Abstract
Introduction
Electrodiagnostic features of demyelination are essential for establishing the diagnosis in demyelinating subtypes of Guillain-Barré syndrome (GBS), but they may also occur in disorders that mimic GBS clinically. Information about their frequency in GBS mimics is sparse.
Methods
Evaluation of electrodiagnostic features from 38 patients with suspected GBS in whom the diagnosis was later refuted (GBS mimics). Their diagnostic accuracy was analyzed by comparison with NCS from 73 confirmed GBS patients.
Results
Disorders that mimicked GBS clinically at the time of hospital admission included other inflammatory, metabolic, toxic, or infectious neuropathies and spinal cord disorders. The sural sparing pattern was the most specific electrodiagnostic feature for demyelinating GBS.
Discussion
Common electrodiagnostic abnormalities in early demyelinating GBS do not usually exclude other rare differential diagnoses. An exception to this is the sural sparing pattern described here, which strongly supports the diagnosis of demyelinating GBS.
Keywords: differential diagnosis, nerve conduction studies, peripheral neuropathy, misdiagnosis, GBS, Guillain-Barré syndrome
INTRODUCTION
Guillain-Barré syndrome (GBS) is one of the most common causes of acute flaccid paralysis worldwide.1 GBS subtypes that can be distinguished by electrodiagnostic and pathological criteria include acute inflammatory demyelinating polyradiculoneuropathy (AIDP) and axonal variants, such as acute axonal motor neuropathy (AMAN) and acute motor sensory axonal neuropathy (AMSAN). AIDP is by far the most common of all GBS variants in Europe and North America accounting for 90–95% of all cases.1
The diagnosis of GBS is usually based on the history and typical clinical symptoms; it is confirmed by characteristic abnormalities in the cerebrospinal fluid (CSF) (albuminocytologic dissociation) and nerve conduction studies (NCS). Discrimination from other causes of flaccid paralysis can be sometimes challenging, and differential diagnoses that may be confused with GBS in the acute stage include spinal cord lesions, metabolic or toxic neuropathies, or an underlying chronic neuropathy.2 Moreover, findings in NCS suggestive of demyelination in early AIDP are nonspecific and may even occur in disorders that mimic GBS in the acute stage. For example, abnormal late responses are well known to occur in patients with acute myelopathy and might be therefore insufficient to confirm or refute the diagnosis of GBS.3 In this study we explored the accuracy of common electrophysiological criteria of demyelination as a discriminator for AIDP and its clinical mimics. We reviewed electrophysiological data from patients suspected to have GBS on hospital admission but in whom the diagnosis was subsequently ruled out. We assessed sensitivity and specificity by comparing the electrodiagnostic findings with those from confirmed demyelinating GBS cases.
METHODS
GBS mimics were identified by retrospective review of clinical and electrophysiological charts from a total of 130 patients with suspected GBS who were referred to 3 different neuromuscular centers. The 3 centers were the Department of Neurology, Heinrich-Heine University, Düsseldorf (center A, time period 2004–2011), the Department of Neurology, University Hospital of Cologne (center B, time period 2008–2012), and the Department of Neurology, University of Texas Medical School at Houston, Houston, TX, USA (center C, time period 2010–2011). In centers A and B, patients were identified by use of the International Statistical Classification of Diseases and Related Health Problems Version 10 (ICD10) code for GBS (G61.0). The suspected diagnoses in those 2 centers were documented at referral in all patients and were coded by ICD10. Patients whose initial diagnosis “GBS” (G61.0) was rejected subsequently, were identified by discordant ICD10 diagnose codes at admission and discharge. In center A we also included 2 patients with acute flaccid paralysis in whom GBS was considered, but no clear diagnosis was made at the initial evaluation. The clinical and electrophysiological data were compared to those from 73 confirmed AIDP cases from the 3 centers (center A, n=39; center B, n=28; center C, n=6).
The confirmation of the diagnosis of AIDP was based on 2 criteria: first, patients must fulfill criteria for GBS developed by the Brighton Collaborative GBS working group (diagnostic certainty levels 1 and 2).4 This case definition requires the presence of bilateral flaccid weakness of the limbs, decreased or absent deep tendon reflexes in weak limbs, a monophasic illness pattern with an interval between onset and nadir of weakness between 12 h and 28 days with subsequent clinical plateau. Electrophysiological findings that are consistent with GBS must be present along with albuminocytologic dissociation (elevation of CSF protein level above laboratory normal value and CSF total white cell count <50 cells/ l) to fulfill level one of diagnostic certainty. If 1 of these 2 criteria was not fulfilled the level of diagnostic certainty is 2. In any patient an identified alternative diagnosis for weakness must not be present. In addition, the electrophysiological studies must be consistent with AIDP according to previously published criteria.5 Patient data which were incomplete or which did not fulfill these 2 criteria were excluded from further analysis. IgG antibodies against the gangliosides GD1a, GM1, GD1b, and GQ1b were tested as described previously.6
Nerve conduction studies
In each patient, electrophysiological studies were carried out within 48h after hospital admission by trained neurologists, as described previously.7 Compound muscle action potentials (CMAP), distal motor latency (DML), motor conduction velocity (MCV), and F-waves were obtained from at least 3 motor (tibial, fibular, median, and /or ulnar) nerves, although the selection of nerves varied in each center. Sensory nerve action potentials (SNAP) were recorded from median and ulnar nerves using orthodromic technique (center A) and antidromically from the sural nerve. In centers B and C the ulnar and median sensory nerves were assessed antidromically. H-reflex, facial neurography, and blink reflex were not included in the analysis, since these tests were not examined regularly in each patient.
Statistical methods
Sensitivity, specificity, and positive and negative likelihood ratios (PLR and NLR, respectively) were analyzed for electrophysiological changes for demyelination in GBS mimics and in GBS cases that were validated by diagnostic criteria of the Brighton GBS Working Group.4 The statistical estimates were calculated for the postulates that the presence of common electrophysiological changes for demyelination [prolonged DML, reduced MCV, F-wave abnormality (absent F-waves or prolonged F-wave latencies)] at presentation indicated the diagnosis of GBS. Sensitivity, specificity, PLR, and NLR were also calculated for albuminocytologic dissociation in the CSF.
RESULTS
Spectrum of disorders mimicking GBS
We identified 38 patients (24 men and 14 women) who presented with symptoms consistent with GBS, but in whom the initial diagnosis was subsequently refuted (table 1). Of those, 20 patients were identified in center A, 12 patients in center B, and 6 patients in center C. The mean time from symptom onset to first electrodiagnostic examination was 15 days (range 2–20 days). The age ranged from 21 to 81 years, with a median of 55 years. The most frequent diagnosis among GBS mimics was chronic inflammatory demyelinating polyradiculoneuropathy (CIDP, 24%) followed by chronic axonal neuropathy (13%) and somatoform disorder (13%).
Table 1.
Spectrum of differential diagnosis of Guillain-Barré syndrome
| Other neurological disease diagnosis | No of patients |
|---|---|
| CIDP | 9 |
| Chronic axonal polyneuropathy | 5 |
| Somatoform disorder | 5 |
| Spinal cord infarction | 4 |
| Spinal cord compression | 2 |
| Chronic axonal-demyelinating polyneuropathy | 2 |
| Alcoholic neuropathy | 2 |
| Infectious neuritis | 2 |
| Ganglionitis | 1 |
| Parainfectious myositis | 1 |
| Nerve pressure palsy | 1 |
| Neuroborreliosis | 1 |
| Vasculitic neuropathy | 1 |
| Hypokalemia | 1 |
| Tetrodotoxin poisoning | 1 |
| Total | 38 |
Clinical characteristics of the GBS cohort
Study GBS patients included a total number of 73 patients consisting of 42 men and 31 women. All patients fulfilled diagnostic criteria of the Brighton Collaboration GBS working group. 52% of the patients were categorized as level 1 patients, 48% of the patients were classified as level 2. The age ranged from 22 to 80 years, with a median of 56 years. Anti-ganglioside antibody testing was performed in 37% of patients. Of those one patient was tested positive for anti-GM1 antibodies, one patient was positive for antibodies against GD1a and five patients were positive for antibodies against GQ1b.
Electrophysiological findings at referral and their predictive value for diagnosis
Electrophysiological features that suggest demyelination could also be detected in varying frequency in patients with a non-GBS diagnosis. The most common abnormality that was observed in about 40% of the patients was abnormal late responses. Other abnormal findings that were noted frequently were reduced NCV (34%) and prolonged DML (26%), mostly in patients with a final diagnosis of chronic neuropathy. The most specific finding suggestive of early demyelinating GBS was the presence of a “spared” normal sural SNAP with abnormal ulnar SNAP (measured orthodromically), which had a specificity of 0.95, a sensitivity of 0.41, and a positive likelihood ratio of 8.20 (Table 2). When the ulnar nerve was measured antidromically, the specificity of the sural sparing pattern was still high (0.83), though the positive likelihood ratio was considerably lower (2.00). Although the presence of conduction block was only occasionally noted in patients in whom the final diagnosis was not GBS, its specificity was low, since it was present only in the minority of confirmed GBS patients.
Table 2.
Statistical estimates for electrodiagnstic criteria for demyelination in Guillain-Barré syndrome and its mimics
| Diagnosis (percentage) | Specificity | Sensitivity | PLR | NLR | ||
|---|---|---|---|---|---|---|
| GBS | GBS mimics | |||||
| Prolongation of DML | 71 | 26 | 0.74 | 0.71 | 2.76 | 0.38 |
| Center A | 79 | 25 | 0.75 | 0.79 | ||
| Center B | 57 | 58 | 0.42 | 0.57 | ||
| Center C | 100 | 50 | 0.5 | 1.00 | ||
| Abnormal F-waves | 80 | 42 | 0.57 | 0.80 | 1.91 | 0.34 |
| Center A | 85 | 35 | 0.65 | 0.85 | ||
| Center B | 57 | 58 | 0.42 | 0.57 | ||
| Center C | 100 | 100 | 0 | 1 | ||
| Slow NCV | 49 | 34 | 0.66 | 0.49 | 1.43 | 0.77 |
| Center A | 49 | 25 | 0.75 | 0.49 | ||
| Center B | 48 | 25 | 0.75 | 0.48 | ||
| Center C | 67 | 83 | 0.16 | 0.67 | ||
| SSP (ulnar vs. sural) | 41 | 5 | 0.93 | 0.35 | 5.29 | 0.69 |
| Center A (orthodr.) | 41 | 5 | 0.95 | 0.41 | ||
| Center B (antidr.) | 0 | 0 | 1 | 0 | ||
| Center C (antidr.) | 33 | 17 | 0.83 | 0.33 | ||
| SSP (median vs. sural) | 26 | 8 | 0.91 | 0.26 | 3.12 | 0.81 |
| Center A (orthodr.) | 44 | 17 | 0.83 | 0.44 | ||
| Center B (antidr.) | 18 | 0 | 1 | 0.18 | ||
| Center C (antidr.) | 0 | 17 | 0.83 | 0 | ||
| Elevated CSF protein | 80 | 29 | 0.71 | 0.80 | 2.74 | 0.28 |
| Center A | 87 | 33 | 0.67 | 0.87 | ||
| Center B | 71 | 22 | 0.78 | 0.71 | ||
| Center C | N/A | N/A | N/A | N/A | ||
Sural sparing pattern in GBS and non-GBS patients
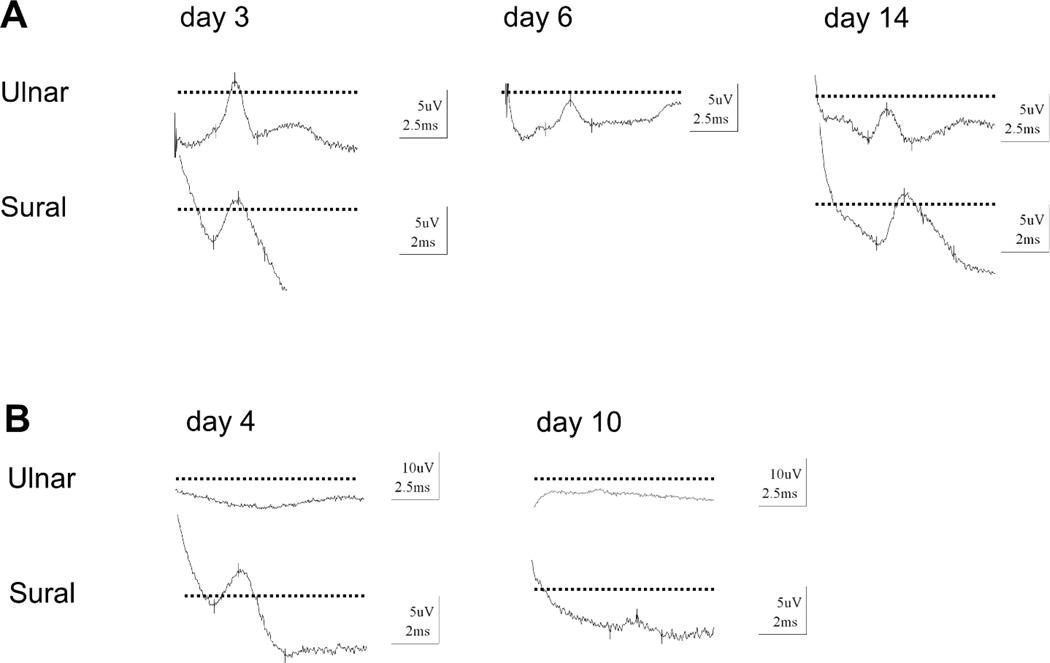
In 41% of the GBS patients the sural nerve was normal, but the SNAP of the ulnar nerve was pathologic (44% compared to an abnormal sensory median nerve). The sural sparing pattern was not present either because of normal sural and ulnar/median sensory NCS (21%) or because of an abnormalc SNAP in the sural nerve and upper limb sensory NCS (38%). GBS patients with the sural sparing pattern did not differ in terms of other clinical or electrophysiological features from GBS patients without this pattern. In the majority of those patients (81%), the sural sparing pattern was already present in the initial electrophysiological evaluation and persisted in subsequent NCS. Occasionally, the sural sparing pattern developed during subsequent measurements (Figure 1A). In some GBS patients (21%) with the sural sparing pattern we noted that the abnormal ulnar SNAP preceded a further decline of the sural SNAP, which also became abnormal later (Figure 1B). In the non-GBS group only 1 patient with a final diagnosis of chronic axonal peripheral neuropathy presented with a normal sural SNAP but abnormal ulnar SNAP.
Figure 1.
Serial sensory nerve action potentials (SNAP) recordings from ulnar and sural nerves in two patients with Guillain-Barré syndrome (GBS). Figure 1A: Patient 1 has normal ulnar and sural sensory recordings at day 3 after symptom onset. Serial nerve conduction studies at day 6 and day 14 revealed a developing “sural nerve sparing pattern” with decreased ulnar SNAP but normal sural SNAP. Figure 1B: Patient 2 had already “sural nerve sparing pattern” with inexcitable ulnar sensory nerve fibers on admittance (day 4 after symptom onset). Repeated electrophysiological testing shows decreasing sural nerve SNAP which became abnormal low at day 10 (dotted line = normal levels).
DISCUSSISON
We identified 38 patients in whom the initial suspected diagnosis of GBS was subsequently refuted. Regarding the large number of reviewed GBS cases and the long time frame of the study, GBS mimics are still rare, and our case review approach probably overestimated their absolute frequency. However, the cohort of GBS mimics is instructive, because it represents a fairly complete range of disorders that are often referred to as relevant differential diagnoses for GBS.1,2
We compared the pattern and frequency of electrodiagnostic changes in this cohort of patients with those from confirmed GBS patients with the goal of providing statistical estimates that may be helpful in the clinical setting in which a patient is referred to a neuromuscular unit because of symptoms suggestive of GBS. To minimize a technical bias that may arise because technique and interpretation of electrodiagnostic testing varies between EMG laboratories, we analyzed NCS from 3 different neuromuscular centers in 2 countries. We focused on electrodiagnostic changes that support the diagnosis of AIDP, the most common GBS subtype in Europe and North America.1 These abnormal NCS findings reflect the underlying multifocal demyelinating process in AIDP and include abnormal F-waves, presence of A-waves, prolonged DML, reduced MCV, and the combination of abnormal upper limb SNAP and normal “spared” sural nerve SNAPs (“sural sparing pattern”).8–14
None of these abnormalities are known to be specific for GBS,3,15–18 and in the early stages these electrodiagnostic findings are often incompletely developed or even entirely absent.13,14 Thus, serial NCS during the course of the disease are usually recommended to ascertain the diagnosis and to improve proper subtype classification.19,20
We found that electrodiagnostic features for demyelination were also present in the cohort of GBS mimics, although their frequency was generally lower as compared to patients with confirmed demyelinating GBS. The most specific discriminating electrodiagnostic feature was the sural sparing pattern, which is recognized widely to be a common abnormality that occurs early in 39 – 67% of GBS patients.13,14,21–23 Compared to patients with a non-GBS diagnosis, the sural sparing pattern had modest sensitivity (0.35) but high specificity (0.93 for a normal ulnar nerve SNAP and 0.91 for normal median nerve SNAP). Specificities do not differ substantially when orthodromic (center A) or antidromic (centers B and C) upper limb sensory nerve conduction measurements were used. Thus we conclude that this finding is relatively robust for these different recording techniques. Our results are in line with a previous report that found a high specificity (0.96) for the sural sparing pattern in GBS patients compared to patients with critical illness polyneuropathy.22
A clear limitation of our study is that electrodiagnostic features of GBS mimics could only be compared to AIDP cases but not to axonal subtypes of GBS. As recently pointed out24, findings of reversible nerve conduction abnormalities in distal nerve segments in addition to CMAP reductions in AMAN merit reconsideration and further validation of the current electrodiagnostic criteria for GBS subtypes.
The clinical implications of this study are that electrodiagnostic features of demyelination such as prolonged DML and abnormal F-waves are less helpful in excluding relevant differentials to demyelinating GBS. In contrast, the occurrence of a sural sparing pattern is highly suggestive of AIDP, and special attention should be paid to this combination during the initial electrophysiological evaluation of patients with suspected GBS.
Acknowledgments
FUNDING:
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
ABBREVIATIONS
- AIDP
acute inflammatory demyelinating polyneuropathy
- AMAN
acute axonal motor neuropathy
- CIDP
chronic inflammatory demyelinating polyradiculoneuropathy
- CMAP
compound muscle action potential
- CSF
cerebrospinal fluid
- DML
distal motor latency
- GBS
Guillain-Barré syndrome
- MCV
motor conduction velocity
- NLR
negative likelihood ratio
- SNAP
Sensory nerve action potentials
- PLR
positive likelihood ratio
Footnotes
COMPETING INTERESTS:
There are no competing interests.
CONTRIBUTORSHIP STATEMENT:
All authors had full access to all of the data in the study. Study concept and design: AD, HCL, KAS Acquisition of data: AD, CR, PA, PM. Analysis and interpretation of data: AD, BCK, HCL. Drafting of the manuscript: AD, BCK, HPH, SAK, HCL. Critical revision of the manuscript for important intellectual content: HPH, BCK, KAS, HCL.
REFERENCES
- 1.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366(24):2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 2.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. 2008;7(10):939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 3.Syme JA, Kelly JJ. Absent F-waves early in a case of transverse myelitis. Muscle Nerve. 1994;17(4):462–465. doi: 10.1002/mus.880170415. [DOI] [PubMed] [Google Scholar]
- 4.Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, Burwen DR, Cornblath DR, Cleerbout J, Edwards KM, Heininger U, Hughes R, Khuri-Bulos N, Korinthenberg R, Law BJ, Munro U, Maltezou HC, Nell P, Oleske J, Sparks R, Velentgas P, Vermeer P, Wiznitzer M, Group BCGW. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, Swan AV. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Ann Neurol. 1998;44(5):780–788. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- 6.Willison HJ, Veitch J, Swan AV, Baumann N, Comi G, Gregson NA, Illa I, Jacobs BC, Zielasek J, Hughes RA. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur J Neurol. 1999;6:71–77. doi: 10.1046/j.1468-1331.1999.610071.x. [DOI] [PubMed] [Google Scholar]
- 7.van Asseldonk JTH, Van den Berg LH, Kalmijn S, Van den Berg-Vos RM, Polman CH, Wokke JHJ, Franssen H. Axon loss is an important determinant of weakness in multifocal motor neuropathy. J Neurol Neurosurg Psychiatry. 2006;77:743–747. doi: 10.1136/jnnp.2005.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Bergh PY, Piéret F. Electrodiagnostic criteria for acute and chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2004;29(4):565–574. doi: 10.1002/mus.20022. [DOI] [PubMed] [Google Scholar]
- 9.Albers JW, Donofrio PD, McGonagle TK. Sequential electrodiagnostic abnormalities in acute inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 1985;8(6):528–539. doi: 10.1002/mus.880080609. [DOI] [PubMed] [Google Scholar]
- 10.Albers JW, Kelly JJ. Acquired inflammatory demyelinating polyneuropathies: clinical and electrodiagnostic features. Muscle Nerve. 1989;12(6):435–451. doi: 10.1002/mus.880120602. [DOI] [PubMed] [Google Scholar]
- 11.Cornblath DR. Electrophysiology in Guillain-Barre syndrome. Ann Neurol. 1990;27(Suppl):S17–S20. doi: 10.1002/ana.410270706. [DOI] [PubMed] [Google Scholar]
- 12.Vucic S, Cairns KD, Black KR, Chong PS, Cros D. Neurophysiologic findings in early acute inflammatory demyelinating polyradiculoneuropathy. Clin Neurophysiol. 2004;115(10):2329–2335. doi: 10.1016/j.clinph.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Gordon PH, Wilbourn AJ. Early electrodiagnostic findings in Guillain-Barré syndrome. Arch Neurol. 2001;58(6):913–917. doi: 10.1001/archneur.58.6.913. [DOI] [PubMed] [Google Scholar]
- 14.Albertí MA, Alentorn A, Martínez-Yelamos S, Martínez-Matos JA, Povedano M, Montero J, Casasnovas C. Very early electrodiagnostic findings in Guillain-Barré syndrome. J Peripher Nerv Syst. 2011;16(2):136–142. doi: 10.1111/j.1529-8027.2011.00338.x. [DOI] [PubMed] [Google Scholar]
- 15.Curt A, Keck ME, Dietz V. Clinical value of F-wave recordings in traumatic cervical spinal cord injury. Electroencephalogr Clin Neurophysiol. 1997;105(3):189–193. doi: 10.1016/s0924-980x(97)96626-1. [DOI] [PubMed] [Google Scholar]
- 16.Mesrati F, Vecchierini MF. F-waves: neurophysiology and clinical value. Neurophysiol Clin. 2004;34(5):217–243. doi: 10.1016/j.neucli.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Verny C, Prundean A, Nicolas G, Pautot V, Maugin D, Levade T, Bonneau D, Dubas F. Refsum's disease may mimic familial Guillain Barre syndrome. Neuromuscul Disord. 2006;16(11):805–808. doi: 10.1016/j.nmd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769–1773. doi: 10.1212/wnl.58.12.1769. [DOI] [PubMed] [Google Scholar]
- 19.Uncini A, Manzoli C, Notturno F, Capasso M. Pitfalls in electrodiagnosis of Guillain-Barré syndrome subtypes. J Neurol Neurosurg Psychiatry. 2010;81(10):1157–1163. doi: 10.1136/jnnp.2010.208538. [DOI] [PubMed] [Google Scholar]
- 20.Tsang TY, Umapathi T, Yuki N. Serial electrodiagnostic studies increase the diagnostic yield of axonal Guillain-Barré syndrome. Clin Neurophysiol. 2013;124(1):210–212. doi: 10.1016/j.clinph.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Al-Shekhlee A, Robinson J, Katirji B. Sensory sparing patterns and the sensory ratio in acute inflammatory demyelinating polyneuropathy. Muscle Nerve. 2007;35(2):246–250. doi: 10.1002/mus.20660. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shekhlee A, Hachwi RN, Preston DC, Katirji B. New criteria for early electrodiagnosis of acute inflammatory demyelinating polyneuropathy. Muscle Nerve. 2005;32(1):66–72. doi: 10.1002/mus.20342. [DOI] [PubMed] [Google Scholar]
- 23.Wee AS, Abernathy SD. The sural sensory nerve is usually spared in Guillain-Barre syndrome. J Miss State Med Assoc. 2003;44(8):251–255. [PubMed] [Google Scholar]
- 24.Kuwabara S, Yuki N. Axonal Guillain-Barré syndrome: concepts and controversies. Lancet Neurol. 2013;12(12):1180–1188. doi: 10.1016/S1474-4422(13)70215-1. [DOI] [PubMed] [Google Scholar]