Abstract
Despite being considered “good-risk” acute myelogenous leukemia (AML), long term outcomes in core binding factor (CBF) AML suggest room for improvement. We report on a regimen consisting of fludarabine, cytarabine, granulocyte colony stimulating factor, and low dose gemtuzumab ozogamicin (FLAG-GO) as front-line therapy of patients with CBF AML. Forty-five patients were enrolled (median age 48 years). Remission rate was 95% with 5% induction deaths. The overall survival (OS) and relapse free survival (RFS) probability at 3 years are 78% and 85%, respectively. FLAG-GO regimen results in high rates of RFS and OS in CBF AML. Our data along with recent data from several large groups strongly argues in favor of incorporation of gemtuzumab ozogamicin in frontline regimens for CBF AML.
Introduction
Based on retrospective data from Cancer and Leukemia Group B (CALGB), anthracycline- and cytarabine-based induction and repeated cycles of post-remission high dose cytarabine (HDAC) (usually 3–4) have emerged as preferred treatment of core binding factor acute myelogenous leukemia (CBF AML) [1, 2]. The CALGB data indicated that three to four cycles of HDAC is clearly superior to one cycle of HDAC consolidation. Cumulative experiences of several collaborative groups have clearly established benefit of HDAC in CBF AML [3, 4]. Despite the perceived favorable prognosis of patients with CBF AML, large groups that adhere generally to such induction/post-remission strategy report survival probability of 40–50% at 5 years [4]. Even among pediatric patients with CBF AML, long term event free survival (EFS) is only about 55–60% [5]. Although these outcomes are better than AML with intermediate-risk or complex cytogenetics, there is clear need for improvement.
Two approaches toward enhancing treatment outcomes are noteworthy. The first involves addition of fludarabine. Fludarabine administration prior to cytarabine can increase intracellular accumulation of arabinosylcytosine triphosphate [6, 7]. We reported improved EFS in patients with CBF AML with a front-line regimen combining fludarabine, cytarabine, and G-CSF (FLAG) as induction and post-remission therapy compared to the same with idarubicin and cytarabine (IA) [8]. In the Medical Research Council (MRC) AML 15 trial, among patients younger than 60 years of age who completed two cycles of fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-Ida) followed by two cycles of HDAC consolidation, the survival rate was 95% among patients with favorable-risk AML [9].
The second approach uses gemtuzumab ozogamicin (GO). GO is an anti-CD33 monoclonal antibody linked to calicheamycin with single-agent activity among elderly patients with AML in first relapse [10]. In the MRC AML 15 trial [11], patients with newly diagnosed AML, younger than 60 years were randomized to receive single low dose of GO, in induction and/or in post-remission period. Subgroup analysis indicated overall survival (OS) benefit among patients with CBF AML who received GO in induction. Randomized data from the Acute Leukemia French Association (ALFA) [12] also confirmed improvement in OS and EFS with the use of GO in combination with chemotherapy as front-line therapy in older patients with favorable (including CBF AML) and intermediate-risk cytogenetics AML while the Southwest Oncology Group reported improved OS and RFS in younger patients with CBF AML who were randomized to receive GO with “3+7” [13].
This motivated a front-line open label trial of fludarabine, cytarabine, G-CSF in combination with low dose GO (FLAG-GO) in patients with CBF AML. The trial was registered at www.Clinicaltrials.gov as NCT00801489.
Methods
Objective
The primary objectives were to simultaneously assess the safety and the efficacy (remission rate) of FLAG-GO regimen in patients with newly diagnosed AML associated with inversion 16, t(16;16), or t(8;21). Secondary objectives included OS, RFS, and correlating serial quantitative monitoring of fusion transcripts associated with above cytogenetic abnormalities with clinical outcomes.
Eligibility
Patients age ≥18 years (no upper limit) with new diagnosis of AML with t(8;21), Inv(16), or t(16;16), with or without additional cytogenetic abnormalities, were eligible. Poor performance status or organ dysfunctions were not exclusions but dose adjustments were allowed.
Treatment plan
Induction
Filgrastim (G-CSF) 5 mcg/kg was administered subcutaneously (SQ), starting on day 1 and continued until absolute neutrophil count (ANC) recovered to ≥1 × 109/L. Once the chemotherapy part of induction was completed, patients could receive one dose of pegylated filgrastim (6 mg SQ) instead of daily filgrastim.
Chemotherapy comprised of fludarabine 30 mg/m2 intravenously (IV) over approximately 30 min daily on days 1–5 and Cytarabine 2 g/m2 IV over 4 hr daily on days 1–5. Cytarabine infusion started 3.5 hr after the completion of Fludarabine. GO 3 mg/m2 was administered IV over 2 hr on day 1.
Post-remission therapy
Post-remission therapy composed of fludarabine, cytarabine, gemtuzumab ozogamicin, and filgrastim as during induction except that fludarabine and cytarabine were given for 4 days. In general, post-remission courses began once the neutrophil count recovered to ≥1 × 109/L and the platelet count to ≥75 × 109/L. The planned number of post-remission cycles was 6.
Protocol amendments
The protocol was amended to limit the administration of GO to induction and two post-remission cycles, on cycles 3 or 4 and 5 or 6. The administration of fludarabine and cytarabine in post-remission courses was also reduced to 3 days instead of 4. These were implemented to avoid prolonged cytopenias delaying post-remission therapy.
Dose adjustments
For patients with serum creatinine ≥1.5 mg/dL and Eastern Co-operative Oncology Group performance status 3, dose reductions were recommended for both fludarabine and cytarabine. GO was ≥omitted for aspartate amino-transferase (AST) and/or alanine aminotransferase (ALT) levels more than three times the upper limit of normal and/or for bilirubin level >2 mg/dL. For patients above the age of 60 years, the suggested number of days of fludarabine and cytarabine was 4 instead of 5 during induction.
Quantitative polymerase chain reaction for fusion transcripts and mutation analysis
Extracted RNA was analyzed by real-time quantitative polymerase chain reaction (QPCR) for the RUNX1/RUNX1T1 (AML1/ETO) and CBFB/MYH11 fusion transcripts on an ABI HT 7900 platform from Applied Biosystems. Values were expressed as percentage of fusion transcript to normalizing ABL1. The sensitivity of detection is 1 in 100,000. PCR negativity was defined as no detectable fusion transcript with a minimum of 10,000 ABL1 copies. QPCR studies from bone marrow were done at diagnosis, end of induction and of consolidation 2 or 3, and at end of treatment. Follow-up QPCR studies from bone marrow samples were also done every 3–6 months for at least 2 years.
Mutation analysis was carried out in exons 8 and 17 in KIT gene, in codons 12, 14, and 61 in NRAS and KRAS genes using PCR-based DNA sequencing methods, and for internal tandem duplications (ITD) or D835 mutations in FLT3 gene according to published methods [14].
Response criteria
Response criteria were according to the International Working Group 2003 recommendations [15]. Complete remission (CR) required an ANC ≥1 × 109/L, platelet count ≥100 × 109/L, <5% of blast cells in bone marrow; CR with incomplete platelet recovery (CRp) was response as in CR except platelet count <100 × 109/L and partial remission (PR) was defined as CR except for the presence of 5–25% marrow blasts and with a decrease of marrow blast by at least 50%.
Statistical considerations
With safety and response (remission rate) as primary endpoints, the trial was designed using the method by Thall, Simon, and Estey for monitoring multiple outcomes [16]. To implement the design, we used the program Multc Lean Desktop available at the Department of Biostatistics website: http://biostatistics.m-danderson.org/SoftwareDownload/. For both response and toxicity, we used a noninformative prior distribution: beta (1.9, .0.1) for response and beta (0.5, 1.5) for toxicity. The stopping rule for response (CR) was to stop if the posterior probability of the experimental treatment being less responsive is >0.90, that is, Probability [standard response > experimental response] >0.90 and a similar rule was applied for toxicity (clinically significant ≥grade 3 nonhematological toxicity and major infection) occurring during induction: Probability [standard toxicity < experimental toxicity] >0.90.
OS was calculated from diagnosis until death or last follow up, and RFS was assessed from remission until relapse or last follow up. Kaplan-Meier method was used to estimate the probabilities of OS and RFS. Log-rank test [17] was used to compare subgroups of patients in terms of OS or RFS. Univariate and multiple Cox proportional hazards regression models [18] were fit to assess the association between patient characteristics, molecular results, and OS or RFS. All statistical analyses were conducted using SAS or Splus.
Results
Patients
Between April 2007 and June 2010, 45 consecutive patients [Inv(16) = 18, t(8;21) = 27] were enrolled. Median age was 48 years (range, 19–76 years) and 27 (60%) were male. Patient characteristics are summarized (Table I). Twenty (44%) patients had additional autosomal cytogenetic abnormalities; trisomy 8 in 16%, trisomy 22 in 7%, 9q deletion, and −7 in in 4% each. Four patients (9%) had mutations in KIT gene.
TABLE I.
Patient Characteristics (N = 45)
| Patient characteristics | INV(16) |
t(8;21) |
Total cohort N or % |
P | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| No. patients | 18 | 40% | 27 | 60% | 45 | |
| Age (years), median (range) | 55 (28–76) | 44 (19–69) | 48 (19–76) | 0.04 | ||
| Sex | ||||||
| Male | 9 | 50% | 18 | 67% | 58% | 0.35 |
| Race | ||||||
| White | 16 | 89% | 19 | 70% | 0.33 | |
| Non-White | 2 | 11% | 8 | 30% | ||
| Platelets × 109/L, median (range) | 30 (4–307) | 23 (6–426) | 24 (4–426) | 0.25 | ||
| WBC (K/μL) × 109/L, median (range) | 9.9 (2.1–97.2) | 12.6 (1.3–56.3) | 12.3 (1.3–97.2) | 0.6 | ||
| Percentage of BM blasts, median | 42% | 51% | 46% | |||
| KIT mutation | 1 | 6% | 3 | 11% | 9% | |
| FLT3 mutation | 2 | 4% | 3 | 11% | 11% | |
| RAS mutation | 11 | 61% | 5 | 19% | 36% | |
| Response | ||||||
| CR | 16 | 89% | 25 | 93% | 91% | |
| CRP | 2 | 11% | 0 | 0% | 4% | |
| Early death | 0 | 0% | 2 | 7% | 4% | |
Response
Of the 45 patients enrolled in the study, 41 (91%) achieved CR and 2 (4%) CRp. There were two induction deaths [both t(8;21)]. CR/CRp rate was 100% (18/18) among patients with Inv(16) and 93% (25/27) among patients with t(8;21). All patients except one (Inv(16)) achieved remission after one induction. The CR/CRp rate was consistent with historical prior used for statistical design of the study.
Post-remission therapy
The median number of post-remission cycles was 5 (range, 0–6), 12 (24%) completing all 6 planned post-remission cycles. Sixty percent of patients needed dose reductions during post-remission cycles, mostly due to delays in count recovery, with dose reductions occurring after a median of two cycles (range 1–5). Protocol amendments reducing the intensity of post-remission therapy reduced delays; more specifically the median number of days between post-remission cycle 2 and 3 was reduced from 44 days (33–121 days) to 37 days (25–119 days). We chose to look at this time point as most initial dose delays and dose reductions occurred around this time.
The number of GO cycles administered to number of patients are 4 cycles = 5 patients, 3 cycles = 12 patients, 2 cycles = 13 patients, 1 cycle = 8 patients. GO was omitted in induction cycle in three patients; two due to organ dysfunction, and one due to active pneumonia at presentation.
QPCR Data
Median fusion transcript ratio at presentation was >100 for both Inv 16 and t(8;21) patients. Of the 43 patients evaluable (2 induction deaths not evaluable) for this outcome, all except one achieved 3 or more log reduction in transcript ratio (defined as transcript ratio <0.1). The median time to this landmark was 1 month (range, 1–9 months) for the entire cohort and for both cytogenetic subgroups. Thirty-eight of 43 patients achieved three or more log reduction of transcript ratio by end of third cycle (second consolidation) of therapy.
RFS and OS
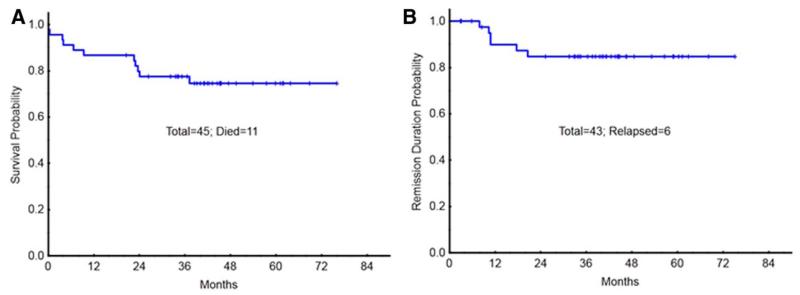
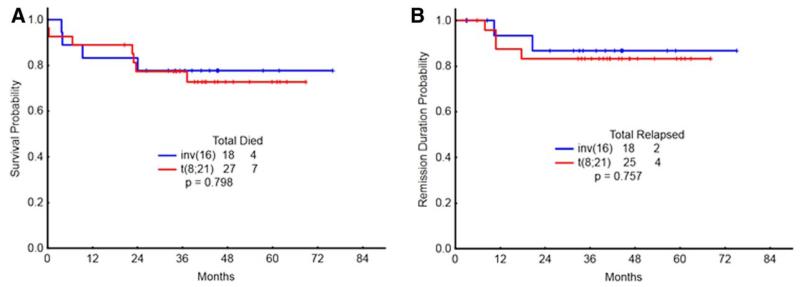
With a median follow up of over 36 months (range, 15–54 months), 6/43 patients (13%) [4/25 of t(8;21) and 2/18 of Inv(16)] have relapsed. One of the four patients with t(8;21) had KIT mutation and both patients with Inv(16) had RAS mutation (one with FLT3-ITD mutation). Median CR duration was 12 months (range, 9–22 months) among the patients who relapsed. Four of the six patients with relapse achieved second remission with salvage therapy, one went to allogeneic stem cell transplant (allo-SCT) with active disease and the last patient with central nervous system (CNS) relapse died of progressive CNS disease. Five nonrelapse deaths on study include two induction deaths and three deaths in remission from septic events. The OS probability was 78%, and RFS probability at 3 years, was 85% (Fig. 1). OS and RFS did not differ between Inv(16) and t(8;21) groups (all P values > 0.7) (Fig. 2). None of the parameters included in the model, for example, age, performance status, white count, platelet count, hemoglobin, marrow or peripheral blood blast percentage, bilirubin, creatinine, lactate dehydrogenase, additional autosomal abnormality, or kinase mutation (KIT, RAS, or FLT3), predicted for OS or RFS in univariate or mutivariate analysis (data not shown) Reduction in transcript ratio of ≥3 log at 1 or 3 months also did not predict for OS (P values 0.08 and 0.5, respectively) or RFS (P = 0.09 and 0.84, respectively).
Figure 1.
OS (A) and RFS (B) of all patients with CBF leukemia treated on study. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
OS (A) and RFS (B) of patients with CBF leukemia treated on study by cytogenetic category. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Toxicities
Grade 3–4 nonhematological toxicities during induction included transaminase elevations (8%), cardiac arrhythmia (4%), renal insufficiency (4%), and respiratory failure (2%) (Table II). Infectious events during induction included pneumonia in 18%, fever of unknown origin in 24%, and sepsis in 10% of patients. The combined incidence of clinically significant ≥grade 3 nonhematological toxicity and major infection as defined in statistical design was lower than historical prior. No event suggestive of veno-occlusive disease (VOD) was encountered. Prolonged myelosuppression as evidenced by delayed recovery of neutrophil/platelet count beyond 6 weeks after induction was encountered in 12% of patients while the same was seen in 70% of patients during the conduct of the study. As the study included use of myeloid growth factor, over 90% of the delays in count recovery were due to thrombocytopenia. The median number of days to count recovery of 47 days after cycle 2 of therapy in our study is similar to the 44 days seen with FLAG-Ida regimen in the MRC AML 15 trial [9].
TABLE II.
Toxicities Encountered During Induction
| Toxicities during induction | Grade 1–2 (%) | Grade 3–4 (%) | Total (%) |
|---|---|---|---|
| Cardiac/Sinus Tachy/Afib | 2 | 4 | 6 |
| Diarrhea | 8 | 2 | 10 |
| Dyspnea | 4 | 0 | 4 |
| Edema | 2 | 0 | 2 |
| Elevated AST/ALT | 64 | 8 | 72 |
| Hyperuricemia | 0 | 2 | 2 |
| Hypotension | 4 | 0 | 4 |
| Lipase | 0 | 2 | 2 |
| Nausea/vomiting | 8 | 0 | 8 |
| Pain | 6 | 2 | 8 |
| Rash | 4 | 0 | 4 |
| Renal | 14 | 4 | 18 |
| Respiratory failure | 0 | 4 | 4 |
Two patients with t(8;21) died during induction. One death due to intracerebral hemorrhage on day 2 was that of a 61-year-old patient with secondary t(8;21) AML who had been treated with prior allogeneic stem cell transplant for MDS (deletion 7 abnormality) with refractory thrombocytopenia prior to starting therapy. The second death was of a 49-year-old male with obstructive sleep apnea, pulmonary hypertension who was briefly intubated in an outside facility for respiratory distress prior to transfer to MD Anderson Cancer Center. His respiratory status deteriorated on the first day of chemotherapy requiring reintubation, and he succumbed to pulmonary hemorrhage. In addition, three patients died during post-remission therapy (remission durations 3, 3, and 6 months); one (age, 51 years) with disseminated fungal infection (comorbidities included prior myocardial infarction, prosthetic aortic valve) and the other two (ages 41 and 69 years) with intravenous catheter related sepsis.
Discussion
Induction/consolidation with FLAG-GO clearly meets the composite end points of safety and efficacy based on historical priors used to design our current study. FLAG-GO has resulted in high OS and RFS rates among patients with newly diagnosed CBF AML. Based on this data as well as randomized data from MRC-15, ALFA trial, and SWOG [11, 13, 19], use of GO in induction/consolidation should be considered for all patients with CBF AML. Our retrospective comparison of sequential, nonrandomized regimens earlier indicated better outcomes among patients with CBF AML treated with FLAG-based regimen when compared with IA [8, 20]. Results with FLAG-GO, along with excellent results from MRC 15 trial particularly in the cohort treated with two cycles of FLAG-Idarubicin followed by HDAC [9], argue in favor of incorporating FLAG regimens in front-line therapy of CBF AML.
The addition of lower dose of GO to chemotherapy in our trial did not result in significant additional liver toxicity. No hepatic VOD was seen. Low incidence of significant VOD in patients treated with a less intense GO schedule than traditionally used has also been the experience in multiple front-line trials of AML across age groups [11, 19, 21, 22].
Despite the fact that our trial did not limit enrollment based on age or performance status, induction death rate of 4% with FLAG-GO regimen is comparable to that of contemporary studies of AML where the eligibility is restricted to patients aged 60 years or less [13]. Delays and dose reductions in post-remission treatment were encountered because of persistent cytopenias and reduction of postremission chemotherapy days from 4 to 3 days improved the number of days to count recovery. While most patients received at least five cycles of post-remission therapy, 60% required dose reductions largely due to cytopenias. The experience is somewhat akin to the MRC 15 trial where patients randomized to FLAG-Ida induction/consolidation arm encountered more myelosuppression [23] but patients who completed therapy with such regimen had better outcome [9]. It is possible that post-remission therapy less than planned in this trial will be adequate but our trial was not powered enough to answer that question. Future trials in CBF AML also need to look at the question of tailoring post-remission therapy based on molecular response.
The frequency of KIT mutations (8%) in exons 8 or 17 is lower in our cohort than reported in other series [24-26]. Most series report KIT exon sequencing after PCR amplification [5] (one followed in our laboratory) while high performance liquid chromatography screening followed by sequencing has been used in others [27, 28]. We did not sequence exons 10 and 11 of KIT gene but frequency of mutations in these exons is low. Whether KIT mutation alone or combination of multiple mutations (RAS, FLT3, KIT) predicts for RFS is an evolving question and highly effective treatment can modulate impact of mutations [5, 29, 30].
Deeper molecular response appears to translate to better RFS [29, 31] in CBF AML. High rate of ≥3 log reduction in transcript ratio in our current trial has likely played a role in the overall favorable outcome. In addition to the MRC data alluded to earlier [29], in the multivariate analysis from French Intergroup CBF-2006 trial, minimal residual disease (≥3 log reduction of transcript) was the sole prognostic factor for relapse [31] to the exclusion of KIT, FLT3-ITD mutations. This raises the possibility of designing randomized trial comparing front-line regimens in CBF AML using molecular end points of transcript reduction. The current limitation in comparing transcript ratio data across laboratories lies in the lack of uniformity in reporting and adoption of international standardization as done in chronic myelogenous leukemia is likely to help.
In summary, our current trial and cumulative experience indicates that a FLAG-based induction/consolidation regimen with incorporation of GO results in excellent outcome among patients with CBF AML and is associated with acceptable toxicity profile.
Acknowledgments
Dr. Koller passed away July 7, 2013 and should be acknowledged for his contribution to this manuscript.
Contract grant sponsor: MD Anderson Cancer Center Support; Contract grant number: CA016672.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Byrd JC, Dodge RK, Carroll A, et al. Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol. 1999;17:3767–3775. doi: 10.1200/JCO.1999.17.12.3767. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Ruppert AS, Mrozek K, et al. Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): Results from CALGB 8461. J Clin Oncol. 2004;22:1087–1094. doi: 10.1200/JCO.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Marcucci G, Mrozek K, Ruppert AS, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:5705–5717. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Kopecky KJ, Tallman MS, et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006;135:165–173. doi: 10.1111/j.1365-2141.2006.06276.x. [DOI] [PubMed] [Google Scholar]
- 5.Pollard JA, Alonzo TA, Gerbing RB, et al. Prevalence and prognostic significance of KIT mutations in pediatric patients with core binding factor AML enrolled on serial pediatric cooperative trials for de novo AML. Blood. 2010;115:2372–2379. doi: 10.1182/blood-2009-09-241075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi V, Estey E, Keating MJ, et al. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993;11:116–124. doi: 10.1200/JCO.1993.11.1.116. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi V, Estey E, Keating MJ, et al. Biochemical modulation of arabinosylcytosine for therapy of leukemias. Leuk Lymphoma. 1993;10(Suppl):109–114. doi: 10.3109/10428199309149122. [DOI] [PubMed] [Google Scholar]
- 8.Borthakur G, Kantarjian H, Wang X, et al. Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer. 2008;113:3181–3185. doi: 10.1002/cncr.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–3368. doi: 10.1200/JCO.2012.47.4874. [DOI] [PubMed] [Google Scholar]
- 10.Larson RA, Boogaerts M, Estey E, et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin) Leukemia. 2002;16:1627–1636. doi: 10.1038/sj.leu.2402677. [DOI] [PubMed] [Google Scholar]
- 11.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 12.Castaigne S, Pautas C, Terre C, et al. Fractionated doses of gemtuzumab ozogamicin (GO) combined to standard chemotherapy (CT) improve event-free and overall survival in newly-diagnosed de novo AML patients aged 50–70 years old: A prospective randomized phase 3 trial from the Acute Leukemia French Association (ALFA) ASH Annual Meeting Abstracts. 2011;118:6. [Google Scholar]
- 13.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Jones D, Medeiros LJ, et al. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. Br J Haematol. 2005;130:726–728. doi: 10.1111/j.1365-2141.2005.05666.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Bai Y, Yu H, Qiu YN, et al. Acute minimal differentiated myeloid leukemia: Report of three cases. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:76–77. [PubMed] [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 20.Borthakur G, Jabbour E, Wang X, et al. Effect of front-line therapy with fludarabine, cytarabine, filgrastim, and gemtuzumab ozogamicin (FLAG-GO) on outcome in core-binding factor associated acute myelogenous leukemia (CBF-AML) ASCO Meeting Abstracts. 2010;28:6552. [Google Scholar]
- 21.Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27:75–81. doi: 10.1038/leu.2012.229. [DOI] [PubMed] [Google Scholar]
- 22.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with alltrans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnett AK, Hills RK, Milligan D, et al. Attempts to optimise induction and consolidation chemotherapy in patients with acute myeloid leukaemia: Results of the MRC AML15 trial. ASH Annual Meeting Abstracts. 2009;114:484. [Google Scholar]
- 24.Cairoli R, Beghini A, Grillo G, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: An Italian retrospective study. Blood. 2006;107:3463–3468. doi: 10.1182/blood-2005-09-3640. [DOI] [PubMed] [Google Scholar]
- 25.Park SH, Chi HS, Min SK, et al. Prognostic impact of c-KIT mutations in core binding factor acute myeloid leukemia. Leuk Res. 2011;35:1376–1383. doi: 10.1016/j.leukres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Paschka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): A Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24:3904–3911. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 27.Luck SC, Russ AC, Du J, et al. KIT mutations confer a distinct gene expression signature in core binding factor leukaemia. Br J Haematol. 2010;148:925–937. doi: 10.1111/j.1365-2141.2009.08035.x. [DOI] [PubMed] [Google Scholar]
- 28.Goemans BF, Zwaan CM, Miller M, et al. Mutations in KIT and RAS are frequent events in pediatric core-binding factor acute myeloid leukemia. Leukemia. 2005;19:1536–1542. doi: 10.1038/sj.leu.2403870. [DOI] [PubMed] [Google Scholar]
- 29.Yin JA, O’Brien MA, Hills RK, et al. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML-15 trial. Blood. 2012;120:2826–2835. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 30.Allen CG, Hills RK, Evans CM, et al. Mutations in a large cohort of young adult patients with core binding factor acute myeloid leukemia: Impact on outcome and the selection of patients for alternative treatment including transplantation in first complete remission. ASH Annual Meeting Abstracts. 2011;118:419. [Google Scholar]
- 31.Jourdan E, Boissel N, Chevret S, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121:2213–2223. doi: 10.1182/blood-2012-10-462879. [DOI] [PubMed] [Google Scholar]