug;39(8):1011–1017. doi: 10.1212/wnl.39.8.1011. [DOI] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Pearlman J. A., Chamberlain J. S., Caskey C. T. Expression of recombinant dystrophin and its localization to the cell membrane. Nature. 1991 Jan 24;349(6307):334–336. doi: 10.1038/349334a0. [DOI] [PubMed] [Google Scholar]
- Lemaire C., Heilig R., Mandel J. L. The chicken dystrophin cDNA: striking conservation of the C-terminal coding and 3' untranslated regions between man and chicken. EMBO J. 1988 Dec 20;7(13):4157–4162. doi: 10.1002/j.1460-2075.1988.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M., Masumura T., Nishio H., Nakajima T., Kitoh Y., Takumi T., Koga J., Nakamura H. Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy kobe. J Clin Invest. 1991 Jun;87(6):2127–2131. doi: 10.1172/JCI115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe E. R., Towbin J., Chamberlain J., Baumbach L., Witkowski J., van Ommen G. J., Koenig M., Kunkel L. M., Seltzer W. K. Complementary DNA probes for the Duchenne muscular dystrophy locus demonstrate a previously undetectable deletion in a patient with dystrophic myopathy, glycerol kinase deficiency, and congenital adrenal hypoplasia. J Clin Invest. 1989 Jan;83(1):95–99. doi: 10.1172/JCI113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Roberts R. G., Barby T. F., Manners E., Bobrow M., Bentley D. R. Direct detection of dystrophin gene rearrangements by analysis of dystrophin mRNA in peripheral blood lymphocytes. Am J Hum Genet. 1991 Aug;49(2):298–310. [PMC free article] [PubMed] [Google Scholar]
- Roberts R. G., Bentley D. R., Barby T. F., Manners E., Bobrow M. Direct diagnosis of carriers of Duchenne and Becker muscular dystrophy by amplification of lymphocyte RNA. Lancet. 1990 Dec 22;336(8730):1523–1526. doi: 10.1016/0140-6736(90)93305-9. [DOI] [PubMed] [Google Scholar]
- Récan D., Chafey P., Leturcq F., Hugnot J. P., Vincent N., Tomé F., Collin H., Simon D., Czernichow P., Nicholson L. V. Are cysteine-rich and COOH-terminal domains of dystrophin critical for sarcolemmal localization? J Clin Invest. 1992 Feb;89(2):712–716. doi: 10.1172/JCI115640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Wessels A., Ginjaar I. B., Moorman A. F., van Ommen G. J. Different localization of dystrophin in developing and adult human skeletal muscle. Muscle Nerve. 1991 Jan;14(1):1–7. doi: 10.1002/mus.880140102. [DOI] [PubMed] [Google Scholar]