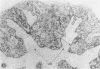
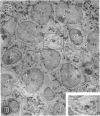
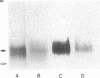
Abstract
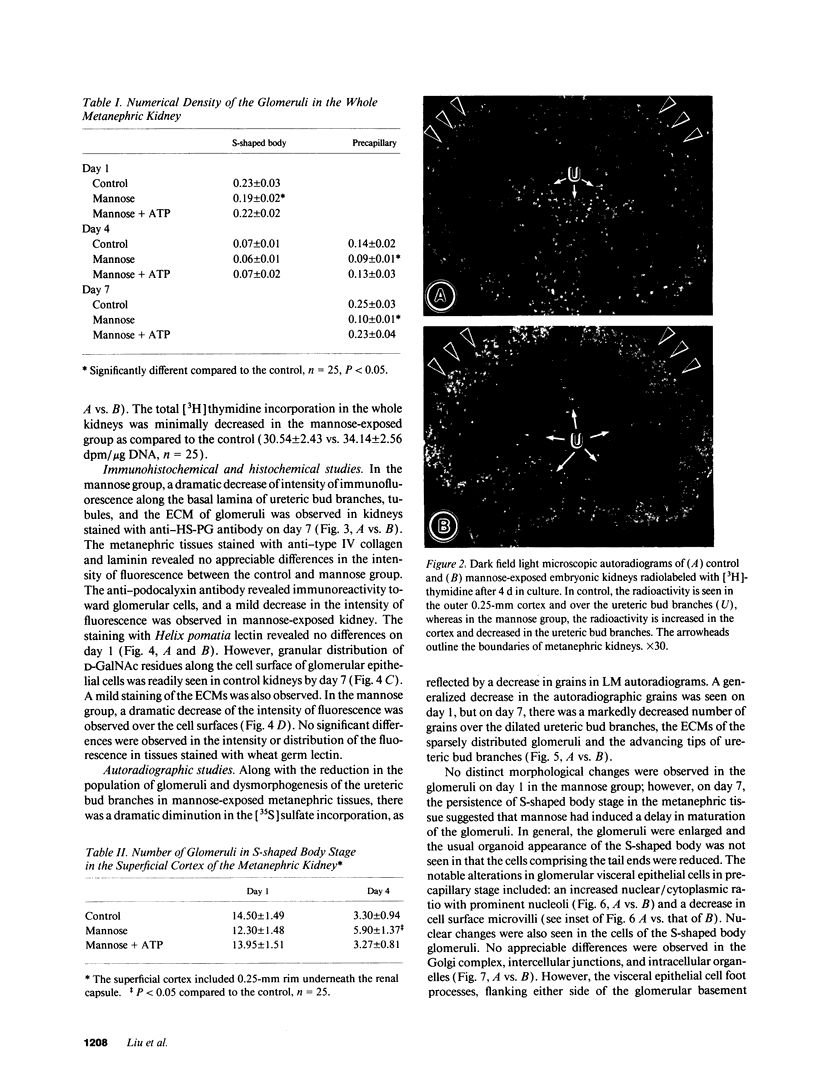
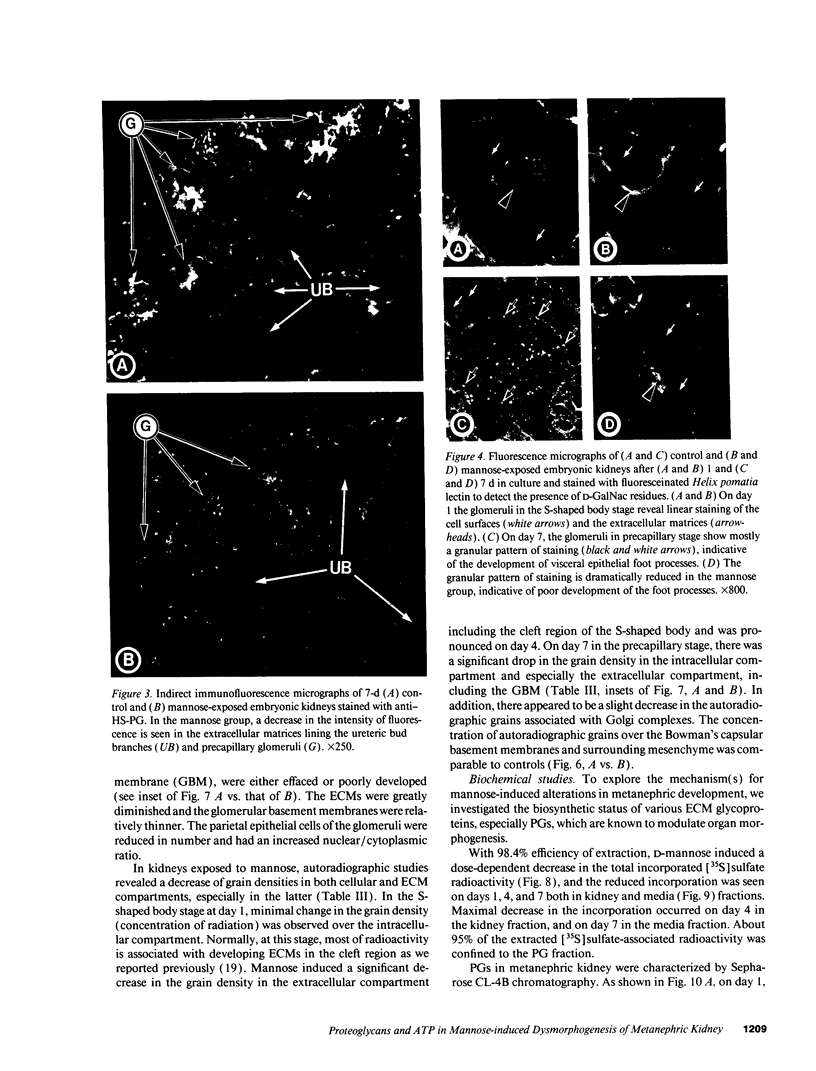
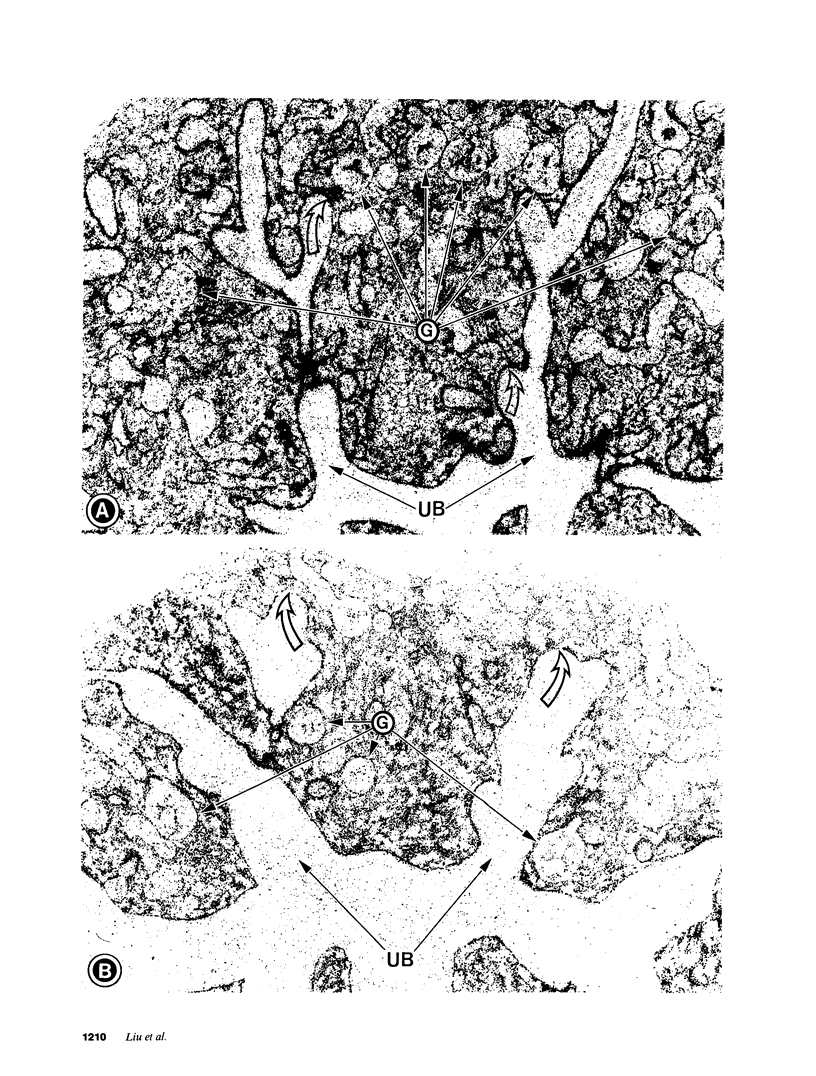
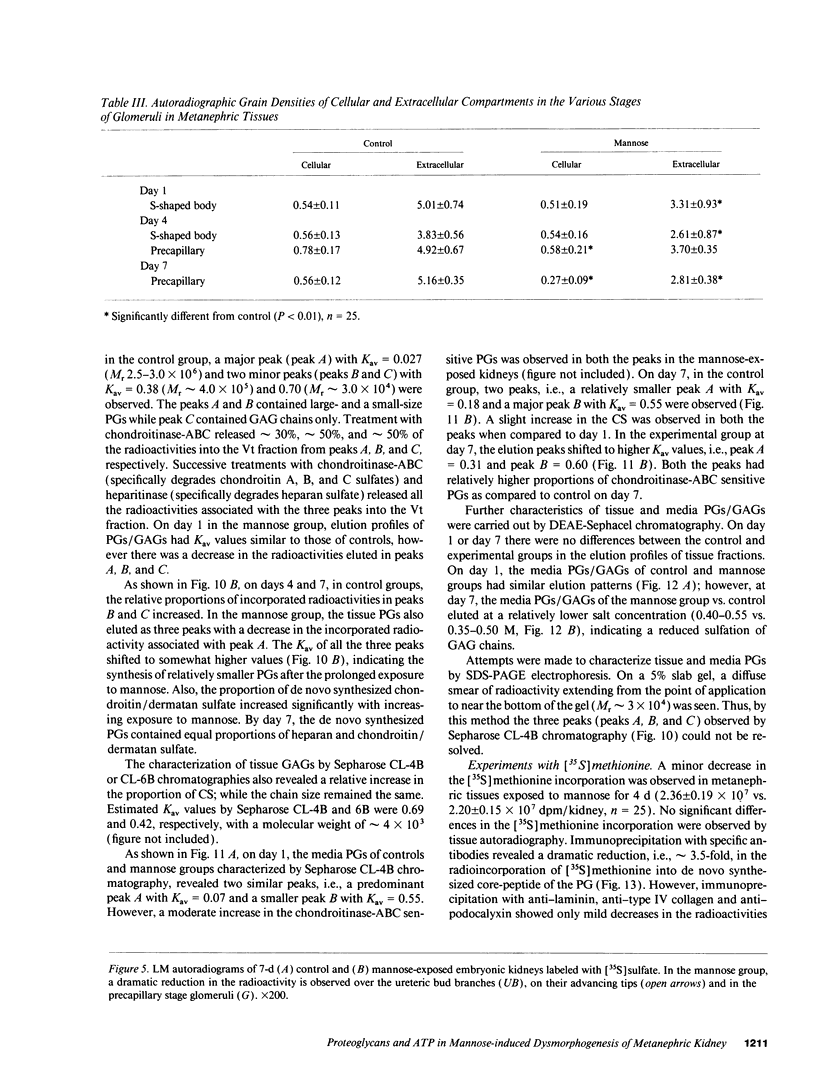
Because various fetal anomalies are seen in diabetic offspring, we examined the effects of sugars on proteoglycans (PGs): extracellular matrix (ECM) macromolecules modulating morphogenesis. 13-d-old mouse metanephric kidney explants were exposed to mannose for 7 d and labeled with [35S]sulfate, [35S]-methionine, or [3H]thymidine. Mannose exposure caused reduction in kidney size and disorganization of ureteric bud branches with inhibition of glomerulogenesis. Tissue autoradiographic and immunofluorescence studies indicated decreased expression of sulfated PGs in ECMs. Helix pomatia lectin binding to D-GalNAc residues of glomerular epithelial cells was also reduced. Biochemical studies revealed decreased synthesis of sulfated PGs. PGs were of lower molecular weight with reduced charge density and increased chondroitin/heparan sulfate ratio. Immunoprecipitation of [35S]methionine-labeled proteins confirmed the reduction of PG core peptides. Intracellular ATP levels were reduced. The addition of 0.1 mM ATP to culture media restored kidney size, the population of glomeruli, and the synthesis and characteristics of PGs to almost normal, with no detectable effect on the replication of cells as determined by [3H]thymidine incorporation. The effect of ATP could be partially blocked by the P2y-purinoreceptor, i.e., reactive blue-2. Data suggest that mannose causes energy depletion by cellular ATP consumption and thus selectively alters the synthesis of heavily glycosylated proteins with rapid turnover, such as PGs, resulting in renal dysmorphogenesis.
Full text
PDF

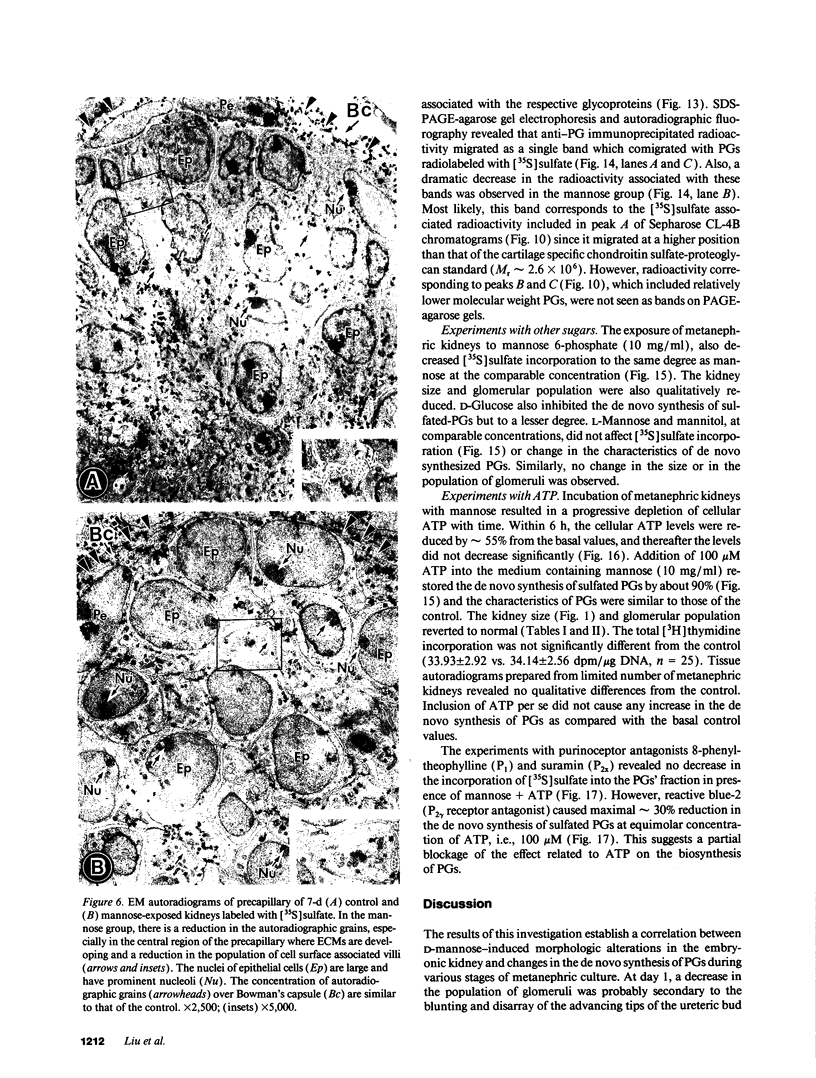

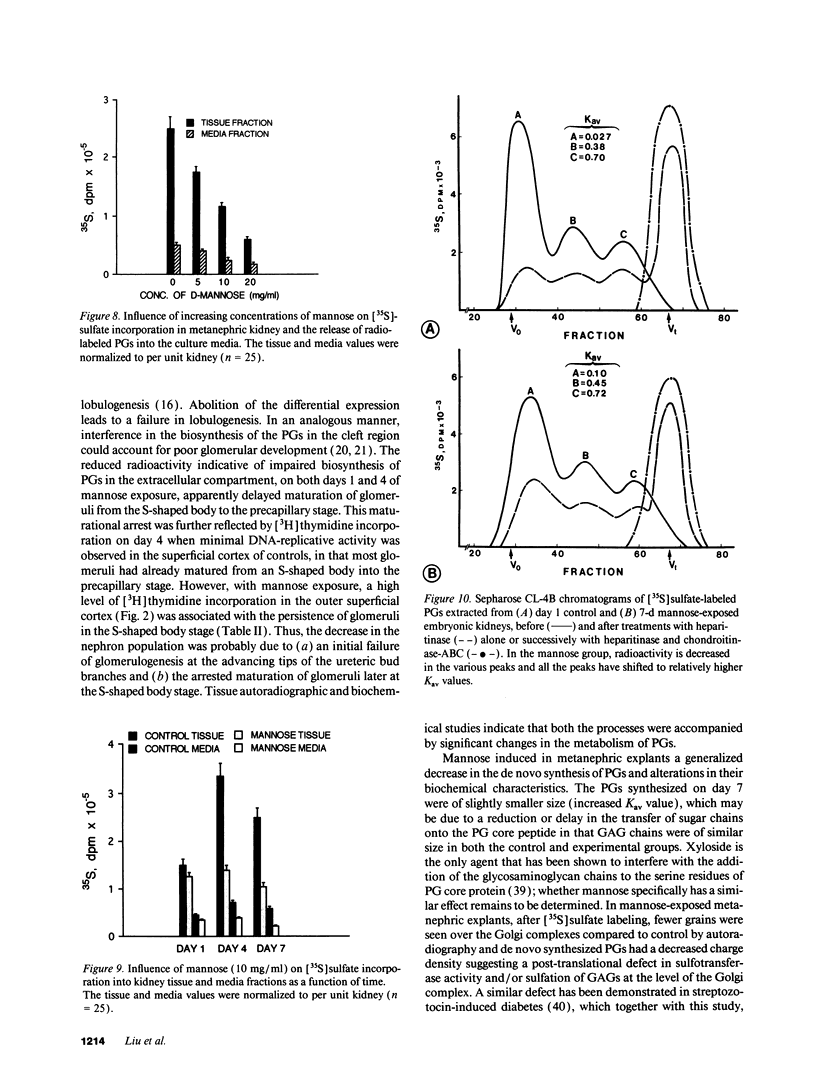
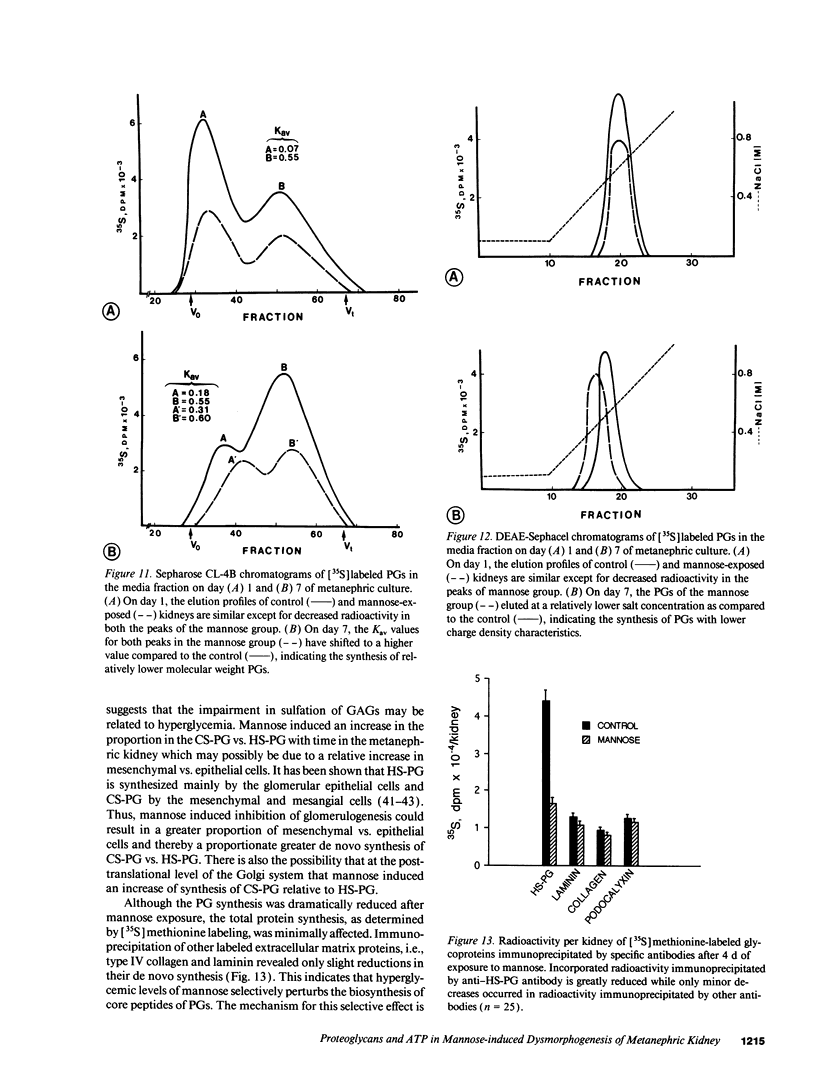
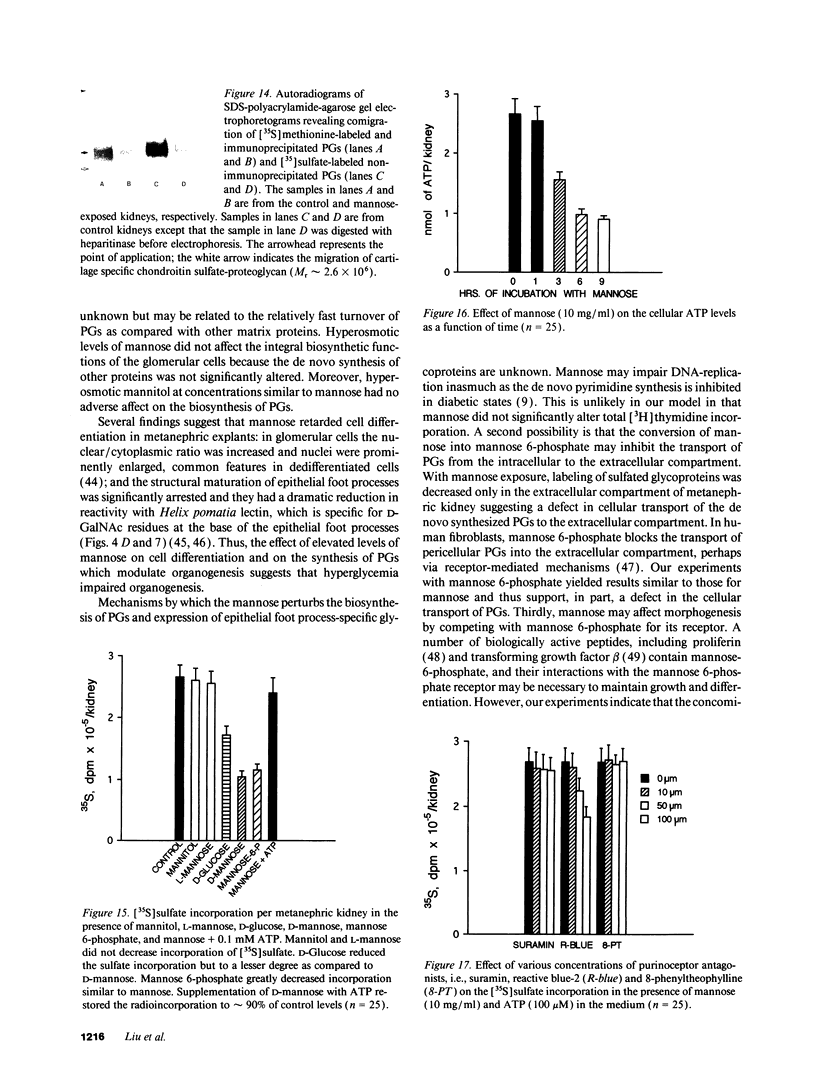


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avner E. D., Ellis D., Temple T., Jaffe R. Metanephric development in serum-free organ culture. In Vitro. 1982 Aug;18(8):675–682. doi: 10.1007/BF02796422. [DOI] [PubMed] [Google Scholar]
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J., Cheng F., Roszka J. Glomerular differentiation in metanephric culture. Lab Invest. 1981 Aug;45(2):183–190. [PubMed] [Google Scholar]
- Brenner B. M. Determinants of epithelial differentiation during early nephrogenesis. J Am Soc Nephrol. 1990 Aug;1(2):127–139. doi: 10.1681/ASN.V12127. [DOI] [PubMed] [Google Scholar]
- Buchanan T. A., Freinkel N. Fuel-mediated teratogenesis: symmetric growth retardation in the rat fetus at term after a circumscribed exposure to D-mannose during organogenesis. Am J Obstet Gynecol. 1988 Mar;158(3 Pt 1):663–669. doi: 10.1016/0002-9378(88)90050-6. [DOI] [PubMed] [Google Scholar]
- Comess L. J., Bennett P. H., Burch T. A., Miller M. Congenital anomalies and diabetes in the Prima Indians of Arizona. Diabetes. 1969 Jul;18(7):471–477. doi: 10.2337/diab.18.7.471. [DOI] [PubMed] [Google Scholar]
- Deuchar E. M. Embryonic malformations in rats, resulting from maternal diabetes: preliminary observations. J Embryol Exp Morphol. 1977 Oct;41:93–99. [PubMed] [Google Scholar]
- Dixit S. N. Isolation, purification and characterization of intact and pepsin-derived fragments of laminin from human placenta. Connect Tissue Res. 1985;14(1):31–40. doi: 10.3109/03008208509089841. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Volberg T., Sabanay I., Thiery J. P., Geiger B. Spatial and temporal distribution of the adherens-junction-associated adhesion molecule A-CAM during avian embryogenesis. Development. 1988 Jun;103(2):325–344. doi: 10.1242/dev.103.2.325. [DOI] [PubMed] [Google Scholar]
- Eriksson U. J., Lewis N. J., Freinkel N. Growth retardation during early organogenesis in embryos of experimentally diabetic rats. Diabetes. 1984 Mar;33(3):281–284. doi: 10.2337/diab.33.3.281. [DOI] [PubMed] [Google Scholar]
- Freinkel N., Lewis N. J., Akazawa S., Roth S. I., Gorman L. The honeybee syndrome - implications of the teratogenicity of mannose in rat-embryo culture. N Engl J Med. 1984 Jan 26;310(4):223–230. doi: 10.1056/NEJM198401263100404. [DOI] [PubMed] [Google Scholar]
- Fuhrmann K., Reiher H., Semmler K., Fischer F., Fischer M., Glöckner E. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983 May-Jun;6(3):219–223. doi: 10.2337/diacare.6.3.219. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Jakubowski M. L., Gibbons J. T. Effect of beta-D-xyloside on the glomerular proteoglycans. I. Biochemical studies. J Cell Biol. 1984 Aug;99(2):715–722. doi: 10.1083/jcb.99.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Jakubowski M. L. Distribution of de novo synthesized sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Lab Invest. 1983 Aug;49(2):216–225. [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Linker A., Jakubowski M. L. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2272–2275. doi: 10.1073/pnas.80.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Bielefeld D., Hook M. Reduced sulfation of liver heparan sulfate in experimentally diabetic rats. Diabetes. 1983 Apr;32(4):337–342. doi: 10.2337/diab.32.4.337. [DOI] [PubMed] [Google Scholar]
- Kunz A., Brown D., Vassalli J. D., Kontturi M., Kumpulainen T., Orci L. Ultrastructural localization of glycocalyx domains in human kidney podocytes using the lectin-gold technique. Lab Invest. 1985 Oct;53(4):413–420. [PubMed] [Google Scholar]
- Lee S. J., Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988 Mar 5;263(7):3521–3527. [PubMed] [Google Scholar]
- Lelongt B., Makino H., Dalecki T. M., Kanwar Y. S. Role of proteoglycans in renal development. Dev Biol. 1988 Aug;128(2):256–276. doi: 10.1016/0012-1606(88)90289-8. [DOI] [PubMed] [Google Scholar]
- Lelongt B., Makino H., Kanwar Y. S. Maturation of the developing renal glomerulus with respect to basement membrane proteoglycans. Kidney Int. 1987 Oct;32(4):498–506. doi: 10.1038/ki.1987.238. [DOI] [PubMed] [Google Scholar]
- Liu Z. Z., Dalecki T. M., Kashihara N., Wallner E. I., Kanwar Y. S. Effect of puromycin on metanephric differentiation: morphological, autoradiographic and biochemical studies. Kidney Int. 1991 Jun;39(6):1140–1155. doi: 10.1038/ki.1991.145. [DOI] [PubMed] [Google Scholar]
- Makino H., Gibbons J. T., Reddy M. K., Kanwar Y. S. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane--I. J Clin Invest. 1986 Jan;77(1):142–156. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Reversible and selective antagonism by suramin of ATP-activated inward current in PC12 phaeochromocytoma cells. Br J Pharmacol. 1990 Sep;101(1):224–226. doi: 10.1111/j.1476-5381.1990.tb12117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki I., Ito Y., Fukatsu A., Suzuki N., Yoshida F., Watanabe Y., Sakamoto N., Matsuo S. A plasma membrane antigen of rat glomerular epithelial cells. Antigenic determinants involving N-linked sugar residues in a 140-kilodalton sialoglycoprotein of the podocytes. Lab Invest. 1990 Nov;63(5):707–716. [PubMed] [Google Scholar]
- PEDERSEN L. M., TYGSTRUP I., PEDERSEN J. CONGENITAL MALFORMATIONS IN NEWBORN INFANTS OF DIABETIC WOMEN. CORRELATION WITH MATERNAL DIABETIC VASCULAR COMPLICATIONS. Lancet. 1964 May 23;1(7343):1124–1126. doi: 10.1016/s0140-6736(64)91805-7. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Brown D. M., Granlund K., Oegema T. R., Klein D. J. Proteoglycan metabolism associated with mouse metanephric development: morphologic and biochemical effects of beta-D-xyloside. Dev Biol. 1987 Oct;123(2):293–306. doi: 10.1016/0012-1606(87)90388-5. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Cooper J. A., Brunner A. M., Lioubin M. N., Gentry L. E., Kovacina K. S., Roth R. A., Marquardt H. Identification of mannose 6-phosphate in two asparagine-linked sugar chains of recombinant transforming growth factor-beta 1 precursor. J Biol Chem. 1988 Oct 5;263(28):14211–14215. [PubMed] [Google Scholar]
- Reeves W., Caulfield J. P., Farquhar M. G. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978 Aug;39(2):90–100. [PubMed] [Google Scholar]
- Roff C. F., Wozniak R. W., Blenis J., Wang J. L. The effect of mannose6-phosphate on the turnover of cell surface glycosaminoglycans. Exp Cell Res. 1983 Apr 1;144(2):333–344. doi: 10.1016/0014-4827(83)90412-3. [DOI] [PubMed] [Google Scholar]
- Roth J., Brown D., Orci L. Regional distribution of N-acetyl-D-galactosamine residues in the glycocalyx of glomerular podocytes. J Cell Biol. 1983 May;96(5):1189–1196. doi: 10.1083/jcb.96.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982 Mar;90(1):215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Smeaton T. C., Eichner R. D. Measurement of DNA with an automatic spectrophotometer. Anal Biochem. 1983 Jun;131(2):394–396. doi: 10.1016/0003-2697(83)90189-6. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Hilfer S. R., Searls R. L., Nathanson M. A., Allodoli M. D. Effects of beta-D-xyloside on differentiation of the respiratory epithelium in the fetal mouse lung. Dev Biol. 1990 Mar;138(1):42–52. doi: 10.1016/0012-1606(90)90175-i. [DOI] [PubMed] [Google Scholar]
- Soler N. G., Walsh C. H., Malins J. M. Congenital malformations in infants of diabetic mothers. Q J Med. 1976 Apr;45(178):303–313. [PubMed] [Google Scholar]
- Striker G. E., Lange M. A., MacKay K., Bernstein K., Striker L. J. Glomerular cells in vitro. Adv Nephrol Necker Hosp. 1987;16:169–186. [PubMed] [Google Scholar]
- Thiery J. P., Duband J. L., Rutishauser U., Edelman G. M. Cell adhesion molecules in early chicken embryogenesis. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6737–6741. doi: 10.1073/pnas.79.21.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Kemler R., Ekblom P. Cell-adhesion molecule uvomorulin during kidney development. Dev Biol. 1985 Nov;112(1):213–221. doi: 10.1016/0012-1606(85)90135-6. [DOI] [PubMed] [Google Scholar]
- WATANABE G., INGALLS T. H. Congenital malformations in the offspring of alloxan-diabetic mice. Diabetes. 1963 Jan-Feb;12:66–72. doi: 10.2337/diab.12.1.66. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. The value of stereology in analysing structure and function of cells and organs. J Microsc. 1972 Feb;95(1):3–13. doi: 10.1111/j.1365-2818.1972.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Yaoita E., Oguri K., Okayama E., Kawasaki K., Kobayashi S., Kihara I., Okayama M. Isolation and characterization of proteoglycans synthesized by cultured mesangial cells. J Biol Chem. 1990 Jan 5;265(1):522–531. [PubMed] [Google Scholar]