Abstract
Cancer stem cells are rare chemotherapy resistant cells within a tumor which can serve to populate the bulk of a tumor with more differentiated daughter cells and potentially contribute to recurrent disease. Ovarian cancer is a disease for which at the time of initial treatment we can obtain complete clinical remission in the majority of patients. Unfortunately, most will relapse and succumb to their disease. This clinical course is in line with the cancer stem cell model. In the past five years a significant amount of work has been done to identify cells with characteristics of ovarian cancer stem cells. This review will focus specifically on the markers used to define human ovarian cancer stem cells, the prognostic implications of the expression of these cancer stem cell markers in patient's primary tumors, and the potential of these cancer stem cell markers to serve as therapeutic targets.
Introduction
Within an ovarian cancer, all tumor cells are not created equal; tumor cells display a great deal of heterogeneity. More specifically, within a given tumor (or even tumor cell line), there are abundant distinct tumor cell populations expressing different markers. These unique cell populations have differential capacities for growth, survival, metastasis and resistance to chemotherapy and radiation therapy. Cancer stem cells make up a small proportion of malignant cells within a tumor, typically 0.01–1.0%. Cancer stem cells have the capacity to undergo either symmetric or asymmetric divisions to recreate a tumor with the complete original complex pool of tumor cells in immune-suppressed mice [1; 2]. Moreover, these highly specialized cell populations reportedly have un-limited division potential and therefore are capable of serial passages in vitro and in vivo. These cells have been termed cancer stem cells (CSC), tumor initiating cells (TICs), cancer initiating stem cells (CIC) and tumor propagating cells (TPC). For the purpose of this review we will refer to these cells as CSC.
Ovarian CSC are, for the most part, shown to be resistant to chemotherapy and radiation therapy [3; 4; 5; 6]. Based on their resistance to traditional cancer therapies and presumed ability to recapitulate the original tumor, CSC are believed to be the source of recurrent ovarian cancer. Consequently, there is a strong interest to identify, functionally characterize the pathobiology of, and eventually target ovarian CSC. To date, the study of CSC in ovarian cancer has been extremely challenging. It has been postulated that CSC may arise from genetic changes in normal stem cells [7; 8]. Thus, one way to identify CSC is to characterize cells within a tumor which express known stem cell markers for the tissue of origin. This approach for the identification of ovarian CSC is limited as the exact origin of ovarian cancer is unclear. In addition to the more traditional idea that ovarian carcinoma arises from the surface epithelial in response to cellular damage acquired from incessant ovulation [9], recent pathology data suggests that many ‘ovarian cancers’ may actually be arising in the distal portion of fallopian tube. Ovarian cancer may also arise in the setting of endometriotic lesions [10; 11]. Specific cells within or immediately juxtaposed to the ovarian surface reportedly display characteristics of stem cells [12], though the exact surface markers characterizing these normal ovarian surface epithelial cells remains unclear. Similarly, while cells with the characteristics of stem cells have been reported in endometrial tissues and endometriosis, little is known about their specific cell surface markers [13; 14].
As an added complexity, ovarian cancer is not limited to one subtype. This is evidenced by the multiple histophenotypes and their differential growth patterns as well as response to treatment. Moreover, it is not uncommon that a tumor can present with more than one histophenotype further supporting the concept that ovarian cancer is one of the more heterogenic tumors. The high metastatic potential of ovarian cancer indicates the plasticity of these cells and their capacity to undergo epithelial to mesenchymal transition and the inverse [15]. Associated with this, stem cells can assume quiescent or proliferative states depending on the cellular microenvironment and cellular stresses such as chemotherapy [16; 17].
Given these challenges, it is no surprise that there is significant controversy regarding the markers which define ovarian CSC. Here we will review the current studies on putative markers which define ovarian CSC, the potential functional implications of these CSC markers, and the therapeutic targeting of ovarian CSC markers.
CD133 and Aldehyde Dehydrogenase
One of the most widely described ovarian CSC markers is CD133. CD133 or Prominin is a membrane glycoprotein encoded by the CD133/Prom-1 gene. It was first detected as a marker of hematopoietic stem cells and since then has been demonstrated to be a marker of numerous normal and cancer stem cell populations [18; 19; 20; 21; 22; 23; 24]. In one of the first indications that CD133 may be a marker of ovarian CSC, Ferrandina et al. analyzed expression of CD133 in 41 ovarian tumors, 8 normal ovaries, and 5 benign ovarian tumors [25]. They found that primary ovarian cancer CD133+CK7+ cells had greater colony forming potential and had a higher proliferative potential than CD133−CK7+ cells [26]. Interestingly they also found that normal ovaries and benign tumors had a significantly lower expression of CD133 than ovarian carcinomas [26]. In one of the first functional characterizations of CD133 as an ovarian CSC marker, Baba and colleagues demonstrated that CD133+ cells from established cell lines had greater tumor initiating capacity than CD133− cells [4]. Similarly, consistent with a CSC phenotype, CD133+ cells demonstrated greater resistance to chemotherapy. They also reported that CD133 expression in tumor cells was regulated at the level of promoter methylation, suggesting that epigenetic events could be responsible for the induction of tumor ‘stemness’.
The study by Baba and colleagues relied primarily on established cell lines. Curley et al. used an alternate approach to study CSC in primary human tumor samples [27]. They established 11 primary xenografts from freshly isolated human ovarian carcinomas and then characterized the ability of different cell populations derived from the primary xenografts to initiate new tumors and then undergo serial passage in immunocompromised mice. For both serous and clear cell ovarian cancers, the CD133+ high expressing cell fraction demonstrated greater tumor initiating capacity than CD133− cells. In addition CD133+ cells gave rise to CD133− cells and a tumor with histologic characteristics of the primary tumor. In one instance a 99% pure CD133- fraction gave rise to a tumor; albeit, with a much longer latency period. However an interesting point was the resulting tumor had just over 10% CD133 positive cells suggesting that either CD133− cells can become CD133+ cells or the < 1% CD133+ fraction was sufficiently amplified to become 10% of the resulting tumor. Intriguingly, none of the primary xenografts demonstrated expression of CD117, another putative CSC marker (see below). Whether this lack of expression was due, to passage through the mouse as a xenograft or cellular isolation techniques is not known.
Finally, our group and one other group recently analyzed the combined expression of CD133 and the stem cell marker aldehyde dehydrogenase (ALDH) as ovarian CSC markers [3; 28]. We found that in cell lines and primary human ovarian tumors in which tumor cells lacked CD133 expression, FACS isolated ALDH+ cancer cells were capable of initiating tumors in mice whereas limiting dilutions of ALDH− cells were not. This is in accordance with the work of Landen and colleagues demonstrating ALDH1A1+ cells are ~50 fold more tumorigenic than ALDH1A1- cells [5]. In established cell lines and primary human tumors in which tumor cells expressed CD133, CD133+ALDH+ cells had far greater tumor initiating capacity and shorter tumor latencies. Interestingly, while CD133+ALDH− cells isolated from cell lines were highly tumorigenic, CD133+ALDH− cells isolated from primary tumors were unable to initiate tumors in immunocompromised mice. Whether these cells truly have restricted tumor initiating capacity, or are more sensitive to isolation procedures remains to be determined. Both ALDH+ CSC and CD133+ALDH+ human ovarian CSC were highly angiogenic. This is consistent with studies suggesting ovarian CSC from tumor metastases are capable of attracting endothelial progenitors to promote angiogenesis [29].
CD44 and CD117
CD44 is the receptor for hyaluronic acid and has been identified as a marker of CSC in breast [30], prostate [21], colorectal [31], pancreatic [32], head and neck squamous cell carcinomas [33]. CD117, also known as c-kit, is another well characterized stem cell marker which has been implicated as a CSC marker in several solid tumors. In the first study to consider ovarian CSC, Szotek and colleagues use hoechst dye exclusion to identify side-population (SP) cells with CSC characteristics within a murine ovarian cancer cell line [34]. These SP cells were enriched for CD117 expression. However, they found human ovarian cancer ascites SP cells lacked CD117 expression. In contrast, Bapat et al. isolated tumor cells from the ascites of a patient diagnosed with serous ovarian cancer and established 19 spontaneously immortalized clones [35]. Molecular characterization of these clones identified the expression of CD44, CD117, and scatter factor - the ligand for CD117. Interestingly, only one of these clones demonstrated tumorigenicity in vivo, suggesting the presence of cells with different tumor initiating capacity within the ovarian tumor associated ascites. Alternatively, one could speculate this clone had acquired additional genetic changes due to in vitro culture. Consistent with this, a second clone underwent spontaneous transformation during culture.
Similarly, Zhang et al. analyzed tumor spheroids generated from the ascites of 5 patients with serous ovarian cancer [36]. After ~10 serial passages in a stem cell based media they observed that the remaining spheroid cells were highly enriched for CD44 and CD117 expression. Like the studies above for CD133 [3; 4], CD44+CD117+ spheroid cells were resistant to chemotherapy, and were able to initiate and serially propagate tumors in mice. Finally, using primary tumor xenografts similar to the CD133 study above [27], Luo et al. reported that tumorigenic CD117+lineage- cells were isolated from 3 of 14 tumor xenografts. These cells were capable of serial transplantation, asymmetric division, and the presence of these cells was correlated with chemoresistance [37].
Subsequently, Alvero et al. analyzed epithelial ovarian cancers from 147 patients prior to chemotherapy and found that all expressed CD44, however the expression of CD44 was higher in metastatic tumors and tumor ascites [38]. They then generated primary cell lines from human tumors or ascites and then injected CD44+ cells from the lines into mice. They found that CD44+ cells recapitulated the original tumor and were able to undergo multiple passages in vivo [38]. Complicating the interpretation of this study, they injected a large number of cells (1x106th) and they did not test the tumorigenicity of CD44− cells. Expression analysis of CD44+ and CD44− cells revealed that Myeloid Differentiation Factor 88 (MyD88), an activator of the NFkB signaling pathway was upregulated 10-fold in CD44+ cells, potentially linking CD44 expressing cells and chronic inflammatory responses of cancer [38]. In a follow-up study, the same group showed that xenograft tumors derived from CD44+ cells gave rise to human CD34 expressing blood vessels suggesting that these tumor cells have the potential to differentiate into vessels or direct other cells for the formation of vessels [39].
Finally, a study performed using vital dyes to identify ‘label retaining cells’ i.e. quiescent CSC, demonstrated that nearly 100% of the label retaining cells were CD44+CD117+[40]. However, this study was performed with a murine tumor cell line so the applicability to human cancer remains uncertain. The expression of other CSC makers such as CD133 and CD24 was not reported.
CD24
CD24 is a mucin-like cell surface glycoprotein marker that has been identified as a CSC marker in pancreatic [32], and liver cancer [41]. Interesting, in breast cancer, CSC are reported to be CD24(−) or CD24dim. The differences in CD24 expression in different CSC may relate to the different tissues of origin. While the function of CD24 remains unclear, a recent report suggests that CD24 regulates expression of the stem cell regulator Nanog which drives tumor initiating capacity in CSC [41].
Gao et al. recently reported CD24 as a putative CSC maker in ovarian cancer. They established primary ovarian cancer cell lines from serous and mucinous cystadenocarcinomas and found cells with the CSC characteristics of quiescence, chemoresistance, and tumor initiation capacity were enriched for CD24expression [16]. CD24+ cells expressed increased levels of stem cell related genes such as Nestin, Beta-catenin, Bmi-1, Oct4, Oct3/4, Notch1, and Notch4 when compared to CD24− cells. Interestingly, they found expression of both CD133 and CD117 in the majority of cell clones generated. Approximately 1% of CD24+ cells co-expressed CD133 and approximately 1% of CD24+ cells co-expressed CD117. More recently, this same group analyzed cell clones generated from cells located in the center of a tumor and cells at the tumor periphery. They hypothesized that cells at the tumors leading edge would be enriched for CSC. They found that cells from the leading edge had a higher proportion of side population cells [42]. Within the side-population cells they found enriched expression of CD24 and CD117 (CD133 expression was not reported). Once again approximately 1% of cells co-expressed CD24 and CD117. The tumorigenicity of these cells was not assessed.
Finally, CD24 expression in combination with CD44 and the epithelial cell adhesion molecule (EpCam) was also assessed in conjunction with side-population cells [43]. In established cell lines treated with chemotherapy, the percentage of cells expressing all three cell surface markers was found to increase. In addition, when compared to CD24-, CD44-, EpCam- cells, these ‘triple positive’ cells demonstrated greater invasion in matrigel, and more rapid tumor growth in vivo. This triplet of markers was not functionally assessed in human tumors.
Using the Ovarian Cancer Markers to Establish a Hierarchy of Ovarian Cancer Stem Cells
Several studies have now provided convincing evidence for different cell surface markers which can identify populations of cells with the properties of ovarian cancer stem cells; the ability to initiate tumors in vivo, the ability to serially propagate tumors, the ability to undergo asymmetric division, and increased resistance to chemotherapy. One of the next challenges will be to determine how these markers are related to one another. Studies in breast cancer have been able to identify distinct breast CSC markers for different tumor types, i.e. luminal, ductal, or Her2 positive tumors [44]. Perhaps most importantly, modeling after studies on normal mammary glands, these stem cells are organized in a hierarchy of CSC/progenitors. The establishment of such a hierarchy then allows the ability to identify the factors which regulate CSC self-renewal vs. proliferation and differentiation. Such factors represent potential import therapeutic targets.
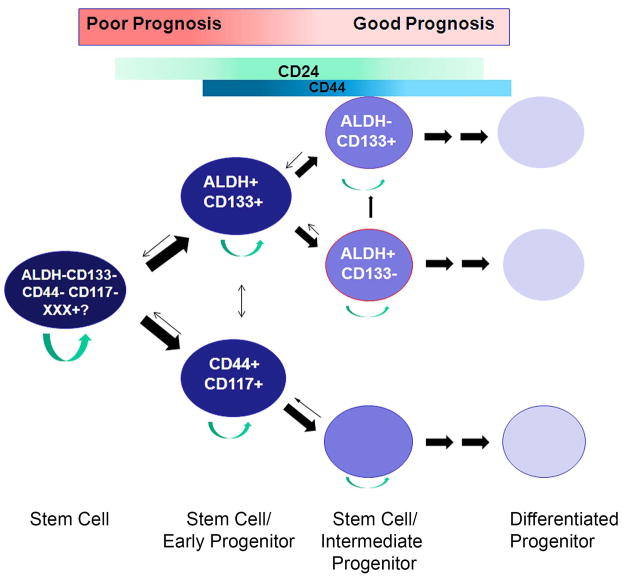
Using ALDH and CD133 as CSC markers, we were able to define a simple hierarchy of ovarian CSC. We found that, parallel to the hematologic system, ALDH and CD133 could be used alone and in combination to identify distinct chemoresistant ovarian CSC populations [3]. Both ALDH+CD133+ cells and ALDH+CD133- human ovarian tumor cells could initiate tumors in mice. However, consistent with a hierarchy, primary human ALDH+CD133+ CSC generated poorly-differentiated, highly aggressive tumors in vivo within ~4 months. ALDH+CD133+ cell initiated tumors contained ALDH+CD133+, ALDH+CD133−, ALDH−CD133+, and ALDH−CD133− tumor cell populations. In contrast ALDH+CD133− CSC generated more well differentiated tumors which required 6–12 months to develop in vivo. We have observed that 50,000–100,000 ALDH−CD133− cells can occasionally initiate tumors in mice with both ALDH+/− and CD133+/− cells. Similarly Curley et al. observed that CD133− cells could sometimes give rise to CD133+ cells. While we cannot rule out that this is due to contaminating CSC we speculate the existence of a rare ALDH−CD133− CSC with as yet undefined markers (Figure 1). It is also possible that differentiated progeny are able to ‘de-differentiate’ to create upstream cells, however this remains highly contested.
Figure 1. Proposed hierarchy of ovarian cancer stem cell differentiation based on the current literature.
A rare common ovarian CSC, for which markers have yet to be defined can give rise to either an ALDH+CD133+ early progenitor or a CD44+CD117+ early progenitor. Each of which can subsequently divide into more differentiated progenitors. The potential for expression of CD24 and CD44 in both lineages is indicated by the colored bars. Rounded arrows indicate cells with self-renewal capacity which have the potential to serve as CSC. Small arrows indicate the unproven potential for 'de-differentiation'.
Interestingly, serous and clear cells tumors both demonstrated CD133+ CSC. Thus it is possible that serous and clear cell tumors share a common stem cell ancestor. Given the different molecular profiles of serous versus clear cell ovarian tumors, different mutations of the same stem cell (or progenitors from the same stem cell) may yield different histological phenotypes [45; 46]. Alternatively, the different microenvironments (endometriosis vs. peritoneal surface) may influence the differentiation and histology of the CSC progeny. Finally, there may be distinct stem cells for each tumor type that each happen to express CD133.
How this CD133 hierarchy relates to CD117+ (either CD44+ or CD24+) CSC remains to be established. Several lines of study would suggest that these CSC could be related in a lineage. First in our characterization of 14 human ovarian cancers, all tumors which had detectable levels of CD117 also had detectable CD133 expression. Further supporting a potential relationship, Ma et al. reported that both CD117 and CD133 expression could be identified in SKOV3 sphere cells after chemotherapy selection [47]. Finally, as noted above, CD24+ CSC had expression overlap with both CD133 and CD117 [16]. Taking all of these observations together, we propose the two CSC pathways may be linked via an undefined common ovarian CSC. This common ovarian CSC can undergo asymmetric division to give rise to either an ALDH+CD133+(CD24+/−) early progenitor CSC, or CD44+CD117+(CD24+/−) early progenitor CSC. Each of these early progenitor CSC populations can subsequently undergo asymmetric divisions to produce additional progenitor cells which could give rise to tumors with a more differentiated histology.
Ovarian CSC Markers and Patient Prognosis
Recently Steffensen et al. reported on 117 patients, and found that 57.1% of stage I patients had tumors expressing >20% CD44+ cells, whereas the majority of tumors from patients with stage II, III, and IV disease had a lower expression of CD44+ cells [48]. This study suggested that patients with early stage tumors have a higher population of ovarian CSC/early progenitors driving tumor growth. As the tumor progresses these CSC either lose their CSC marker expression or, become ‘diluted’ as a greater number of differentiated cells are produced. This group also found that early-stage ovarian cancer patients whose tumors contained > 20% CD44+ epithelial ovarian CSC had a shorter progression-free survival compared to patients with tumors having <20% of these cells [48]. This would also be consistent with the relative chemo-resistance of CSC and suggest that tumors with a greater percentage of CSC are more aggressive in nature.
ALDH has also been studied as a prognostic biomarker in ovarian cancer, although with conflicting results. Both Landen et. al. and Deng et. al. reported that increased ALDH1 expression in ovarian tumor cells is correlated with poor prognosis [5; 49]. The study by Landen and colleagues analyzed tumors from 65 patients with untreated high grade papillary-serous ovarian tumors. Deng and colleagues analyzed a panel of 439 serous ovarian tumors, though information on tumor grade was not provided. In contrast, in a study of 442 samples, Chang et al reported that the presence of increased levels of ALDH1 expression in tumor islets was associated with an improved patient prognosis [50]. This study included a wide panel of tumor histologies; 40% were non-serous tumors. The inclusion of low grade tumors, which tend to have a prolonged survival, and a large number of Type I vs. Type II tumors may have impacted the outcomes of this study.
We analyzed the expression CD133 and ALDH in combination from 56 patient samples. We found that patients whose tumors had CD133+ALDH+ tumor cells had a significantly poorer outcome than those whose tumors lacked CD133+ALDH+ tumor cells [3]. Zhang et al also reported that in samples analyzed from a tumor bank of over 400 ovarian cancers, CD133 expression by ovarian tumor cells is a negative prognostic factor [51]. More recently, Landen and colleagues analyzed several stem cell markers in primary tumor specimens, specimens collected after chemotherapy, and specimens at first recurrence [52]. They found that CD133, ALDH1A1, and CD44 were present at low numbers in primary samples. These same markers were increased in tumor samples taken immediately following chemotherapy treatment, and then reduced back to initial cell numbers in recurrent tumor samples. This suggests that these markers identify chemoresistant cells. Interestingly, only CD133 was significantly increased in tumors collected from recurrent platinum-resistant patients. Concurrent with the increases in ALDH1A1, CD133, and CD44 the authors identified increases in several stem cell pathways, supporting the hypothesis that these are CSC markers. Finally, while less studied, the expression of CD24 has been correlated with poor prognosis [53].
CSC Markers as Therapeutic Targets
Based strictly on the stem cell specific expression of the CSC markers, they represent potential important therapeutic targets. In addition, these markers may be functionally important for CSC making them even more attractive as therapeutic targets. CD44 functions in cells as an adhesion molecule binding to hyaluronic acid (HA). This binding of CD44 to HA may regulate cellular migration and metastasis [54]. Several groups have exploited this high affinity interaction of CD44 with HA to develop CD44 targeted therapeutics. HA or HA analogs have been coupled to various chemotherapeutics and shown to have significant therapeutic efficacy versus CD44+ tumor cells both in vitro and in vivo [55; 56; 57; 58; 59; 60; 61; 62]. Targeting CD44 is associated with a decrease in metastases in several tumor models [62].
As noted above, CD117 is a receptor tyrosine kinase expressed on stem cells. CD117 is a well-documented oncogene, making it an attractive target for cancer therapy. As a tyrosine kinase, CD117 it is a very ‘drugable target’. Inhibitors of CD117 kinase activity such as imatinib have been developed and demonstrated to be highly effective in tumors in gastrointestinal stromal tumors and chronic myelogenous leukemia, [63]. Imatinib has demonstrated some efficacy against ovarian cancer cell lines in vitro. Unfortunately, phase 2 clinical trials using imatinib both in recurrent disease and as a maintenance agent in patients following a complete clinical remission demonstrated no efficacy [64; 65]. Similarly a combination of docetaxel and imatinib for the treatment of platinum resistant ovarian cancer demonstrated few responses [66]. It is possible that these disappointing results are because trials were performed in non-selected patient populations; only ~30% of ovarian cancer patient’s tumors are CD117+ and might be expected to respond to CD117 targeted therapy.
Like CD117, CD133 is a marker of many normal stem cells. The exact function of CD133 remains unclear. CD133 mutations in humans are associated with retinal degeneration as part of Retinitis Pigmentosa or Stargardt’s disease, both of which have been associated with the formation of tumors [67; 68; 69]. Some studies indicate CD133 functions to promote stem cell self-renewal and suppress differentiation thereby maintaining ’stemness’. However, this is controversial [70; 71; 72; 73; 74]. Preclinical studies demonstrate anti-CD133 antibodies will concentrate in ovarian tumor xenografts suggesting they may be used as imaging agents or therapeutics [75; 76]. Therapeutic approaches targeting CD133 directly may be complicated by the expression on CD133 on hematopoietic stem cells.
Aldehyde-dehydrogenase is an enzyme that is functional in stem cells and may play a role in cellular detoxification and retinoic acid metabolism [77]. Increased expression of ALDH1A1, the putative ovarian CSC marker, has been linked with the induction of chemoresistance suggesting an important function for ALDH in the ovarian CSC [5]. Preclinical studies demonstrate that knockdown of ALDH1A1 can restore chemosensitivity in ovarian cancer cell lines [5]. Several ALDH inhibitors are currently clinically available. Disulfiram, an ALDH inhibitor has demonstrated significant activity against CSC in breast cancer, prostate cancer and others [78; 79; 80; 81]. We have found that Disulfiram is preferentially toxic to ALDH+ ovarian CSC and that Disulfiram is highly synergistic with cisplatin therapy against ovarian CSC in vitro (Yang and Buckanovich unpublished data). As Disulfiram is an FDA approved drug, it represents an important drug for testing the proof of principle of ALDH inhibitors as CSC targeting agents in ovarian cancer. Importantly Disulfiram has been safely used in cancer patients in combination with chemotherapy suggesting that normal stem cells will tolerate ALDH targeted therapies [82].
Finally, CD24 is felt to play an important role in cancer cell metastasis and survival [83]. In animal models short hairpin RNA knockdown of CD24 was associated with a reduction in ovarian tumor xenograft growth [84]. Reduction of CD24 was also associated with a decrease in microvascular density, decreased cellular proliferation, and increased apoptosis. Unfortunately, the ability to use such an approach in humans could be challenging due to the relatively broad expression of CD24.
Other approaches have been used to target ovarian CSC utilizing mechanisms not reliant on CSC specific markers. Notably the developmental regulator Mullerian inhibitory substance has demonstrated significant activity in vitro and in vivo [43; 85]. We recently demonstrated that bone morphogenetic protein (BMP) inhibitor Noggin, can prevent CSC self-renewal and reduce tumor growth in vivo [86]. Studies targeting stem cell related pathways have shown some efficacy. Recently, it was demonstrated that treatment of ovarian tumor explants with a hedgehog antagonist reduced tumor growth but more importantly appeared to inhibit recurrent tumor growth following treatment with carboplatin and paclitaxel [87]. These and other therapeutic studies have been reviewed elsewhere [7].
Conclusions
In the past five years there has been a significant amount of work investigating markers which identify ovarian CSC. In the coming years, one of the major challenges will be to determine how these different ovarian CSC markers relate to one another. As cell lines often act quite differently than human tumor cells, we believe the development of improved models to study human ovarian CSC in vivo will be essential for this understanding and for the development of CSC specific therapeutics. To date, in vivo engraftment rates of ovarian CSC have been very poor. Limited studies use CSC derived from primary tumors. Most studies to date used cell lines for in vivo studies and only used human samples in vitro. At best, flank tumor engraftment rates of isolated human CSC have been 20 to 40% [3]. Additionally, the slow growth rates limit experimentation. One manner to improve engraftment rates could be to use intraperitoneal injection of CSC. Intraperitoneal models may be superior to flank models in that the peritoneum is a natural environment for ovarian CSCs. However Curley and colleagues demonstrated that flank models had similar engraftment rates when compared to intraperitoneal injection [27]. Flank models were relatively superior to intraperitoneal injection with respect to ease of monitoring. While intra-ovarian bursal injection is an option, this is labor intensive and requires regular in vivo imaging to assess tumor development. Moreover, the human female does not have a bursa. Therefore whether or not intra-ovarian bursal injection in the mouse would provide for direct extrapolation to humans is debatable.
Ultimately it will be critical to apply what we have learned about ovarian CSC to clinical trials. As we attempt to target ovarian CSC, selecting the appropriate patient population for the studies will be critical. Limited numbers of tumors express the different stem cell markers; 34 to 40% express CD133 and 30 to 40% express CD117 [3; 4; 25]. While markers such as CD24 and CD44 may have more broad expression, targeting these cells in vivo may be limited by the broad expression of these molecules in normal tissue. However, it may be possible to target these stem cell markers safely using tumor specific ’zip codes’ to localize therapy specifically to the tumor microenvironment and minimize systemic exposure. In addition, the ability to administer therapy intraperitoneally may be exploited to limit systemic exposure of CSC targeted drugs for ovarian cancer. While ovarian CSC targeted therapy may be several years away, it is our hope that ovarian CSC targeted therapies will significantly improve the survival of ovarian cancer patients, something we have been unable to obtain in the past 30 years.
References
- 1.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 2.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 3.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, Wicha MS, Buckanovich RJ. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 5.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Jr, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 7.Murphy SK. Targeting ovarian cancer-initiating cells. Anticancer Agents Med Chem. 2010;10:157–163. doi: 10.2174/187152010790909272. [DOI] [PubMed] [Google Scholar]
- 8.Naora H. Developmental patterning in the wrong context: the paradox of epithelial ovarian cancers. Cell Cycle. 2005;4:1033–1035. doi: 10.4161/cc.4.8.1906. [DOI] [PubMed] [Google Scholar]
- 9.Fleming JS, Beaugié CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: Revisiting old hypotheses. Molecular and Cellular Endocrinology. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Helbing T, Rothweiler R, Ketterer E, Goetz L, Heinke J, Grundmann S, Duerschmied D, Patterson C, Bode C, Moser M. BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood. 2011 doi: 10.1182/blood-2011-03-339762. [DOI] [PubMed] [Google Scholar]
- 11.Osada A, Kiyozumi D, Tsutsui K, Ono Y, Weber CN, Sugimoto N, Imai T, Okada A, Sekiguchi K. Expression of MAEG, a novel basement membrane protein, in mouse hair follicle morphogenesis. Experimental cell research. 2005;303:148–159. doi: 10.1016/j.yexcr.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- 13.Chan RWS, Gargett CE. Identification of Label-Retaining Cells in Mouse Endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 14.Sasson IE, Taylor HS. Stem Cells and the Pathogenesis of Endometriosis. Annals of the New York Academy of Sciences. 2008;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberauer R, Rist W, Lenter MC, Hamilton BS, Neubauer H. EGFL6 is increasingly expressed in human obesity and promotes proliferation of adipose tissue-derived stromal vascular cells. Mol Cell Biochem. 2010;343:257–269. doi: 10.1007/s11010-010-0521-7. [DOI] [PubMed] [Google Scholar]
- 16.Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 17.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–9253. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 18.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 19.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med. 2003;13:201–206. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 20.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 21.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 22.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, Eades WC, Creer MH, Nolta JA. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentry T, Deibert E, Foster SJ, Haley R, Kurtzberg J, Balber AE. Isolation of early hematopoietic cells, including megakaryocyte progenitors, in the ALDH-bright cell population of cryopreserved, banked UC blood. Cytotherapy. 2007;9:569–576. doi: 10.1080/14653240701466347. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 25.Ferrandina G, Martinelli E, Petrillo M, Prisco MG, Zannoni G, Sioletic S, Scambia G. CD133 antigen expression in ovarian cancer. BMC Cancer. 2009;9:221. doi: 10.1186/1471-2407-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S, Scambia G. Expression of CD133-1 and CD133-2 in ovarian cancer. International Journal of Gynecological Cancer. 2008;18:506–514. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 27.Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, Foster R. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Liu S, Roh M, Vatan L, Szeliga W, Wei S, Banerjee M, Mao Y, Kotarski J, Wicha MS, Liu R, Zou W. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 2011 doi: 10.1002/ijc.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusumbe AP, Mali AM, Bapat SA. CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculature. Stem Cells. 2009;27:498–508. doi: 10.1634/stemcells.2008-0868. [DOI] [PubMed] [Google Scholar]
- 30.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 33.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo L, Zeng J, Liang B, Zhao Z, Sun L, Cao D, Yang J, Shen K. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp Mol Pathol. 2011;91:596–602. doi: 10.1016/j.yexmp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, Oidtman J, Silasi DA, Mor G. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27:2405–2413. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Research. 2009;69:9245–9253. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 41.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Choi YP, Shim HS, Gao M-Q, Kang S, Cho NH. Molecular portraits of intratumoral heterogeneity in human ovarian cancer. Cancer Letters. 307:62–71. doi: 10.1016/j.canlet.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 43.Wei X, Dombkowski D, Meirelles K, Pieretti-Vanmarcke R, Szotek PP, Chang HL, Preffer FI, Mueller PR, Teixeira J, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proc Natl Acad Sci U S A. 2010;107:18874–18879. doi: 10.1073/pnas.1012667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anglesio MS, George J, Kulbe H, Friedlander M, Rischin D, Lemech C, Power J, Coward J, Cowin PA, House CM, Chakravarty P, Gorringe KL, Campbell IG, Okamoto A, Birrer MJ, Huntsman DG, de Fazio A, Kalloger SE, Balkwill F, Gilks CB, Bowtell DD. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res. 2011;17:2538–2548. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 46.Tsuda H, Ito YM, Ohashi Y, Wong KK, Hashiguchi Y, Welch WR, Berkowitz RS, Birrer MJ, Mok SC. Identification of overexpression and amplification of ABCF2 in clear cell ovarian adenocarcinomas by cDNA microarray analyses. Clinical Cancer Research. 2005;11:6880–6888. doi: 10.1158/1078-0432.CCR-05-0751. [DOI] [PubMed] [Google Scholar]
- 47.Ma L, Lai D, Liu T, Cheng W, Guo L. Cancer stem-like cells can be isolated with drug selection in human ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin (Shanghai) 2010;42:593–602. doi: 10.1093/abbs/gmq067. [DOI] [PubMed] [Google Scholar]
- 48.Steffensen KD, Alvero AB, Yang Y, Waldstrom M, Hui P, Holmberg JC, Silasi DA, Jakobsen A, Rutherford T, Mor G. Prevalence of epithelial ovarian cancer stem cells correlates with recurrence in early-stage ovarian cancer. J Oncol. 2011;2011:620523. doi: 10.1155/2011/620523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang Y, Montone K, Butzow R, Coukos G, Zhang L. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS ONE. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang B, Liu G, Xue F, Rosen DG, Xiao L, Wang X, Liu J. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22:817–823. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2011 doi: 10.1038/modpathol.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steg AD, Bevis KS, Katre AA, Ziebarth A, Alvarez RD, Zhang K, Conner MB, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clinical Cancer Research. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristiansen G, Denkert C, Schluns K, Dahl E, Pilarsky C, Hauptmann S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161:1215–1221. doi: 10.1016/S0002-9440(10)64398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M. A6 Peptide Activates CD44 Adhesive Activity, Induces FAK and MEK Phosphorylation, and Inhibits the Migration and Metastasis of CD44-Expressing Cells. Mol Cancer Ther. 2011;10:2072–2082. doi: 10.1158/1535-7163.MCT-11-0351. [DOI] [PubMed] [Google Scholar]
- 55.Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, Price RE, Ravoori M, Kundra V, Freedman RS, Klostergaard J. Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia. 2007;9:479–486. doi: 10.1593/neo.07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MG, Moghimi SM, Peer D. Hyaluronan-coated nanoparticles: The influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J Control Release. 2011;156:231–238. doi: 10.1016/j.jconrel.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 57.Casagrande F, Cocco E, Bellone S, Richter CE, Bellone M, Todeschini P, Siegel E, Varughese J, Arin-Silasi D, Azodi M, Rutherford TJ, Pecorelli S, Schwartz PE, Santin AD. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of clostridium perfringens enterotoxin. Cancer. 2011;117:5519–5528. doi: 10.1002/cncr.26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serafino A, Zonfrillo M, Andreola F, Psaila R, Mercuri L, Moroni N, Renier D, Campisi M, Secchieri C, Pierimarchi P. CD44-targeting for antitumor drug delivery: a new SN-38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosis. Curr Cancer Drug Targets. 2011;11:572–585. doi: 10.2174/156800911795655976. [DOI] [PubMed] [Google Scholar]
- 59.De Stefano I, Battaglia A, Zannoni GF, Prisco MG, Fattorossi A, Travaglia D, Baroni S, Renier D, Scambia G, Ferlini C, Gallo D. Hyaluronic acid-paclitaxel: effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother Pharmacol. 2011;68:107–116. doi: 10.1007/s00280-010-1462-2. [DOI] [PubMed] [Google Scholar]
- 60.Somasunderam A, Thiviyanathan V, Tanaka T, Li X, Neerathilingam M, Lokesh GL, Mann A, Peng Y, Ferrari M, Klostergaard J, Gorenstein DG. Combinatorial selection of DNA thioaptamers targeted to the HA binding domain of human CD44. Biochemistry. 2010;49:9106–9112. doi: 10.1021/bi1009503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slomiany MG, Dai L, Tolliver LB, Grass GD, Zeng Y, Toole BP. Inhibition of Functional Hyaluronan-CD44 Interactions in CD133-positive Primary Human Ovarian Carcinoma Cells by Small Hyaluronan Oligosaccharides. Clin Cancer Res. 2009;15:7593–7601. doi: 10.1158/1078-0432.CCR-09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li SD, Howell SB. CD44-targeted microparticles for delivery of cisplatin to peritoneal metastases. Mol Pharm. 2010;7:280–290. doi: 10.1021/mp900242f. [DOI] [PubMed] [Google Scholar]
- 63.Hassan HT. c-Kit expression in human normal and malignant stem cells prognostic and therapeutic implications. Leuk Res. 2009;33:5–10. doi: 10.1016/j.leukres.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Juretzka M, Hensley ML, Tew W, Konner J, Aghajanian C, Leitao M, Iasonos A, Soslow R, Park K, Sabbatini P. A phase 2 trial of oral imatinib in patients with epithelial ovarian, fallopian tube, or peritoneal carcinoma in second or greater remission. European Journal of Gynaecological Oncology. 2008;29:568–572. [PubMed] [Google Scholar]
- 65.Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, Miner Z, Vanderhyden BC. Phase II Evaluation of Imatinib Mesylate in the Treatment of Recurrent or Persistent Epithelial Ovarian or Primary Peritoneal Carcinoma: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2008;26:3418–3425. doi: 10.1200/JCO.2007.14.3420. [DOI] [PubMed] [Google Scholar]
- 66.Matei D, Chang DD, Jeng MH. Imatinib mesylate (Gleevec) inhibits ovarian cancer cell growth through a mechanism dependent on platelet-derived growth factor receptor alpha and Akt inactivation. Clinical Cancer Research. 2004;10:681–690. doi: 10.1158/1078-0432.ccr-0754-03. [DOI] [PubMed] [Google Scholar]
- 67.Margalit E, Sunness JS, Green WR, Kelman SE, Schachat AP, Fiergang D, Allikmets R. Stargardt disease in a patient with retinoblastoma. Arch Ophthalmol. 2003;121:1643–1646. doi: 10.1001/archopht.121.11.1643. [DOI] [PubMed] [Google Scholar]
- 68.Li HK, Shields JA, Shields CL, Maguire JI, Garg SJ. Retinal vasoproliferative tumour as the initial manifestation of retinitis pigmentosa. Clin Experiment Ophthalmol. 2008;36:895–897. doi: 10.1111/j.1442-9071.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 69.Yang C, Liu Y, Lu X, Qian F, Zhao L. Sporadic bilateral retinitis pigmentosa sine pigmento associated with atypical Peutz-Jeghers syndrome. Can J Ophthalmol. 2010;45:184–185. doi: 10.3129/i09-191. [DOI] [PubMed] [Google Scholar]
- 70.Bourseau-Guilmain E, Griveau A, Benoit JP, Garcion E. The importance of the stem cell marker prominin-1/CD133 in the uptake of transferrin and in iron metabolism in human colon cancer Caco-2 cells. PLoS One. 2011;6:e25515. doi: 10.1371/journal.pone.0025515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takenobu H, Shimozato O, Nakamura T, Ochiai H, Yamaguchi Y, Ohira M, Nakagawara A, Kamijo T. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene. 2011;30:97–105. doi: 10.1038/onc.2010.383. [DOI] [PubMed] [Google Scholar]
- 72.Dong L, Qi N, Ge RM, Cao CL, Lan F, Shen L. Overexpression of CD133 promotes the phosphorylation of Erk in U87MG human glioblastoma cells. Neurosci Lett. 2010;484:210–214. doi: 10.1016/j.neulet.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 73.Feng HL, Liu YQ, Yang LJ, Bian XC, Yang ZL, Gu B, Zhang H, Wang CJ, Su XL, Zhao XM. Expression of CD133 correlates with differentiation of human colon cancer cells. Cancer Biol Ther. 2010;9:216–223. doi: 10.4161/cbt.9.3.10664. [DOI] [PubMed] [Google Scholar]
- 74.Yao J, Zhang T, Ren J, Yu M, Wu G. Effect of CD133/prominin-1 antisense oligodeoxynucleotide on in vitro growth characteristics of Huh-7 human hepatocarcinoma cells and U251 human glioma cells. Oncol Rep. 2009;22:781–787. doi: 10.3892/or_00000500. [DOI] [PubMed] [Google Scholar]
- 75.Xu M, Rettig M, Sudlow G, Wang B, Akers W, Cao D, Mutch D, Dipersio J, Achilefu S. Preclinical evaluation of mab CC188 for ovarian cancer imaging. Int J Cancer. 2011 doi: 10.1002/ijc.27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferrandina G, Petrillo M, Bonanno G, Scambia G. Targeting CD133 antigen in cancer. Expert Opinion on Therapeutic Targets. 2009;13:823–837. doi: 10.1517/14728220903005616. [DOI] [PubMed] [Google Scholar]
- 77.Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Reviews. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 78.Kast RE, Belda-Iniesta C. Suppressing glioblastoma stem cell function by aldehyde dehydrogenase inhibition with chloramphenicol or disulfiram as a new treatment adjunct: an hypothesis. Curr Stem Cell Res Ther. 2009;4:314–317. doi: 10.2174/157488809789649241. [DOI] [PubMed] [Google Scholar]
- 79.Morrison BW, Doudican NA, Patel KR, Orlow SJ. Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res. 2010;20:11–20. doi: 10.1097/CMR.0b013e328334131d. [DOI] [PubMed] [Google Scholar]
- 80.Lin J, Haffner MC, Zhang Y, Lee BH, Brennen WN, Britton J, Kachhap SK, Shim JS, Liu JO, Nelson WG, Yegnasubramanian S, Carducci MA. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate. 2011;71:333–343. doi: 10.1002/pros.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verma S, Stewart DJ, Maroun JA, Nair RC. A randomized phase II study of cisplatin alone versus cisplatin plus disulfiram. Am J Clin Oncol. 1990;13:119–124. doi: 10.1097/00000421-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Lee JH, Kim SH, Lee ES, Kim YS. CD24 overexpression in cancer development and progression: a meta-analysis. Oncol Rep. 2009;22:1149–1156. doi: 10.3892/or_00000548. [DOI] [PubMed] [Google Scholar]
- 84.Su D, Deng H, Zhao X, Zhang X, Chen L, Chen X, Li Z, Bai Y, Wang Y, Zhong Q, Yi T, Qian Z, Wei Y. Targeting CD24 for treatment of ovarian cancer by short hairpin RNA. Cytotherapy. 2009;11:642–652. doi: 10.1080/14653240902878308. [DOI] [PubMed] [Google Scholar]
- 85.Chang HL, Pieretti-Vanmarcke R, Nicolaou F, Li X, Wei X, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance inhibits invasion and migration of epithelial cancer cell lines. Gynecol Oncol. 2011;120:128–134. doi: 10.1016/j.ygyno.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, Buckanovich RJ. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCann CK, Growdon WB, Kulkarni-Datar K, Curley MD, Friel AM, Proctor JL, Sheikh H, Deyneko I, Ferguson JA, Vathipadiekal V, Birrer MJ, Borger DR, Mohapatra G, Zukerberg LR, Foster R, MacDougall JR, Rueda BR. Inhibition of Hedgehog Signaling Antagonizes Serous Ovarian Cancer Growth in a Primary Xenograft Model. PLoS One. 6:e28077. doi: 10.1371/journal.pone.0028077. [DOI] [PMC free article] [PubMed] [Google Scholar]