Summary
Objective
Calcium absorption is an important determinant of calcium retention and bone metabolism. However, most methods of measuring calcium absorption, including the well-established dual stable isotope method, are costly and cumbersome to implement. We evaluated whether an oral calcium tolerance test (OCTT), which involves measuring calcium excretion in a fasting 2-h urine collection and two 2-h collections following an oral calcium dose, may be a useful index of calcium absorption in older adults consuming a fixed calcium intake of 30 mmol/day.
Design
After a 10-day metabolic diet containing 30 mmol/day of calcium, subjects had calcium absorption measured using the dual stable isotope method and the OCTT.
Participants
Eleven healthy subjects aged 54–74 years.
Measurements
Fractional calcium absorption (FCA), calcium excretion in a fasting 2-h urine collection and two 2-h collections in response to a 10-mmol calcium dose (total intake 30 mmol/ day).
Results
Calcium excretion from several combinations of the urine collections was examined in relation to FCA. The most predictive of FCA was calcium excretion 4 h following the calcium dose. This measure was significantly correlated with FCA (r = 0·735, P = 0·010), fitting 54% of the variability in FCA.
Conclusion
Urinary calcium excretion during the 4 h after a 10-mmol calcium dose is a useful index of calcium absorption among older adults consuming recommended calcium intakes. This test is inexpensive, easy to implement and potentially useful in large clinical studies.
Introduction
Intestinal calcium absorption is an important determinant of net calcium balance (retention) and bone metabolism.1 It is also a valuable measurement in clinical investigation. Dual stable isotopic tracers of calcium are used as a well-established methodology for measuring calcium absorption.2 This technique involves use of an oral and an intravenous calcium tracer. Typically, the oral tracer is given to fasting subjects with a calcium-fortified meal followed by the intravenous tracer.3 After administration of the tracers, a 24-h urine is collected, and the relative fractions of the two tracers are measured in the urine.3 This method is highly accurate, but the tracers and the equipment needed to measure them are costly and not widely available.
Broadus et al. described an oral calcium tolerance test (OCTT), which was used as an index of calcium absorption in both normal subjects and patients with hypercalciuria.4 This test consisted of three sequential 2-h urine collections. Eighteen normal subjects were placed for 3–5 days on a metabolic diet that provided 10 mmol/day of calcium.4 After a 2-h fasting urine was collected (collection 1), subjects were given a 25-mmol oral calcium dose (17·5 mmol of calcium as gluconate and 7·5 mmol of calcium in milk). After that, urine collections 2 and 3 were obtained.4 Calcium excretion was measured in all three collections. Broadus found a positive correlation between the change in calcium excretion from collection 1 to collection 3 corrected for glomerular filtration rate (GFR) and fractional calcium absorption (FCA) as determined by forearm count (r = 0·75, P < 0·001).4
Our study presents a variant of Broadus’ method in that it examines older subjects on currently recommended calcium intakes (30 mmol/day)5 instead of a low intake (10 mmol/day), and it assesses the calciuric response to a 10-mmol calcium dose rather than a 25-mmol dose. The latter change was made to better approximate the calcium content of a typical meal. We also examined calcium excretion from several combinations of the three urine collections obtained in the OCTT to determine which measurement best predicted FCA as measured by the dual stable calcium isotope method.
Methods
Study design and subjects
We studied 11 healthy subjects who comprised the placebo group of a randomized intervention study that examined effects of potassium bicarbonate on bone and muscle indices. Subjects consumed a metabolic diet containing a fixed calcium intake of 30 mmol/day for 10 days. On day 10 of the diet, calcium absorption was measured by the two methods being compared.
Healthy men and postmenopausal women age ≥50 were screened with a medical history, physical examination, and fasting blood and urine tests within 6 months of the study start date. Exclusion criteria included use of thiazide diuretics, use of osteoporosis medications in the last 2 years, use of oestrogen therapy in the last 6 months, a history of kidney stones, active hyperparathyroidism, creatinine clearance <50 ml/min/1·73 × m2 of body surface area, 24-h urinary calcium excretion >7·5 mmol/day, abnormal serum calcium and serum 25-hydroxyvitamin D [25(OH)D] level <40 nmol/l. We did not measure testosterone levels in the men in this study; however, no one reported a history of hypogonadism. None of the subjects had a fracture in the last year. None of the subjects had a history of malabsorption. The Tufts Medical Center – Tufts University Health Sciences Campus Institutional Review Board approved the study, and written informed consent was obtained from each subject.
Diet and supplements
During the 10-day metabolic diet, all food and caloric beverages were provided by the Metabolic Research Unit. The calcium content of the diet was 15 mmol/day. The diet also had a fixed phosphorus content of 35 mmol/day and a fixed sodium content of 113 mmol/day. The subjects were nonsmokers and were asked to abstain from alcohol during the study. The metabolic diet allowed up to 12 ounces of caffeinated beverages daily. Subjects also took a tablet containing 15 mmol of calcium (as tricalcium phosphate) and 125 IU of vitamin D3 (Posture D; US Rhodia, Cranbury, NJ, USA) and a multivitamin (CVS brand) containing 400 IU of vitamin D3 with the evening meal.
Biochemical measurements
Screening blood was drawn after a 12-h overnight fast and between 7:00 and 10:00 am. All samples from individual subjects were batched for analyses. Urinary calcium excretion was measured by direct-current plasma emission spectroscopy (Beckman Spectra-Span VI Direct Current Plasma Emission Spectrophotometer; Beckman Instruments, Fullerton, CA, USA) with a coefficients of variation (CV) of 3–5%. Serum calcium and urine creatinine were measured on an automated clinical chemistry analyser (Olympus AU400; Olympus America Inc., Melville, NY, USA) with CVs of 3–6%. Serum 25(OH)D level was measured with Diasorin radioimmunoassay kits (Stillwater, MN, USA) with CVs of 5·6–7·7%. Serum intact PTH was measured by chemiluminescent immunoradiometric assay on an automated immunoassay system (IMMULITE® 1000; Diagnostic Product Corporation, Los Angeles, CA, USA), with CVs of 5·5–6·6%.
Dual stable calcium isotope method
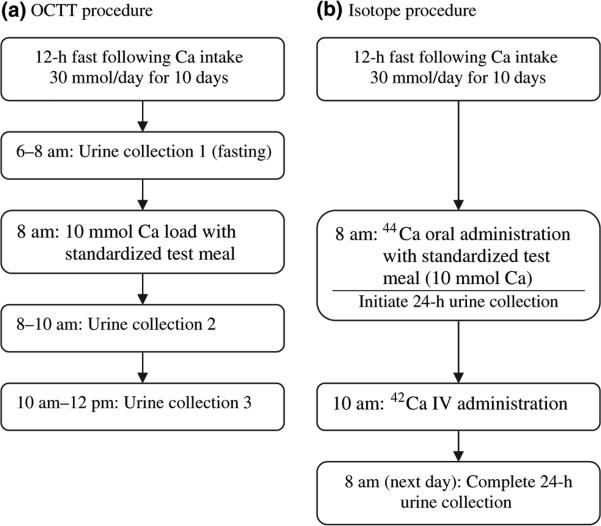
FCA was measured in each subject on the last day of the metabolic diet period.3 Figure 1 provides a flow chart of the procedure. Subjects arrived at the centre after an overnight fast, had a peripheral intravenous catheter placed and were given breakfast at 8:00 am. Towards the end of breakfast, subjects were given 44Ca (0·375 mmol for subjects weighing <80 kg and 0·575 mmol for those ≥80 kg) that had been mixed in 240 ml of calcium-fortified Minute Maid orange juice (8·5 mmol of calcium as phosphate and lactate). The breakfast and tracer drink combined contained a total of 10 mmol of calcium. Two hours after breakfast, 42Ca (0·025–0·038 mmol for subjects weighing <80 kg and 0·0575 mmol for those weighing ≥80 kg) was infused over 2 min intravenously. A 24-h urine collection began immediately after the oral tracer was administered with breakfast. When the collection was completed, an aliquot was prepared and analysed by the method of Chen et al.6 The 42Ca/44Ca ratio was measured by magnetic sector inductively coupled plasma mass spectrometry (ICP-MS, Bremen, Germany). FCA was determined as the ratio of the cumulative oral tracer recovery to the cumulative intravenous tracer recovery in the 24-h urine collections obtained postdosing. The precision of this method is <1%. The stable isotopes were purchased from Trace Sciences International Corporation (Richmond Hill, ON, Canada). The isotopic enrichments for these tracers were >95%. Tracers were prepared by the Tufts Medical Center Research Pharmacy and were tested for sterility and pyrogenicity prior to use.
Fig. 1.
Flow chart of the (a) oral calcium tolerance test (OCTT) procedure and (b) stable isotope procedure.
Oral calcium tolerance test
This test was performed on each subject on the same morning that the dual tracer method was performed (Fig. 1). From 6:00 to 8:00 am, a 2-h fasting urine collection (collection 1; -2 to 0 h) was obtained. At 8:00 am, the subject was given breakfast, which contained a total of 10 mmol of calcium as described earlier. Immediately following breakfast, urine was collected for two consecutive 2-h periods – collection 2 (0 to +2 h) and collection 3 (+2 to +4 h). An aliquot from each collection was analysed for calcium. Then, collections 2 and 3 were added to the 24-h urine obtained for the dual tracer study.
Statistical analysis
Point estimates are reported as mean ± SD. Pearson correlation coefficients and linear regression were used to describe the association between the two calcium absorption methods. Two-sided P-values less than 0·05 were considered to indicate statistical significance. Statistical analyses were conducted with spss version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Three men and eight women were studied. Mean age was 62 ± 7 years, mean weight was 64·1 ± 3·7 kg, mean 24-h urinary calcium excretion was 2·97 ± 1·88 mmol, mean 25(OH)D level was 64·1 ± 16·7 nmol/l, mean intact PTH level was 4·75 ± 0·94 pmol/l and mean serum calcium level was 2·28 ± 0·09 mmol/l.
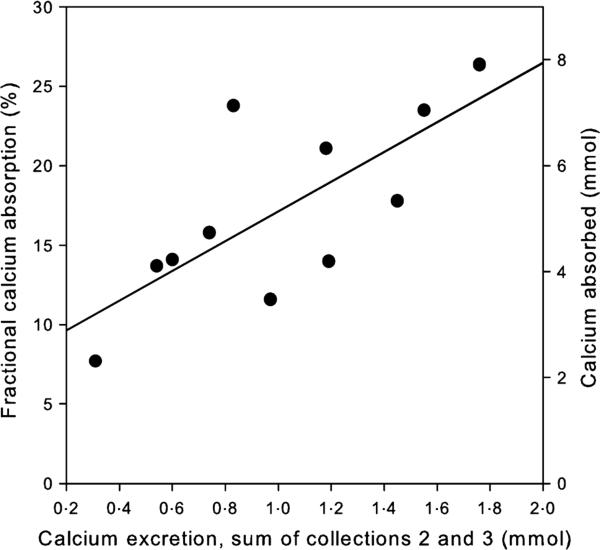
Mean FCA was 17·2 ± 5·8%. Table 1 provides the mean values of calcium excretion for individual urine collections, combined collections 2 and 3 and changes in calcium excretion from collection 1 (in order to express calciuric responses as a change from baseline). As expected following the calcium dose, calcium excretion increased in collections 2 and 3. The best predictor of FCA was the sum of calcium excretion from collections 2 and 3, representing calcium excretion during the 4 h after the calcium dose. The correlation of this measure with FCA was 0·735, P = 0·010. A scatterplot of this association is shown in Fig. 2. The regression equation relating the two measures is: FCA = 7·76 + 9·36 × (the sum of calcium excretion from collections 2 and 3 in mmol), and the r2 for the model is 0·54. Standardization of the calcium excretion for creati-nine in the combined collections 2 and 3 did not improve the correlation between the methods.
Table 1.
Calcium excretion (mean ± SD) during the 10 mmol oral calcium tolerance test
| Collection 1 (mean ± SD) | Collection 2 (mean ± SD) | Collection 3 (mean ± SD) | Collections 2 and 3 (mean ± SD) | |
|---|---|---|---|---|
| Calcium excretion (mmol) | 0·17 ± 0·15 | 0·50 ± 0·25 | 0·51 ± 0·23 | 1·01 ± 0·46 |
| Change from collection 1 (mmol) | 0·33 ± 0·18 | 0·34 ± 0·15 | 0·84 ± 0·36 |
Fig. 2.
Relationship between the sum of calcium excretion from collections 2 and 3 and fractional calcium absorption on the left Y-axis and calcium absorbed (fractional calcium absorption/100 × fixed calcium intake of 30 mmol/day) on the right Y-axis.
Calcium excretion levels from individual collections were modestly less well correlated with FCA (collection 2: r = 0·692, P = 0·018; collection 3: r = 0·714, P = 0·014). We also subtracted collection 1 from the combined collections 2 and 3, and although the resulting measure was also positively correlated with FCA (r = 0·702, P = 0·016), it was not better than the combined collections 2 and 3 alone. Changes in calcium excretion from collection 1 to collection 2 (r = 0·501, P = 0·116) and collection 3 (r = 0·495, P = 0·121) were not significantly correlated with FCA. Calcium excretion expressed as a fraction of body weight did not substantially alter the relationship between the two methods.
The right axis in Fig. 2 indicates calcium absorption in mmol, calculated as FCA/100 times the calcium daily intake (30 mmol in all subjects). Mean estimated calcium absorption by dual stable calcium isotope method was 5·16 ± 1·74 mmol/day, and the mean combined calcium excretion from collections 2 and 3 was about 20% of this amount (1·01 ± 0·46 mmol).
Discussion
Various methods have been developed to estimate intestinal calcium absorption in humans. One of the earliest methods used was calcium balance, which calculated dietary calcium consumed and calcium lost in urine and faeces over a matching period. Its major limitation has been its inability to differentiate unabsorbed dietary calcium in the faeces from absorbed calcium that is resecreted in the gastrointestinal tract. Therefore, it measures net calcium balance rather than true FCA.7,8 Several isotopic methods have been developed that can measure true FCA. These methods have employed radioisotopes, stable isotopes, or a combination of the two and have involved faecal, urine or plasma collections. Radio-isotopic methods, commonly using 45Ca (β emitter) and/or 47Ca (γ emitter), have had good precision (1–2%); however, the disadvantages include exposure to radioactivity and the cost of the tracers and equipment.9 The dual stable isotope tracer method with a 24-h timed urine collection is probably the most precise (<1%) and reliable of the currently available methods, and it avoids the need for a faecal collection.7,8 However, the dual isotope tracer method remains a time-consuming and expensive method to perform, especially in large clinical trials.
The calcium absorption index developed by Broadus et al. 4 presents a practical method for estimating calcium absorption in large studies that would not otherwise be able to include any measure of calcium absorption. In subjects habitually consuming a low-calcium diet (10 mmol/day), Broadus observed a correlation of the OCTT (as change in calcium excretion from collections 1 to 3 corrected for GFR) with FCA of 0·75. The correlation of our modified OCTT (the sum of calcium excretion from collections 2 and 3) with FCA was similar (r = 0·74), suggesting that our calcium absorption index may be equally useful in subjects consuming recommended calcium intakes (30 mmol/day)5. We also observed that subtracting predose calcium excretion (collection 1) did not improve the index. These modifications simplify the implementation of this test, because subjects can avoid the inconvenient 2-h early morning fasting urine collection and the need for a GFR calculation.
The strengths of our study include the fact that the two methods for assessing calcium absorption were performed on the same day and followed a 10-day metabolic diet with a fixed calcium intake. This is important because calcium absorption is influenced by calcium intake. The 10-day metabolic diet period that we used is long enough for older subjects to adapt to a change in calcium intake.10 Limitations of the study are the small sample size and the fact that we, like Broadus, evaluated the OCTT at only one calcium intake level. However, the similar correlations between the OCTT and FCA at a 10-mmol/day calcium intake4 and at a 30-mmol/day intake suggest that the OCTT may be a useful index over this intake range. Observing this correlation between the two methods in subjects on self-selected diets would confirm its application in field studies.
In conclusion, 4-h urinary calcium excretion after a 10-mmol calcium load is a practical index of calcium absorption in healthy older adults consuming 30 mmol/day of calcium. This simple urine test is inexpensive and easy to implement, and could be used in large clinical studies for which the cost of more sophisticated measurements would be prohibitive.
Acknowledgements
We thank the Metabolic Research Unit at the Jean Mayer USDA HNRCA at Tufts University and the staff at the USDA/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine for their work on the study. L. Ceglia was supported by grant DK007651. This research was supported by the Unilever Corporate Research, Bedfordshire, UK.
This material is based upon work supported by the US Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture.
Footnotes
Competing interests/financial disclosure
Nothing to declare.
References
- 1.Norman AW. Intestinal calcium absorption: a vitamin D-hormone-mediated adaptive response. The American Journal of Clinical Nutrition. 1990;51:290–300. doi: 10.1093/ajcn/51.2.290. [DOI] [PubMed] [Google Scholar]
- 2.Abrams SA, Yergey AL, Heaney RP. Relationship between balance and dual tracer isotopic measurements of calcium absorption and excretion. The Journal of Clinical Endocrinology and Metabolism. 1994;79:965–969. doi: 10.1210/jcem.79.4.7962306. [DOI] [PubMed] [Google Scholar]
- 3.Yergey AL, Abrams SA, Vieira NE, et al. Determination of fractional absorption of dietary calcium in humans. The Journal of Nutrition. 1994;124:674–682. doi: 10.1093/jn/124.5.674. [DOI] [PubMed] [Google Scholar]
- 4.Broadus AE, Dominguez M, Bartter FC. Pathophysiological studies in idiopathic hypercalciuria: use of an oral calcium tolerance test to characterize distinctive hypercalciuric subgroups. The Journal of Clinical Endocrinology and Metabolism. 1978;47:751–760. doi: 10.1210/jcem-47-4-751. [DOI] [PubMed] [Google Scholar]
- 5.Food and Nutrition Board . Institute of Medicine Dietary Reference Intakes: Calcium, Magnesium, Phosphorus, Vitamin D and Fluoride. National Academy Press; Washington, DC.: 1997. [Google Scholar]
- 6.Chen Z, Griffin IJ, Kriseman YL, et al. Inductively coupled plasma mass spectrometric analysis of calcium isotopes in human serum: a low-sample-volume acid-equilibration method. Clinical Chemistry. 2003;49:2050–2055. doi: 10.1373/clinchem.2003.025692. [DOI] [PubMed] [Google Scholar]
- 7.Heaney RP. Factors influencing the measurement of bio-availability, taking calcium as a model. The Journal of Nutrition. 2001;131:1344S–1348S. doi: 10.1093/jn/131.4.1344S. [DOI] [PubMed] [Google Scholar]
- 8.Griffin IJ, Abrams SA. Methodological considerations in measuring human calcium absorption: relevance to study the effects of inulin-type fructans. The British Journal of Nutrition. 2005;93(Suppl. 1):S105–S110. doi: 10.1079/bjn20041344. [DOI] [PubMed] [Google Scholar]
- 9.Weaver C. Clinical approaches for studying calcium metabolism and its relationship to disease. In: Weaver C, Heaney R, editors. Calcium in Human Health. Humana Press; Totowa, NJ: 2006. pp. 65–81. [Google Scholar]
- 10.Dawson-Hughes B, Stern DT, Shipp CC, et al. Effect of lowering dietary calcium intake on fractional whole body calcium retention. The Journal of Clinical Endocrinology and Metabolism. 1988;67:62–68. doi: 10.1210/jcem-67-1-62. [DOI] [PubMed] [Google Scholar]