Abstract
Introduction
Irisin is a newly identified 112 amino acid hormone, derived as a product of fibronectin type III domain containing 5 (FNDC5), which is highly related to metabolic activity in skeletal muscle and brown fat. The effects of irisin on cardiovascular functions are unknown.
Purpose
To explore the effects of central and peripheral irisin on cardiovascular functions.
Methods
Irisin was either administrated into 3rd ventricle of rats or intravenously, and its effects on blood pressure and cardiac contractibility measured.
Results
Administration of recombinant human irisin into the 3rd brain ventricle of rats activated neurons in the paraventricular nuclei of the hypothalamus. Central administration of irisin increased blood pressure and cardiac contractibility. Exogenous irisin reversed atenolol-induced inhibition of cardiac contractibility. In contrast, peripheral administration of irisin reduced blood pressure in both control and spontaneously hypertensive rats. Irisin dilated mesenteric artery rings through ATP-sensitive potassium channels.
Conclusion
Our studies indicate that central and peripheral irisin may differentially regulate cardiovascular activities.
Keywords: Irisin, Hypothalamus, Blood pressure, Cardiac contractibility
Introduction
Irisin was originally recognized as a 112 amino acids poly-peptide hormone secreted as a product of fibronectin type III domain containing 5 (FNDC5) from skeletal muscle both in mice and humans [1]. Exercise induces FNDC5 gene expression in skeletal muscle and increases irisin concentration in the circulation [2–5]. Irisin targets white adipocytes to induce browning, activating thermogenesis to increase energy expenditure through cross-talk between skeletal muscle and adipose tissues [1, 3, 6, 7]. Irisin in circulation is associated with insulin resistance [8, 9] and atherosclerosis [9].
Irisin may have a role in the nervous system and heart based upon expression of FNDC5 in these two organs [10, 11]. Studies using antiserum against irisin peptide fragment (42–112) reveal irisin-positive cells in skeletal muscle, heart and Purkinje cells of cerebellum [12]. Aydin et al. also demonstrated the presence of irisin immunoreactivity in both neurons and neuroglia of rat brain [13]. Knockdown of Fndc5 significantly decreases neural differentiation of mouse embryonic stem cells [14], while neurite outgrowth and synaptogenesis increase in a dose-dependent manner by irisin exposure in mouse H19-7 HN cells [15]. Irisin is present in human cerebrospinal fluid (CSF) and in human hypothalamic sections [16]. In paraventricular neurons, co-localization with neuropeptide Y [16] suggests that irisin might exert central effects.
It is currently unknown whether irisin influences cardiovascular functions. Here we demonstrate that central administration of recombinant human irisin (r-irisin) by intracerebroventricular (ICV) injection results in an activation of neurons in the paraventricular nucleus (PVN) region, which is associated with increases in systemic blood pressure and cardiac contractibility. Conversely, peripheral administration of r-irisin reduces blood pressure, while not affecting cardiac contractibility.
Materials and Methods
3rd Ventricle Cannulation
All animal studies were approved by the University of Michigan Committee on the Use and Care of Animals. Animals were housed in a temperature controlled environment with 12 h light and dark cycles, and access to food and water ad libitum. Third ventricular cannulation in rats was prepared as previously described [17]. Male Sprague–Dawley rats weighing 200–250gwereanesthetized with intra-peritoneal ketamine and xylazine and placed on a stereotaxic device with the incisor bar 3.3 mm below the interaural line according to Paxinos and Watson. A stainless steel 26 gauge guide cannula was implanted into the third ventricle using the following stereotaxic coordinates: 2.4 mm posterior to the bregma, 8.4 mm ventral to the surface of the skull and directly along the midline. The cannula was anchored to the skull with four screws and dental cement. An internal cannula was placed into the guide cannula to maintain patency. Rats were allowed to recover for 1 week. Guide cannula patency was assessed by injection of 10 ng angiotensin II in 5 μl of saline[18]. Cannulas were considered patent if rats consumed at least 5 ml of water within 1 h of injection. Rats with correct third ventricle cannulation were used 5 days later. All rats were handled daily to minimize stress reactions to manipulation. Animals were randomly divided into two groups: IgG Fc peptide or r-irisin dissolved in cerebrospinal fluid. Each group contained 5–8animals.
Measurements of Blood Pressure
Blood pressure was measured by a 1.4 F micro-tip catheter sensor (model SPR-671, Millar Instruments Inc, Houston, TX) inserted to the bifurcation of iliac artery and connected to a transducer (850–5101 AEC-10D, Millar Instruments Inc, Houston, TX).±dp/dt was the synthetic derivative of left cardiac ventricular pressures which were monitored by insertion of another microtip catheter sensor into the left cardiac ventricle via the right carotid artery. Data were acquired and analyzed by Powerlab 8/30 data-acquisition system and chart software (AD Instruments, Colorado Springs, CO) [19].
Mesenteric Artery Contraction and Relaxation Assays
Mesenteric arteries were isolated from Sprague–Dawley rats (200–250 g) and placed on ice in a physiological saline solution (PSS) consisting of 10 mmol/L glucose, 24.9 mmol/L NaHCO3, 118 mmol/L NaCl, 1.18 mmol/L Mg2SO4, 4.7 mmol/L KCl, 1.18 mmol/L KH2PO4, 2.5 mmol/L CaCl2, and 0.03 mmol/L EDTA. Second order mesenteric arteries (2 mm long) were mounted onto a wire myograph (Model 610 M, DMT). The wall tension developed by the vessel was measured as previously described [20]. The endothelium of a subgroup of rings was removed by scraping using a forceps tip before mounting onto wire myographs. After normalization, pre-stimulation with potassium and balance period, vessels were pre-constricted with 10−6 M phenylephrine (PE) and then treated with increasing concentrations of recombinant human irisin. Data acquisition was performed using the DMT Normalization Module (AD Instruments). Data are shown as mean ± SEM, n=6 in each group.
Data Analysis
Mean ± SEM values were analyzed using Prism (version 6). Statistical comparisons between two groups were performed by Student's t test, and among three groups were performed by one-way ANOVA. The curves of locomotor and metabolic activity, and vasoactivity of artery rings were adjusted with nonlinear regression first, and then were compared by twoway ANOVA and Bonferroni post-tests. Significance was accepted as p<0.05.
Results
Central Administration of Irisin Activates Hypothalamic Neurons
Activation of the paraventricular nucleus (PVN) in the hypothalamus has been reported to increase sympathetic outflow, with resultant rise in energy expenditure, blood pressure and cardiac output [21–23]. We thus examined hypothalamic nuclei activated by irisin using the c-fos oncogene as a marker. As shown in Fig. 1, administration of r-irisin(2.5 μg) by ICV injection produced a rapid induction of c-fos immunoreactivity in the PVN. Both the intensity and total area of c-fos immunoreactivity increased significantly relative to control injection with IgG Fc peptide. No significant difference in c-fos immunoreactivity was observed in other hypothalamic nuclei, including the arcuate nuclei, ventromedial nuclei, dorsomedial nuclei and lateral nuclei.
Fig. 1.
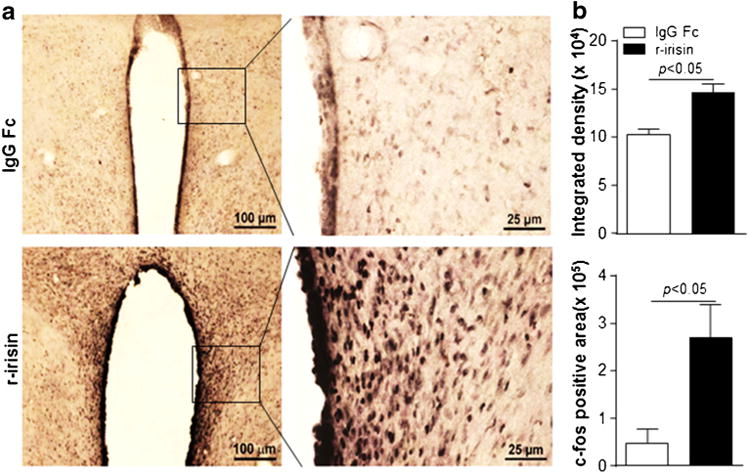
Activation of c-fos in PVN neurons (a) c-fos immnuoreactivity was observed only in paraventricular nuclei (PVN) of the hypothalamus 90 min after administration of recombinant human irisin (r-irisin) at 2.5 μg/rat. (b) c-fos positive signal intensity and area expressed as mean±SEM. p<0.05 denotes statistical significance
Pressor Response to Central Administration of Irisin
We next examined the effects of centrally administrated irisin on the blood pressure. Our study showed that blood pressure was increased immediately following injection of 0.625–2.5 μg/rat r-irisin in the 3rd ventricle. Elevation of blood pressure was not caused by Fc segment in the recombinant human irisin (Fig. 2a). When compared to basal levels, 0.625, 1.25 and 2.5 μg/rat r-irisin increased systolic blood pressure by 8 ± 37, 24 ± 9 and 50 ± 8 mmHg, respectively (Fig. 2b). Diastolic blood pressure was increased as well. When compared to the basal pressures, r-irisin at 0.625, 1.25 and 2.5 μg/rat elevated diastolic blood pressure by 5±2, 17±10 and 37±15 mmHg, respectively (Fig. 2b). Heart rate increased dose-dependently, 413± 80, 423±78 and 468±41 bpm from a baseline of 331± 42 bpm after administration of 0.625, 1.25 and 2.5 μg/rat of r-irisin, respectively. Administration of control peptide IgG Fc did not alter heart rate.
Fig. 2.
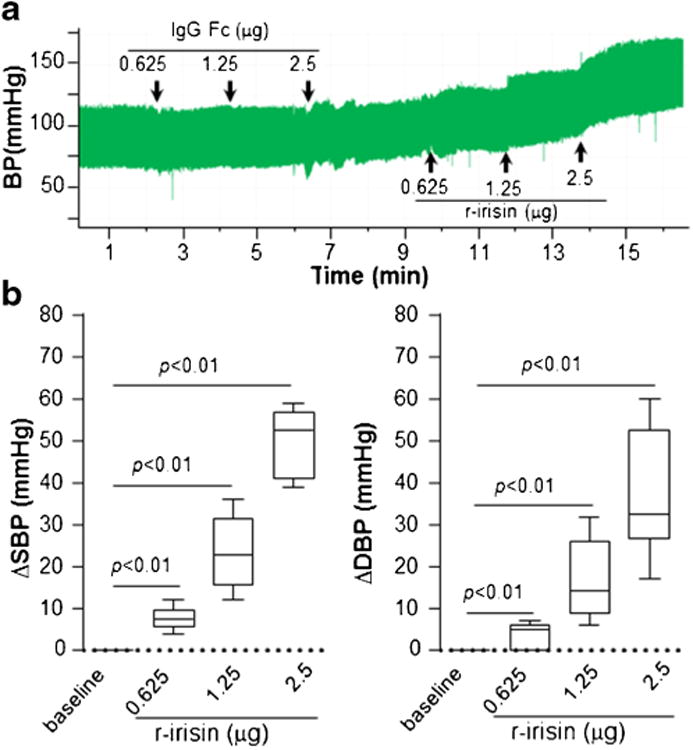
Pressor response to central administration of irisin (a) Blood pressure was recorded with IgG Fc peptide or r-irisin at indicated dosage dissolved in 2 μl artificial cerebral-spinal fluid. All drugs were delivered via cannula injection starting at the time indicated by arrows. (b) Systolic and diastolic blood pressure was averaged for 2 min periods. Change of each treatment from baseline was calculated. Data are plotted as minimum to maximum (n=6 in each group). Irisin (0.625–2.5 μg) significantly increased systolic (ΔSBP) and diastolic blood pressure (ΔDBP), respectively, while IgG Fc peptide had no effect
Next, we investigated whether central administration of ririsin elevated blood pressure through peripheral vasopressor substances such as adrenaline. As showed in Fig. 3a, intravenous injection of 0.5 mg/kg phenoxybenzamine, a non-selective α-adrenergic receptor antagonist, completely blocked epinephrine-induced elevation of blood pressure, but demonstrated no effect on the increase of blood pressure induce by 0.625 or 1.25 μg of r-irisin. However, phenoxybenzamine partially blocked pressor effects of r-irisin at the dose of 2.5 μg/rat (Fig. 3b).
Fig. 3.
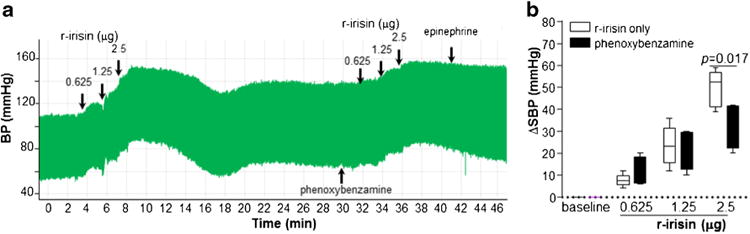
Vasopressor effects of central irisin (a) Blood pressure was monitored as indicated. Five minutes after intravenous injection of phenoxybenzamine (0.5 mg/kg), or recombinant irisin (0.625–2.5 μg) were delivered via cannula injection starting at the time indicated by arrows. (b) Systolic and diastolic blood pressures were averaged for 2 min periods. Change of each treatment from baseline was calculated. Data are plotted as minimum to maximum (n=6 in each group)
Central Irisin Enhanced Cardiac Output which was Associated with Pressor Effects
Cardiac contractility (+dp/dt) increased by 1674±918, 6905± 1293 and 13062±2648 mmHg/s relative to basal + dp/dt with increasing doses of r-irisin (Fig. 4); −dp/dt was reduced by 1650±884, 6545±2534 and 12138±2908 mmHg/s relative to basal -dp/dt(Fig. 4a, b). Intravenous administration of 2 mg/kg atenolol, a β-adrenergic receptor antagonist, significantly reduced baseline cardiac contractility (Fig. 4c, d, upper panels). Systemic blood pressure was reduced in response to administration of atenolol as well (Fig. 4c, d lower panels). Central administration of r-irisin reversed the reduction of ± dp/dt caused by atenolol (Fig. 4c,d, upper panels). Similarly, depressor effects of atenolol were significantly reversed by central r-irisin (Fig. 4c,d, lower panels).
Fig. 4.
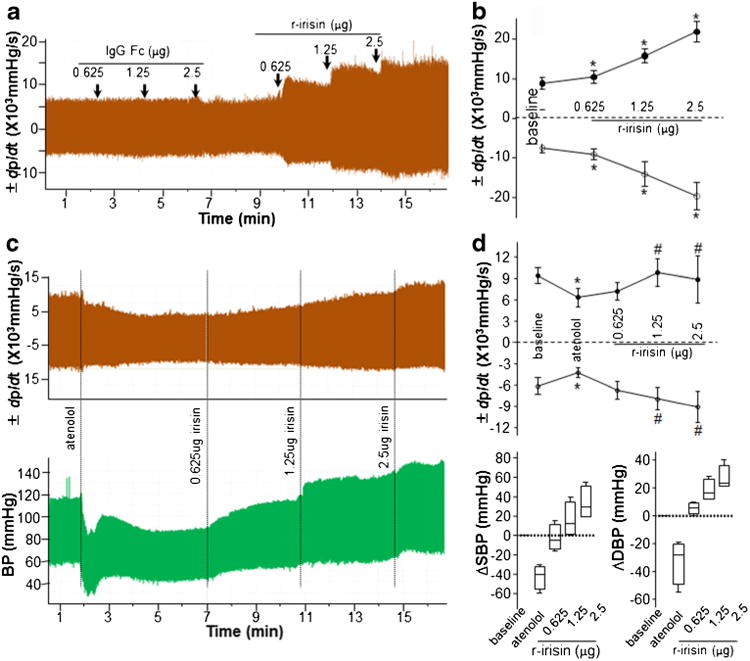
Increased cardiac contractility by irisin (a) Cardiac contractibility was monitored following treatment with IgG Fc peptide or r-irisin as indicated. All drugs were delivered via cannula injection starting at time indicated by arrows. (b) ± dp/dt in was averaged for 2 min periods. Data are shown as mean ± SEM (n=6 in each group). (c) Five minutes after intravenous injection of atenolol (2 mg/kg), r-irisin (0.625–2.5 μg) was delivered via cannula injection starting at the time indicated. (d) ± dp/dt, systolic and diastolic blood pressure was averaged for 2 min periods. Change of each treatment from baseline was calculated. Data of ± dp/dt are shown as mean ± SEM, and data of blood pressure are plotted as minimum to maximum (n=4 in each group)
Peripheral Administration of Irisin Reduced Blood Pressure
Peptides do not readily enter the central nervous system from blood due to poor transport through the blood–brain barrier [24]. Irisin is a peptide with molecular weight ∼25Kd, suggesting that skeletal muscle-derived irisin might exist within the circulation. It is currently unknown whether irisin can cross the blood–brain barrier. Therefore, we investigated the peripheral effects of irisin on blood pressure. Intravenous injection of recombinant human irisin reduced both systolic and diastolic blood pressure, while injection of control IgG Fc peptide did not affect blood pressure either in control Sprague Dawley (SD) rats (Fig. 5a, upper panel) or spontaneously hypertensive rats (SHR) (Fig. 5b). Distinct from the effects of centrally administered r-irisin on cardiac contractibility, peripheral administration of r-irisin did not change cardiac contractibility (Fig. 5a, lower panel), suggesting that cardiac output was not involved in depressor effects of peripheral irisin. In vitro, administration of r-irisin dilated mesenteric artery rings of control animals with or without endothelium of control rats (Fig. 5c). The r-irisin-induced dilation in vessel rings without endothelium was less than that observed in intact endothelium rings, indicating that both endothelial cells and smooth muscle cells were involved in r-irisin-induced vessel dilation. KATP channels are major contributors to r-irisin-induced vessel dilation, as evidenced by the effects of glibenclamide, a KATP channel blocker, which significantly blocked r-irisin-induced dilation in vessel rings with or without endothelium (Fig. 5c). Of interest, r-irisin did not dilate mesenteric artery rings which were pre-contracted by 60 mM KCl (Supplemental Figure 1), suggesting that K+ may positively involve in irisin-induced relaxation of vessel rings.
Fig. 5.
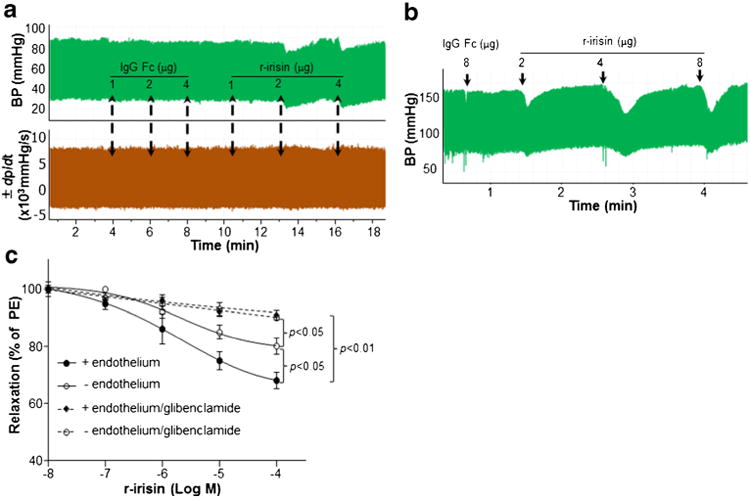
Peripheral administration of irisin reduced blood pressure. Blood pressure and cardiac contractility were measured in control (SD) or spontaneously hypertensive (SHR) rats. IgG Fc peptide and r-irisin were delivered via femoral vein injection. (a) Control IgG Fc peptide did not change blood pressure; r-irisin (2–4 μg) significantly reduced blood pressure (upper panel). Neither IgG Fc nor r-irisin changed cardiac contractility (lower panel). (b) r-irisin(2–8 μg) significantly reduced blood pressure in SHR. (c) Vasoactivity of mesenteric artery rings with or without endothelium was measured. The rings were pre-constricted with 10−6 M phenylephrine (PE). When the contraction of rings reached plateau level, relaxation in response to r-irisin was assayed in the presence or absence of 10 μM glibenclamide (a KATP channel blocker)
Discussion
Irisin is a newly identified hormone that is derived from skeletal muscle in response to exercise and cold stimuli [25]. Growing evidence suggests that irisin is related to obesity and diabetes in humans [16, 26], although current data are inconsistent due to limitations of methods used for detection of plasma irisin [27]. A previous study reported that circulating irisin was elevated in humans with exercise, and it was postulated that exercise-induced production of irisin in skeletal muscle affected metabolic activity [3].
Our study suggests that irisin may activate the central nervous system to coordinate the cardiovascular functions. This conclusion is supported by the following observations. A robust and sustained rise in cardiac output and blood pressure followed 3rd ventricular injection of irisin, while PVN neurons were activated by irisin. This effect occurs within minutes of irisin administration, suggesting a rapid communication between the irisin-induced activation of the central nervous system and the cardiovascular function. This conclusion is consistent with previous studies demonstrating the central effects of irisin on neuronal development [14, 15].
An organism's reaction to changes in ambient environmental conditions requires coordinated action involving skeletal muscle, adipose tissue, the nervous system, and endocrine organs. Mechanisms by which skeletal muscle might signal the central nervous system are largely unknown. Previous studies have identified irisin as a novel cross-organ messenger between skeletal muscle and adipose tissue. However, it is currently unknown whether irisin may function as a messenger between the skeletal muscle and brain. Further exploration should aim to determine whether circulating irisin is able to cross the blood–brain-barrier.
Our study provides novel evidence that irisin may also regulate the cardiovascular functions. The mechanisms by which central activation by irisin exerts cardiovascular effects are unknown. Our data suggest that the pressor effects of central irisin are a consequence of increases in cardiac output by activation of PVN neurons in the hypothalamus. Numerous studies have indicated that the hypothalamus modulates blood pressure by mechanisms involving adrenergic sympathetic activity [21–23], in part via vasoconstrictors such as adenanline and vasopressin. This study demonstrated that plasma vasopressin was significantly increased at 3 min with central irisin administration (data not shown). Similar concentrations of irisin did not increase plasma norepinephrine levels (7.8±0.3 and 8.4±0.4 ng/ml for control animals and those treated with irisin respectively), indicating that vasopressin but not norepinephrine may be responsible for pressor effects of central irisin.
In contrast to the effects of central irisin, peripheral irisin did not alter cardiac contractibility, but reduced blood pressure. Our data suggest that blood vessels are targets for peripheral irisin in terms of regulation of blood pressure, and that both endothelial cells and smooth muscle cells are affected by irisin. Irisin was able to dilate the vessel rings without endothelium, but dilation effect was greater in vessel rings with intact endothelium. Ion channels, especially calcium and potassium channels are involved in regulation of vascular tone and blood pressure. ATP sensitive potassium channel (KATP) was associated with peripheral irisin-lowered blood pressure. Pretreatment vessel rings with glibenclamide, a KATP channel blocker, dramatically abolished irisin-induced vessel dilation.
In summary, brain-derived irisin may function to increase cardiac output and blood pressure by activating hypothalamic PVN neurons, while peripheral irisin derived from skeletal muscle in response to exercise or cold stimuli may prevent the elevation of blood pressure induced by sympathetic out-flow. Irisin may serve as an important cross-organ messenger linking skeletal muscle with the brain, adipose tissue and the cardiovascular system to integrate the energy expenditure with the cardiovascular activity. Our studies demonstrate for the first time that central irisin may function to increase cardiac output and blood pressure by activating the hypothalamic PVN neurons, while peripheral irisin derived from skeletal muscle in response to exercise or cold stimuli may prevent the elevation of blood pressure induced by sympathetic out-flow. Thus, our studies suggest that irisin may serve as an important cross-organ messenger linking skeletal muscle with the brain, adipose tissue and the cardiovascular system to integrate the energy expenditure with the cardiovascular activity. One can thus speculate that irisin may link the exercise with blood pressure and adiposity.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81330010 and 81390354 to W.Z.), American Diabetes Association grant #1-13-BS-225 (to W.Z.), and the National Institute of Health grants 5R37DK043225 (to M. M.), HL105114 (to E. C.) and HL122664 (to L. C.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10557-015-6580-y) contains supplementary material, which is available to authorized users.
Disclosures None.
Contributor Information
Weizhen Zhang, Email: weizhenz@umich.edu, Department of Surgery, University of Michigan, 4618B, MS II, 1301 E. Catherine Street, Ann Arbor, MI 48109, USA; Diabetes Center, Shenzhen University Health Science Center, Shenzhen 518060, China.
Lin Chang, Email: lincha@umich.edu, Department of Internal Medicine, University of Michigan, 2800 Plymouth Rd, Ann Arbor, MI 48109, USA.
Chao Zhang, Department of Surgery, University of Michigan, 4618B, MS II, 1301 E. Catherine Street, Ann Arbor, MI 48109, USA.
Ruthann Zhang, Department of Internal Medicine, University of Michigan, 2800 Plymouth Rd, Ann Arbor, MI 48109, USA.
Ziru Li, Department of Surgery, University of Michigan, 4618B, MS II, 1301 E. Catherine Street, Ann Arbor, MI 48109, USA.
Biaoxin Chai, Department of Surgery, University of Michigan, 4618B, MS II, 1301 E. Catherine Street, Ann Arbor, MI 48109, USA.
Jiyao Li, Department of Surgery, University of Michigan, 4618B, MS II, 1301 E. Catherine Street, Ann Arbor, MI 48109, USA.
Eugene Chen, Department of Internal Medicine, University of Michigan, 2800 Plymouth Rd, Ann Arbor, MI 48109, USA.
Michael Mulholland, Department of Surgery, University of Michigan, 4618B, MS II, 1301 E. Catherine Street, Ann Arbor, MI 48109, USA.
References
- 1.Bostrom P, Wu J, Jedrychowski MP, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenmoehl J, Albrecht E, Komolka K, et al. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int J Biol Sci. 2014;10:338–49. doi: 10.7150/ijbs.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on pgc-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–49. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 4.Hecksteden A, Wegmann M, Steffen A, et al. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med. 2013;11:235. doi: 10.1186/1741-7015-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraemer RR, Shockett P, Webb ND, Shah U, Castracane VD. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm Metab Res. 2014;46:150–4. doi: 10.1055/s-0033-1355381. [DOI] [PubMed] [Google Scholar]
- 6.Lee P, Linderman JD, Smith S, et al. Irisin and fgf21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Li R, Meng Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 map kinase and erk map kinase signaling. Diabetes. 2014;63:514–25. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Legarrea P, de la Iglesia R, Crujeiras AB, et al. Higher baseline irisin concentrations are associated with greater reductions in glycemia and insulinemia after weight loss in obese subjects. Nutr Diabetes. 2014;4:e110. doi: 10.1038/nutd.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sesti G, Andreozzi F, Fiorentino TV, et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0576-0. [DOI] [PubMed] [Google Scholar]
- 10.Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and frcp2, two novel fibronectin type iii repeat containing genes. Gene. 2002;297:79–83. doi: 10.1016/s0378-1119(02)00828-4. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer-Martinez A, Ruiz-Lozano P, Chien KR. Mouse pep: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224:154–67. doi: 10.1002/dvdy.10099. [DOI] [PubMed] [Google Scholar]
- 12.Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisinimmunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–62. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aydin S, Kuloglu T, Aydin S, et al. A comprehensive immunohisto-chemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61C:130–6. doi: 10.1016/j.peptides.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Hashemi MS, Ghaedi K, Salamian A, et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse h19-7 hippocampal cell lines. Metabolism. 2013;62:1131–6. doi: 10.1016/j.metabol.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piya MK, Harte AL, Sivakumar K, et al. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab. 2014;306:E512–8. doi: 10.1152/ajpendo.00308.2013. [DOI] [PubMed] [Google Scholar]
- 17.Walls EK, Wishart TB. Reliable method for cannulation of the third ventricle of the rat. Physiol Behav. 1977;19:171–3. doi: 10.1016/0031-9384(77)90177-9. [DOI] [PubMed] [Google Scholar]
- 18.Li JY, Chai BX, Zhang W, Wang H, Mulholland MW. Expression of ankyrin repeat and suppressor of cytokine signaling box protein 4 (asb-4) in proopiomelanocortin neurons of the arcuate nucleus of mice produces a hyperphagic, lean phenotype. Endocrinology. 2010;151:134–42. doi: 10.1210/en.2009-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Villacorta L, Chang L, et al. Nitro-oleic acid inhibits angiotensin ii-induced hypertension. Circ Res. 2010;107:540–8.20. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang L, Villacorta L, Li R, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–78. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–52. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 22.Mark AL, Rahmouni K, Correia M, Haynes WG. A leptin-sympathetic-leptin feedback loop: potential implications for regulation of arterial pressure and body fat. Acta Physiol Scand. 2003;177:345–9. doi: 10.1046/j.1365-201X.2003.01085.x. [DOI] [PubMed] [Google Scholar]
- 23.Correia ML, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Role of corticotrophin-releasing factor in effects of leptin on sympathetic nerve activity and arterial pressure. Hypertension. 2001;38:384–8. doi: 10.1161/01.hyp.38.3.384. [DOI] [PubMed] [Google Scholar]
- 24.Pardridge WM. Recent developments in peptide drug delivery to the brain. Pharmacol Toxicol. 1992;71:3–10.25. doi: 10.1111/j.1600-0773.1992.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 25.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:E9–10. doi: 10.1038/nature11364. discussion E10-11. [DOI] [PubMed] [Google Scholar]
- 26.Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091–107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson HP. Irisin and fndc5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte. 2012;2:289–93. doi: 10.4161/adip.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


