Aging-related decrease of the microcirculatory perfusion of the lumbar vertebral marrow preceded the loss of bone material density (BMD) and the onset of intervertebral discal degeneration (IDD), and the changes of computed tomographic perfusion were strongly correlated with both BMD and IDD alterations, indicating their possible causal relationship.
Keywords: bone loss, CT perfusion, intervertebral discal degeneration, lumbar vertebral marrow, microcirculatory function
Abstract
Study Design.
Descriptive study, stratified sampling.
Objective.
Using dynamic computed tomographic perfusion (CTP) to explore the age-related distribution patterns of the microcirculation perfusion in the vertebral marrow, the vertebral bone mineral density (BMD), and the intervertebral discal degeneration (IDD) further to discuss the possible causation between them.
Summary of Background Data.
A latest viewpoint deemed that reduced blood supply of the vertebral marrow was correlated with an increased incidence of IDD and loss of BMD. However, the causative relationship between them needs more investigation.
Methods.
One hundred eighty-six general people were randomly enrolled by stratified sampling and grouped by age: 15 years or less, 16 to 25 years, 26 to 35 years, 36 to 45 years, 46 to 55 years, 56 to 65 years, 66 to 75 years, and 76 years or more. Both CTP and BMD of the third and fourth lumbar vertebral marrow were measured, and the IDD incidence of the third-fourth vertebrae was assessed. The temporal-spatial distribution patterns of the age-related changes of CTP, BMD, and IDD were described, and the correlations between them were calculated.
Results.
Microcirculatory perfusion of the vertebral marrow developed to maturate by 25 years, maintained stable at 35 years, and then declined by age after 35 years. BMD grew to a peak phase in 26 to 45 years and then dropped by years. However, IDD presented a sudden increase after 45 years of age. CTP (blood flow [r = 0.806], blood volume [r = 0.685], and permeability [r = 0.619]) showed strong positive correlations and CTP (time to peak [r = −0.211], mean transit time [r = −0.598]) showed negative correlations with BMD. Meanwhile, CTP (blood flow [r = −0.815], blood volume [r = −0.753], and permeability [r = −0.690]) had strong negative correlations and CTP (time to peak [r = 0.323] and mean transit time [r = 0.628]) had positive correlations with the incidence of IDD.
Conclusion.
Aging-related decrease of the microcirculatory perfusion of the lumbar vertebral marrow preceded the loss of BMD and the onset of IDD, indicating their possible causal relationship.
Level of Evidence: 3
Currently, the therapeutic emphasis in the field of lower back pain associated with lumbar vertebral degeneration has been placed on disc surgery1,2 and lumbar fusion.3,4 However, the disc degenerative disease and subsequent degenerative spinal instability is the downstream problem. So, identifying its origin could potentially be helpful in preventing and/or relieving the disease. A viewpoint about the mechanism of lumbar vertebral degeneration had been raised in previous studies that reduced blood supply of the vertebral marrow was correlated with an increased incidence of intervertebral discal degenerative disease5 and loss of vertebral bone mineral density (BMD).6 However, these studies achieved a “correlation” level rather than proof of a causative relationship. So, this article aimed to explore the temporal-spatial distribution pattern with age of the microcirculation of the vertebral marrow, vertebral BMD, and intervertebral discal degeneration (IDD) by designing an observational hemodynamics study with a computed tomographic perfusion (CTP) technique, expecting to disclose the causation between them.
MATERIALS AND METHODS
Patients
The volunteer subjects enrolled in this study came from the medical examination center of Southeast Hospital (Clinical School of Medical College, Xiamen University) from January 2011 to December 2013. The inclusion criteria—general people and body height/weight ratio—were in accordance with the normal reference standard (subjects who were >20% or <10% of standard weight were excluded). The exclusion criteria included spinal development malformation; any history of spinal surgical procedures; any history of spinal traumatic fracture; and any disease, for example, metabolic disease, renal inadequacy, cardiac insufficiency, connective tissue disorder, and tumor, which potentially would affect the spinal homogeneity of the cohorts and influence the results.
All subjects were enrolled by stratified sampling and were set into 8 subgroups by age: 15 years or younger, 16 to 25 years, 26 to 35 years, 36 to 45 years, 46 to 55 years, 56 to 65 years, 66 to 75 years, and 76 years or older. Each group was planned to set 24 randomly selected subjects with an equal number of males and females. In actuality, the subgroup of 15 years or younger did not enlist volunteers from 1 to 10 years of age but 18 subjects from 11 to 15 years of age. Hence, at the final evaluation, there were a total of 186 subjects.
The hospital ethics committee approved the study. Informed consent was obtained from the subjects in the study.
Computed Tomographic Examinations
In methods selection, many previous studies adopted magnetic resonance tomography perfusion mainly because of its free x-ray radiation. However, magnetic resonance tomography perfusion, whether contrast enhancement or arterial spin label methods, has inevitable evident shortcoming, such as unsatisfaction of the acquisition speed and the temporal and spatial resolution, magnetic susceptibility to bone mine material and paramagnetism contrast agent, lower accuracy and reproducibility due to fluctuation of magnetic field, inflow effect, and nephrogenic systemic fibrosis from gadolinium-based contrast agents.7 As opposed to the magnetic resonance imaging, the advantages of computed tomography are represented by its lower cost, improved availability, the speed of acquisition, and the temporal and spatial resolution. In addition, CTP, with the acquisition of successive multiple phases, provides functional information to better analyze bone and soft tissue. The functional analysis is more reproducible than that of the magnetic resonance imaging. Nevertheless, the development of multidetector computed tomography and recent technological evolutions reduces the dose the patient is exposed to. The best example is the recent appearance of iterative reconstructions that reduce the dose by half with an equivalent image quality.8 With these technological innovations and better control of the optimization of the acquisition parameters, it is now possible to perform computed tomography with a dose almost equal that of the standard x-ray assessment. So, an advanced computed tomographic (CT) dose optimization and reduction scheme9 would be applied when CTP is used in this study.
We selected the third and fourth lumbar vertebral bodies as the region of interest (ROI) due to the vertebrae situating at middle lumbar part where being not susceptible to compression fracture as the first vertebra and degeneration as the fifth vertebra. The CT protocols for measurement consisted of a plain low-dose CT scan and a dynamic enhancement CTP scan. All scans were obtained using a Somatom Definition AS 4D multidetector CT scanner (Somatom Definition AS 4D CT; Siemens). A preliminary plain 5.0-mm thick CT scan of the target vertebral region (from the superior border of the third lumbar vertebral body to the inferior border of the forth lumbar vertebral body) was obtained to guarantee a panoramic view of the entire anatomical region and thus help recognize anatomical landmarks. The table position at the level of the target lumbar vertebra was recorded, and the perfusion scan range was defined by the external laser alignment light. Specially trained technical personnel conducted the scanning to reduce the chance of human error.
Measurement of Structural Parameters
The structural parameters, including bone mineral density (BMD) and intervertebral discal changes, were taken into account because these age-related structural changes are a response to spinal physiological degeneration.
The BMD images and data obtained were transferred to an image-processing workstation (syngo MultiModality workplace, series 46531; Siemens). Three slices, located at the site of upper one-third, middle site, and lower one-third of the third vertebral body, respectively, were selected to measure BMDs, and the averaged value of the 3 measurements was considered as the actual BMD of the vertebral body. Commercially available osteo software licensed by the Siemens company was used for the BMD analysis. The ROIs to measure and results were automatically obtained by the software. The results are expressed by absolute values in milligrams per milliliter.
The CT indexes of intervertebral disc degenerative disease included disc bulge, protrusion, hernia into endplate, “vacuum sign,” ossification, and narrowed intervertebral space.10 Signs or lesions of discal degeneration were assessed on the combination of CT transection and reconstructive sagittal plane of the target vertebral segment.
Measurement of Hemodynamics
Perfusion scheme was set by manual adjustment in accordance with the principle of CT dose optimization and reduction.9 The following parameters were selected for dynamic enhancement CTP: tube voltage, 80 kV; quality reference mA 50; effective mAs; 100 to 203; pitch; 1.5; slice thickness, 3.0 mm; gantry rotation time, 0.33 seconds; and bolus injection of 40 mL (weight ≤50 kg) and 50 mL (weight >50 kg) of nonionic iodinated contrast medium (iopamidol 370 mg I/mL; Bracco Sine, Shanghai, China) at 5.0 mL/s, followed by 50 mL of saline at 2 mL/s via a 20-gauge cannula in the right arm antecubital vein. Contrast material was administered using a dual-head pump injector (Medrad Stellant D, MEDRAD Inc.). After administering the contrast material (iopamidol 370 mgI/mL; Bracco Sine, Shanghai, China), consecutive dynamic CT acquisitions were performed after a 4-second delay from the start of the injection, scanning 1 time every 5-second period at the same field of view with shallow breathing for 90 seconds of total duration and no delays. A standard reconstruction algorithm with no edge enhancement was used for the dynamic scanning. One set of axial images with a slice thickness of 5 mm for perfusion analysis was reconstructed without overlap using a medium smooth-tissue convolution kernel (B20f smooth). All images were then made anonymous and transferred to an external workstation (Multi-Modality Workplace; Siemens) for further analysis.
Based on our experience with the preliminary test, the total perfusion-monitored duration was set at 90 seconds because of perfusion period of lumbar vertebral marrow being longer, and repeating scanning period was set at 5 seconds. The timing and intervals were selected to obtain an adequate number of measurements to ensure good perfusion parameter values and to warrant a correct radiation dose. During each of the dynamic perfusion series, the patients received a total local CTDIvol dose of 69 and 83 mGy for young and old subjects, respectively, within the recommended reference levels of a CTDI(w) of 11 to 88 mGy for lumbar vertebral CT scan.11,12 According to Ng et al,13 motion correction is required to obtain optimal CTP imaging, the least variability, and the best estimations of CTP. A cradle scanning technique pertaining to Siemens Somatom Definition AS 4D computed tomography was used for to-and-fro dynamic scanning to control motion artifacts.
CTP Image Data Analysis
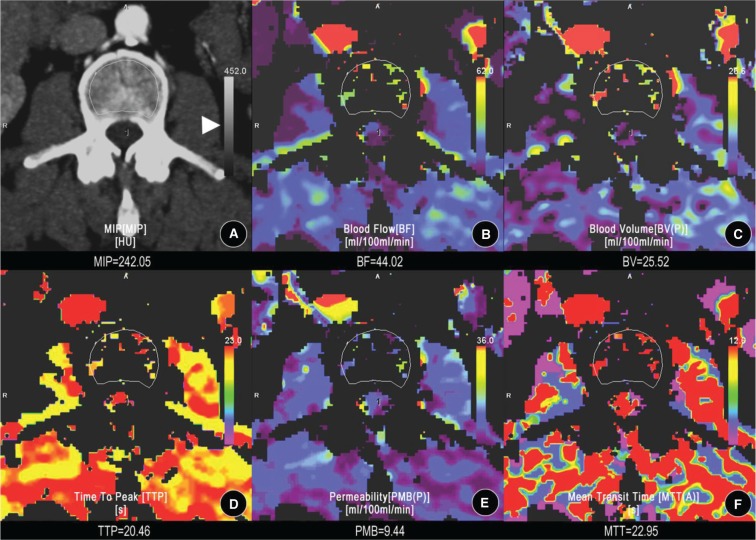
The CTP images and data obtained were transferred to an image-processing workstation and analyzed by 2 expert readers (1 with 17 yr and the other with 11 yr of experience in CT diagnosis). Commercially available software (syngoMMWP version VE36A, Berlin, Germany) was used for the CTP analysis. In one of the series slices, an ROI that was sufficiently small to avoid partial volume effects (2–6 pixels) was placed in the aorta to calculate the arterial input, and an ROI was manually drawn along the subcortex margins of the vertebral body (see Figure 1). The software provided automated calculation of the following perfusion parameters in the ROI areas: blood flow (BF), blood volume (BV), mean transit time (MTT), time to peak (TTP), and permeability (PMB). BF was defined as the volume of blood flowing across 100 g of tumor per minute (mL/min/100 g). BV was defined as the total volume of blood flowing across 100 g of tissue (mL/100 g). MTT was defined as the mean time taken for blood to flow from the artery to the vein, in seconds. TTP was defined as the contrast medium to reach the highest enhancement peak of the ROI, in seconds. PMB was defined as the product of the capillary endothelial space and the sum of their surface area, which reflects the volume of blood flowing from the capillaries to the interstitial space (mL/min/100 g). Values of the perfusion parameters were averaged across 3 slices to minimize the variability resulting from the ROI selection. These 3 ROI slices were selected from the target vertebral body at equal intervals.
Figure 1.
Computed tomographic perfusion (CTP) assessment of the third vertebral body. Images from one of the slices obtained by dynamic perfusion using computed tomography on the third vertebral body of a 28-year-old female. A, A region of interest (ROI) was manually drawn along the subcortical zone of the target vertebral body for calculation of the CTP parameters. B, The blood flow (BF) map of the CTP. C, The blood volume (BV) map. D, The time to peak (TTP) map. E, The permeability (PMB) map. F, The mean transit time (MTT) map. Three ROI slices, respectively, selected from the site of upper one-third, middle, and lower one-third of the third vertebral body were assessed. The CTP parameter (CTPs) values were averaged across these 3 slices and the mean was regarded as the actual CTP values of the target vertebral body. The color scales (triangular arrowhead) were designed automatically by software to show how the perfusion of the ROI was similar to that of the outer tissue.
Statistical Methods
A study had shown that intervertebral disk degeneration related to reduced marrow perfusion of both the adjacent vertebrae.5 So, in this study, the BMD and CTP measurement values of the third and fourth lumbar vertebral marrow were, respectively, averaged, and the mean values were deemed as the last BMD and CTP used in statistical analysis.
Testing for normality was performed for continuous data with the Shapiro-Wilk test. When the 8 age groups had normally distributed data, descriptive statistics, including mean and standard deviation of the CTP parameters (CTPs) and BMD, were presented, respectively. Multivariate tests of the general linear model were used to statistically analyze the differences of CTPs, BMD, and incidence of IDD with age, and their temporal-spatial distribution patterns with age were described as age-related change curves, and correlations between them were calculated by the Pearson correlation. And a 1-way analysis of variance method was applied to statistically analyze the comparisons between CTPs and BMD with and without IDD subjects for each subgroup. Multiple comparisons were adjusted with the Bonferroni test. The statistical analyses were performed using SPSS 17.0 statistical software (SPSS, Chicago, IL). Statistical significance was assessed at a level of P value of less than 0.05.
RESULTS
Temporal-Spatial Distribution Patterns of CTPs, BMD, and Discal Degenerative Disease With Age
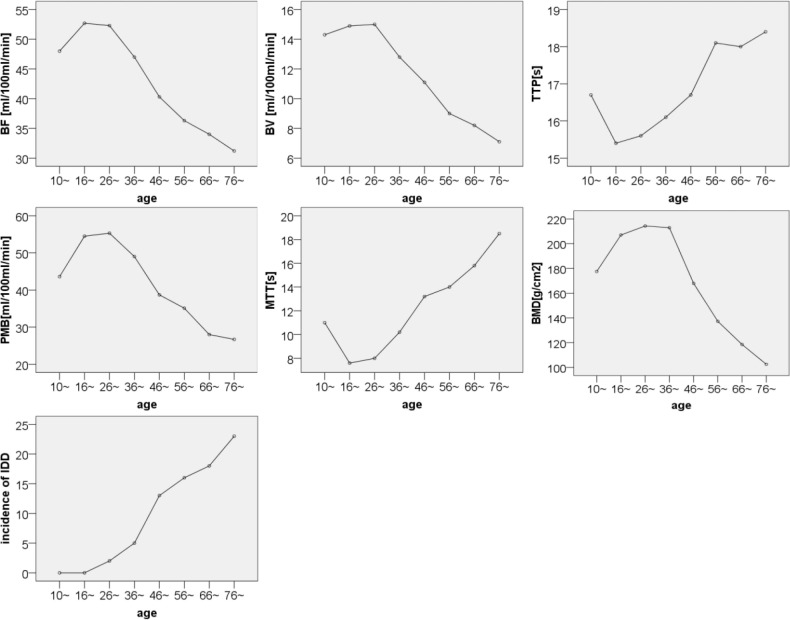
The microcirculation perfusion function embodied by CTPs developed with age, reached to peak by 25 years of age, kept up a stable stage until 35 years of age, and then declined with aging after older than 35 years. BMD had a similar maturity process to perfusion function of the marrow, but it had a longer stable period from 25 to 45 years of age and then decreased with aging. Under 45 years, there was a lower incidence of IDD, much less than 20% for each age group; however, above 45 years, there was a sudden increase of IDD, with an incidence at least more than 50% (see Table 1 and Figure 2) Moreover, in the stage above 45 years, the intervertebral discshowed more and more concurrent multiple degenerative signs with aging, including discal bulge, protrusion, hernia, air vacuum, and ossification (see Table 2).
TABLE 1. Age-Related Changes of the Averaged Computed Tomographic Perfusion and Bone Mineral Density Values of Both the Third and Fourth Lumbar Vertebral Marrow.
| Parameters | Age Groups | Statistical Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 yr (I) | 16 yr (II) | 26 yr (III) | 36 yr (IV) | 46 yr (V) | 56 yr (VI) | 66 yr (VII) | 76 yr (VIII) | Univariate Tests | Pairwise Comparisons (Significance) *† | |
| BMD | 177.5 ± 32.1 | 207.6 ± 35.4 | 214.3 ± 50.4 | 212.9 ± 28.4 | 167.9 ± 39.4 | 137.2 ± 25.5 | 118.5 ± 42.2 | 102.5 ± 55.8 | F = 28.7, P = 0.00 | PV-,II,III,IV, = 0.02,0.00,0.00,0.00 |
| PVI-I,II,III,IV, = 0.00,0.00,0.00,0.00 | ||||||||||
| PVII-I,II,III,IV,V, = 0.00,0.00,0.00,0.00,0.00 | ||||||||||
| PVIII-I,II,III,IV,V, = 0.00,0.00,0.00,0.00,0.00 | ||||||||||
| BF | 48.0 ± 13.2 | 52.7 ± 25.7 | 52.3 ± 20.7 | 47.0 ± 16.2 | 40.3 ± 16.9 | 36.3 ± 11.4 | 34.0 ± 11.6 | 31.2 ± 16.0 | F = 5.7, P = 0.00 | PII -VI,VII,VIII = 0.03,0.01,0.00 |
| PIII-VI,VII,VIII = 0.04,0.01,0.00 | ||||||||||
| BV | 14.3 ± 7.5 | 14.9 ± 9.7 | 15.0 ± 9.8 | 12.8 ± 5.1 | 11.1 ± 7.5 | 9.0 ± 5.8 | 8.2 ± 3.6 | 7.1 ± 6.8 | F = 4.4, P = 0.00 | PII -VII,VIII = 0.05,0.01 |
| PIII-,VII,VIII = 0.04,0.01 | ||||||||||
| TTP | 16.7 ± 2.3 | 15.4 ± 2.0 | 15.6 ± 2.4 | 16.1 ± 2.9 | 16.7 ± 1.9 | 18.1 ± 3.8 | 18.0 ± 3.4 | 18.4 ± 2 .3 | F = 4.7, P = 0.00 | PII -VI,VII,VIII = 0.04,0.01,0.01 |
| PIII-VI,VII,VIII = 0.05,0.01,0.01 | ||||||||||
| PMB | 43.6 ± 13.1 | 54.5 ± 43.6 | 55.3 ± 33.7 | 49.0 ± 18.1 | 38.7 ± 10.6 | 35.1 ± 18.5 | 28.0 ± 10.7 | 26.7 ± 8.4 | F = 5.6, P = 0.00 | PII -VII,VIII = 0.00,0.00 |
| PIII-VII,VIII = 0.00,0.00 | ||||||||||
| PIV-VII,VIII = 0.05,0.03 | ||||||||||
| MTT | 11.0 ± 4.4 | 7.6 ± 4.2 | 8.0 ± 4.4 | 10.2 ± 3.3 | 13.2 ± 4.0 | 14.0 ± 4.9 | 15.8 ± 4.6 | 18.5 ± 5.2 | F = 17.5, P = 0.00 | PI -VII = 0.02, PII-V,VI,VII = 0.00,0.00,0.00 |
| PIII-V,VI,VII, = 0.00,0.00,0.00 | ||||||||||
| PIV-VII, = 0.00, | ||||||||||
| PVIII-I,II,III,IV,V,VI, = 0.00,0.00,0.00,0.00,0.00,0.02 | ||||||||||
| *The mean difference is signifi cant at the 0.05 level. | ||||||||||
| † Adjustment for multiple comparisons: Bonferroni. | ||||||||||
| BMD indicates bone mineral density; BF, blood flow; BV, blood volume; TTP, time to peak; PMB, permeability; MTT, mean transit time. | ||||||||||
Figure 2.
Age-related temporal-spatial distribution pattern of microcirculatory perfusion function (embodied in CTPs) of both the third and fourth lumbar vertebral marrow and their BMD and IDD incidence. BF, blood flow; BV, blood volume; TTP, time to peak; PMB, permeability; MTT, mean transit time; BMD, bone mineral density; IDD, intervertebral discal degeneration.
TABLE 2. Age-Related Distribution Pattern of the Intervertebral Disc Degeneration Between the Third Lumbar and Fourth Lumbar Vertebrae.
| IDD Signs | Age Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 10 yr | 16 yr | 26 yr | 36 yr | 46 yr | 56 yr | 66 yr | 76 yr | |
| Bulge | 0 | 0 | 1 | 5 | 7 | 8 | 6 | 9 |
| Protrusion | 0 | 0 | 0 | 1 | 4 | 7 | 7 | 9 |
| Hernia | 0 | 0 | 1 | 2 | 1 | 1 | 2 | 4 |
| Ossifi cation | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| “Air” sign | 0 | 0 | 0 | 0 | 1 | 2 | 4 | 4 |
| Space—narrowed | 0 | 0 | 0 | 3 | 5 | 8 | 14 | 20 |
| Total cases (incidence) | 0 (0) | 0 (0) | 2 (8%) | 5 (21%) | 13 (54%) | 16 (67%) | 18 (75%) | 23 (96%) |
| IDD indicates intervertebral disc degeneration | ||||||||
Correlation Between CTPs and BMD, Incidence of IDD
CTP (BF [r = 0.806, P = 0.000], BV [r = 0.685, P = 0.005], and PMB [r = 0.619, P = 0.001]) showed strong positive correlations with BMD, and CTP (TTP [r = −0.211, P = 0.322] and MTT [r = −0.598, P = 0.002]) showed negative correlations with BMD. Meanwhile, CTP (BF [r = −0.815, P = 0.000], BV [r = −0.753, P = 0.000], and PMB [r = −0.690, P = 0.000]) had strong negative correlations, and CTP (TTP [r = 0.323, P = 0.126] and MTT [r = 0.628, P = 0.001]) had positive correlations with the incidence of IDD.
DISCUSSION
This study used a CT method called dynamic enhancement scan perfusion imaging to reflect the hemodynamic status of the lumbar vertebral microcirculation. This measurement is potentially helpful for understanding the pathophysiological basis of lumbar vertebral degeneration. The results indicated that the microcirculation of the vertebral marrow alters largely with age; when older than 35 years, the perfusion pressure decreases, velocity of BF slows, BV decreases, and capacity of blood diffusion in tissues lowers.
A long-standing goal for most researchers in the field of lower back pain associated with lumbar vertebral degeneration has been to discover its underlying pathogenesis or pathophysiology. Identifying its origin could potentially be helpful for preventing and/or relieving the disease. Mauno14 had discussed in a detailed manner the association of impaired blood supply with painful lumbar disc degeneration that stenosis of lumbar arteries is significantly associated with decreased diffusion of lumbar discs and may thus play an important role as a mechanism of disc degeneration, that stenosis of lumbar arteries is associated with disc degeneration among patients with sciatica, and the grade of occlusion of lumbar arteries and the severity of disc degeneration are significantly higher among patients with sciatica than among asymptomatic subjects. Moreover, we do know that a decreasing BMD in the lumbar vertebral body is closely correlated with the development and progression of the IDD and that decline of microcirculation perfusion of the vertebral bone marrow is highly related to BMD loss.6,15 However, these studies could not disclose the causation but correlation between them. Because multiple factors contribute to IDD within a same temporal-spatial domain, an experiment design containing a simple factor in vivo was considered often impossible to carry out. This is inspired by a study by Bajwa et al,16 which who found that lumbar degeneration precedes hip degeneration and may be a causative factor for hip osteoarthritis. So, we attempted to investigate the temporal-spatial distribution patterns of microcirculation perfusion and BMD of the vertebral bone marrow and IDD for searching the causation between them.
These hemodynamic and physiological properties can be measured serially using the functional CTP technique and multiplanar imaging maps.17 CTP imaging is a developing technique for quantitatively evaluating tissue blood perfusion.13 This study demonstrated that the microcirculation perfusion of the vertebral bone marrow developed to mature until 25 years of age and maintained a stable stage from 26 to 35 years of age and then declined with aging after older than 35 years of age. BMD showed a similar maturity process to microcirculation perfusion that growth stage was up to the time of 25 years of age, but stationary phase lasted for a longer time from 26 to 45 years of age and then decreased by age. However, IDD-age curve presented no platform period. The age of 45 years was the dividing line age. Above 45 years of age, the IDD sharply developed into a phase of higher incidence with age (see Figure 2).
The CTPs had a good correlation with BMD in each age group (all Ps < 0.05) and also with the incidence of IDD; furthermore, CTP changes preceded both of the latters (see Table 1 and Figure 2). These results demonstrated that alterations in microcirculation of the lumbar vertebral marrow had an influence on its BMD and intervertebral discal healthy status. Some studies had been conducted with an aim to prevent and cure osteoporosis18 and discal degenerative disease19 through maintaining the microcirculation perfusion to the bone marrow with drugs or cell therapy. These successful experimental therapeutics also supported our hypothesis that microcirculation perfusion dysfunction of the vertebral marrow is a cause leading to bone mass loss and intervertebral disc degeneration. On the basis of these findings, a view could be established that reduced blood supply to the vertebral marrow leads to vertebral endplate sclerosis and degeneration of the intervertebral disc because the disc's nutrition depends on the blood supply of the vertebral body passing through the cartilage endplate by diffusion. The microcirculatory dysfunction of the lumbar vertebral marrow brings about the intervertebral disc cell injury, which might be the intermediate procedure transferring the hemodynamic disorder to the IDD.20
The results of this study showed that the BF and BV of the lumbar vertebral body, which reflects the blood supply to the lumbar vertebral marrow, decreased with aging after the maturation phase. This phenomenon might be explained by arterial sclerosis, vascular tortuosity, vertebral compression causing vertebral artery stenosis or occlusion, and/or reduced cardiac output and BV. CTP of the MTT can be used to reflect the blood recirculation status in the microcirculation. MTT is considered a marker of perfusion pressure. Any perfusion pressure decrease appears as the increased MTT on perfusion CT maps.21 This study also observed that MTT was significantly prolonged with aging, which indicated that the microcirculatory blood recirculation slowed with aging. Certain factors might contribute to this pathophysiological change, including the increased diastolic blood pressure that occurs with aging, compression of a vein owing to vertebral body compression, venous thrombosis, a torus on an intervertebral disc oppressing an extravertebral vein, or organized tissue or sterile adhesive drawing, moving, and oppressing an extravertebral vein, among others. These factors lead to increasing venous pressure in the internal vertebral body and subsequently increased interstitial fluid augmentation and pressure, which, in turn, causes a problem for the arterial blood supply. Our results showed that the BF, BV, and PMB decreased and TTP and MTT became prolonged with aging, which were reflected in the BF velocity slowing in the microcirculation of the lumbar vertebral marrow, weakening the perfusion function of dispersed PMB. Subsequently, the metabolism in the vertebral marrow slowed, resulting in BMD decline. Furthermore, in the presence of osteoporosis, which makes bone trabeculae susceptible to distortion (even fracture), vertebral body compression occurs, veins are compressed and become winding or stenotic, blood recirculation is dysfunctional, and venous pressure increases, making the arterial blood supply problematic. These factors form a vicious circle. Later, the endplate and the intervertebral disc are implicated successively. Dysfunction of nutrition passage in the endplate from the marrow to the disc brings about intervertebral discal malnutritional degeneration. And, with aging, degression of microcirculation function and BMD of the vertebral marrow has more and more effect on its intervertebral disc degeneration, which is reflected by more and more concurrent multiple intervertebral discal degenerative signs including discal bulge, protrusion, hernia, air vacuum, and ossification. This cascade reaction helps partially explain the observed recessive lumbago that is so difficult to alleviate in elderly patients.
But there are some limitations in this study: (1) In the preliminary tests, because the blood perfusion time in the vertebral body was prolonged by 90 seconds to reduce radiation exposure during the examination, the study had planned to obtain 1 scan during a 5-second period, so fewer sampling points might affect the precise estimation of CTP values. (2) Up to now, no ideal experimental model could all alone evaluate any 1 factor causing intervertebral disc degeneration because multiple factors integrate to contribute to the degeneration within a same temporal-spatial domain. Interestingly, the study had disclosed the age-related alteration regularity of the microcirculation perfusion function and BMD of the lumbar vertebral marrow and IDD, and in temporal-spatial distribution pattern, the decline of microcirculation function preceded the decrease of BMD and increase of IDD. Based on this finding and knowledge learned from previous studies, a hypothesis was put forward that dysfunction of the microcircution perfusion was a potential cause for the loss of BMD and acceleration of IDD, which perhaps provides a new therapeutic direction for the hard to curative disease.
Key Points
Aging-related decrease of the microcirculatory perfusion of the lumbar vertebral marrow preceded the loss of BMD and the onset of IDD.
CTP (BF, BV, and PMB) of the lumbar vertebral marrow showed strong positive correlations and CTP (TTP and MTT) showed negative correlations with BMD.
CTP (BF, BV, and PMB) of the lumbar vertebral marrow had strong negative correlations and CTP (TTP and MTT) had positive correlations with the incidence of IDD.
Acknowledgments
The authors thank professor Zhen-qi Ding, Director of the Department of Orthopaedics, for his important intellectual concepts and help in clinical diagnosis of lumbar vertebral degenerative disease, as well as Yuan-miao Shou and Tong-ke Su, 2 professors of the Imaging Department, for their help in image analysis. They also thank Yongshi Hu, Director of Science and Education Office of Southeast Hospital Affiliated to Xiamen University, China, for his directions in research design in work.
Footnotes
Acknowledgment date: December 25, 2014.
The device(s)/drug(s) is/are FDA approved or approved by corresponding national agency for this indication.
Zhangzhou City Government supported this study during the Eleventh Five-Year Plan (No. Z07019: Multiple Mode Imaging Methods Diagnosing Degenerative Lumbago Caused by Joint Action of Bone-Arthrosis-Soft Tissue).
No relevant financial activities outside the submitted work.
References
- 1.Shahmohammadi M, Asgharzadeh Shirazi H, Karimi A, et al. Finite element simulation of an artificial intervertebral disk using fiber reinforced laminated composite model. Tissue Cell 2014;46:299–303. [DOI] [PubMed] [Google Scholar]
- 2.Grunert P, Gebhard HH, Bowles RD, et al. Tissue-engineered intervertebral discs: MRI results and histology in the rodent spine. J Neurosurg Spine 2014;20:443–51. [DOI] [PubMed] [Google Scholar]
- 3.Mummaneni PV, Dhall SS, Eck JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine 2014;21:67–74. [DOI] [PubMed] [Google Scholar]
- 4.Eck JC, Sharan A, Ghogawala Z, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: lumbar fusion for intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine 2014;21:42–7. [DOI] [PubMed] [Google Scholar]
- 5.Liu YJ, Huang GS, Juan CJ, et al. Intervertebral disk degeneration related to reduced vertebral marrow perfusion at dynamic contrast-enhanced MRI. AJR Am J Roentgenol 2009;192:974–9. [DOI] [PubMed] [Google Scholar]
- 6.Griffith JF, Wang YX, Zhou H, et al. Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology 2010;254:739–46. [DOI] [PubMed] [Google Scholar]
- 7.Crownover BK, Bepko JL. Appropriate and safe use of diagnostic imaging. Am Fam Physician 2013;87:494–501. [PubMed] [Google Scholar]
- 8.Gervaise A, Osemont B, Lecocq S, et al. CT image quality improvement using adaptive iterative dose reduction with wide-volume acquisition on 320-detector CT. Eur Radiol 2012;22:295–301. [DOI] [PubMed] [Google Scholar]
- 9.Gervaise A, Teixeira P, Villani N, et al. CT dose optimisation and reduction in osteoarticular disease. Diagn Interv Imaging 2013;94:371–88. [DOI] [PubMed] [Google Scholar]
- 10.Wang YZ.ed. Musculoskeletal system volume. In: Wu EH, ed. Chinese Medical Imaging. Beijing, People's Public Health Publishing Company; 2002:155–165. [Google Scholar]
- 11.Pantos I, Thalassinou S, Argentos S, et al. Adult patient radiation doses from noncardiac CT examinations: a review of published results. Br J Radiol 2011;84:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas D, Bible JE, Bohan M, et al. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am 2009;91:1882–9. [DOI] [PubMed] [Google Scholar]
- 13.Ng CS, Chandler AG, Wei W, et al. Reproducibility of CT perfusion parameters in liver tumors and normal liver. Radiology 2011;260:762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauno K. Association of impaired blood supply with painful lumbar disc degeneration. Acta Univ Oul D 2003;732–40. [Google Scholar]
- 15.Ma HT, Griffith JF, Zhao X, et al. Relationship between marrow perfusion and bone mineral density: a pharmacokinetic study of DCE-MRI. Conf Proc IEEE Eng Med Biol Soc 2012;2012:377–9. [DOI] [PubMed] [Google Scholar]
- 16.Bajwa NS, Toy JO, Young EY, et al. Disk degeneration in lumbar spine precedes osteoarthritic changes in hip. Am J Orthop (Belle Mead NJ) 2013;42:309–12. [PubMed] [Google Scholar]
- 17.Qin HY, Sun HR, Wang XF, et al. Correlation between CT perfusion parameters and microvessel density and vascular endothelial growth factor in adrenal tumors. PLoS One 2013;8:e79911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YXJ, Ko CH, Griffith JF, et al. Organic nitrate maintains bone marrow blood perfusion in ovariectomized female rats: a dynamic, contrast- enhanced magnetic resonance imaging (MRI) study. Pharmaceutics 2013;5:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendtsen M, Bünger CE, Zou X, et al. Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration. 26. Spine (Phila Pa 1976) 2011;36:E373–9. [DOI] [PubMed] [Google Scholar]
- 20.Seki S, Tsumaki N, Motomura H, et al. Cartilage intermediate layer protein promotes lumbar disc degeneration. Biochem Biophys Res Commun 2014;446:876–81. [DOI] [PubMed] [Google Scholar]
- 21.Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am 2009;47:161–78. [DOI] [PubMed] [Google Scholar]