The case
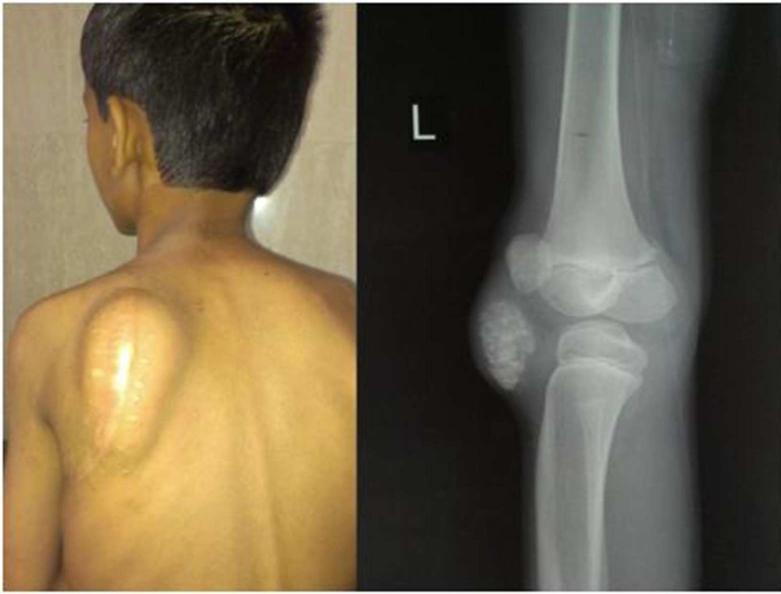
A nine year old boy, born of third degree consanguinity, presented with a hard left scapular swelling for 6 months. There was no history of fever, trauma or weight loss. There was no pain, redness or discharge. This mass was excised but recurred over the next 6 months (Left panel of Figure 1). A similar swelling had been excised from the left knee one year before presentation, but had recurred over 6 months. Family history was negative for similar lesions. Development was normal for age and he had no other medical problems.
Figure 1.
Panel on left: The mass in the scapular region had recurred after surgical excision. Panel on right: Radiograph showing a recurrent calcified lesion lateral to left knee
On examination, pulse was 84 per minute, blood pressure 96/68 mmHg, weight 22 kg and height 122 cm (both between 10th and 25th percentile). A 6 × 4 cm mass was noted in the right scapular region and a 3 × 3 cm mass at the lateral aspect of the left knee. Both of these masses were firm to hard, globular, nontender, and fixed to the bone. The overlying skin was without erythema or local warmth, though scars from the previous resections were noted. Lymphadenopathy was absent. The rest of the examination was unremarkable.
Radiographs of the knee showed a lobular, inhomogenously but densely calcified lesion in the anterolateral region of the left knee (Right panel of Figure 1). Serum calcium was 9.3 mg/dl (2.32 mmol/l), phosphate 8.9 mg/dl (2.87 mmol/l, normal range 3.7-5.6 mg/dl), creatinine 0.7 mg/dl (61.88 μmol/l), alkaline phosphatase 133 units/L, intact parathyroid hormone 5.8 ng/l (16-67 ng/l), 25-hydroxyvitamin D 20.9 ng/ml (>20 ng/ml- sufficiency), 1,25-dihydroxyvitamin D 30.9 pg/ml (19.6-54.3 pg/ml). Tubular maximum reabsorption of phosphate corrected for GFR (TMP/GFR) was 8.5 mg/dl (2.74mmol/l, normal range 3.8-5 mg/dl).
What is the diagnosis? What is the treatment?
The Diagnosis: Familial hyperphosphatemic tumoral calcinosis
Familial hyperphosphatemic tumoral calcinosis (FHTC) is a rare autosomal recessive disorder of phosphate metabolism characterized by hyperphosphatemia and deposition of calcium phosphate in the soft tissues.1
Fibroblast growth factor 23 (FGF23) inhibits sodium-phosphate cotransport in the proximal tubule leading to phosphaturia, and inhibits production of 1,25-dihydroxyvitamin D.2 Decreased activity of FGF23 occurs due to recessive loss-of-function mutations in the FGF23 gene or GALNT3 gene (an enzyme that glycosylates FGF23) or due to mutation in KLOTHO which encodes a cofactor necessary for FGF23 binding to its receptor.1 InFGF23 or GALNT3 mutations , the ability to secrete intact biologically active FGF23 is impaired, due to increased proteolytic cleavage into inactive fragments. With KLOTHO mutations, end-organ response to FGF23 is impaired, causing an FGF23-resistant state. In either situations the diminished FGF23 activity leads to increased renal phosphate reabsorption indicated by increased TMP/GFR, and sometimes elevated 1,25-dihydroxyvitamin D. Progressive soft tissue calcium-phosphate deposition occurs near joints and sometimes in the vessel walls..
Radiography identifies a calcified, often lobular lesion. Hyperphosphatemia with normal renal function, normal or high serum calcium and 1, 25-dihydroxyvitamin D, and normal or suppressed parathyroid hormone are clues to the diagnosis. In the setting of either FGF23 or GALNT3 mutations, C-terminal FGF23 levels (measuring fragments plus intact FGF23) are elevated consistent with physiologic response to hyperphosphatemia. However, intact FGF23 concentrations are low.. However, in the setting of KLOTHO mutation, as an FGF23 resistant state, both C-terminal and intact FGF23 concentrations were elevated in the only case reported to date.4
After surgical resection lesions frequently recur, since the underlying phosphate metabolism defect persists. Lesions may enlarge over time and vascular calcifications may occur as well. Aggressive management with dietary phosphate restriction and phosphate binders may be of benefit, though evidence is limited to case reports.1, 3 Acetazolamide induces phosphaturia and has been used with success. 5
Genetic analysis of this patient revealed a previously described homozygous mutation in exon 3 of FGF23 causing an amino acid change: S129P. 6 C-terminal FGF23 concentration was 1050 RU/ml (normal < 180 RU/ml) and intact FGF23 concentration was 22 pg/ml (normal <70 pg/ml), but a “low normal” concentration is an inappropriate physiologic response to hyperphosphatemia 2, consistent with the expected pattern due to FGF23 mutations causing FHTC. After six months of treatment with sevelamer carbonate and aluminium hydroxide, the patient's serum phosphate levels have decreased to 6 mg/dl, and the lesions have resolved.
Acknowledgements
This work was supported in part by grants from the by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers K23AR057096 (EAI) and R01AR42228 (MJE).
Sources of support: This work was supported in part by grants from the by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers K23AR057096 (EAI) and R01AR42228 (MJE).
References
- 1.Farrow EG, Imel EA, White KE. Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and αKlotho). Best Pract Res Clin Rheumatol. 2011;25:735–747. doi: 10.1016/j.berh.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imel EA, Econs MJ. Approach to the Hypophosphatemic Patient. J Clin Endocrinol Metab. 2012;97:696–706. doi: 10.1210/jc.2011-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson T, Yu X, Davis SI, et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammoglia JJ, Mericq V. Familial tumoral calcinosis caused by a novel FGF23 mutation: response to induction of tubular renal acidosis with acetazolamide and the non-calcium phosphate binder sevelamer. Horm Res. 2009;71:178–184. doi: 10.1159/000197876. [DOI] [PubMed] [Google Scholar]
- 6.Bergwitz C, Banerjee S, Abu-Zahra H, et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94:4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]