Abstract
Background
Nutritional deficits in early life have been associated with a higher prevalence of metabolic syndrome (MetS) in adulthood. Early childhood diarrhea contributes to under-nutrition and may potentially increase the risk for adult non-communicable diseases. Our objective was to examine associations between early childhood diarrhea burden and later development of MetS.
Methods
We studied individuals who participated in the Institute of Nutrition of Central America and Panama Nutritional Supplementation Longitudinal Study (1969–1977) and were followed up in 2002–04. We used logistic regression to determine associations of diarrhea burden at ages 0–6 mo, 6–12 mo, and 12–24 mo with odds of MetS and elevations in its components as adults.
Results
Among 389 adults age 25–42 years at follow-up the prevalence of MetS was 29%. Adjusting for several confounders including adult BMI, each absolute 1% increase in diarrhea burden at age 0–6 mo (but not at other time periods) was associated with increased odds of MetS (odds ratio 1.03; 95% CI 1.01–1.06). This was attributable primarily to associations with elevated BP (OR 1.03, 1.00–1.06) and waist circumference (OR 1.03, 1.00–1.06).
Conclusions
Childhood diarrhea burden 0–6 months is associated with MetS in adulthood after controlling for childhood growth parameters and adult BMI.
Keywords: metabolic syndrome, enteric disease, infection, malnutrition, early origins of adult disease
Introduction
The developmental origins of health and disease paradigm postulates that perturbations in the homeostasis of a developing fetus or child result in long-term changes affecting that individual’s risk of future disease[1]. Individuals born at low birth weight[2–9] and those with low BMI-for-age z-scores in early childhood[10–12] have been found to be more likely to develop high blood pressure (BP), dyslipidemia, and insulin resistance—all features of the metabolic syndrome (MetS). While the mechanisms behind these long-term outcomes is unknown, one plausible mechanism is through epigenetic changes in response to nutrient deficiency[13] and inflammatory signals[14].
The potential for early childhood events to impact adult disease is significant because a large proportion of children in developing countries continue to suffer nutritional and inflammatory insults due to enteric diseases associated with poor environmental sanitation and hygiene[15]. Enteric diseases may result in overt diarrhea and contribute in the short-term to a low BMI-for-age z-score (BMIZ) and in the longer-term to growth retardation and stunting, measured as a height-for-age z-score (HAZ) that is <-2[16–19].
Prior studies from developing countries have documented associations between low BMIZ at 24 months (BMIZ24) and risk for adult disease[10, 11]. Among 1492 individuals followed from birth in New Delhi, India, lower BMIZ24 was associated with the prevalence of pre-diabetes as adults[10]. These associations were stronger following adjustment for adult BMI, with lower BMIZ24 being associated with higher triglycerides, systolic BP, insulin resistance and MetS[10]. In a meta-analysis of cohorts studies from developing countries lower childhood BMIZ24 was associated with higher fasting glucose and systolic BP after adjustment for adult BMI[11].
The cohorts described in prior studies lived in areas in which enteric infections were endemic[10, 11, 20]. While data are not available for these cohorts regarding enteric disease, enteric infections may have contributed to lower BMIZ24 among these children. If diarrheal disease is an underlying factor in the development of MetS, it might explain associations of low BMIZ24 with risk of MetS. There may be critical periods during which diarrhea may exert effects. While these early data have implicated BMI-z-score at 24 months specifically, it is possible that any effect of diarrhea could be restricted to earlier time periods[21]. Any potential relationships among diarrheal illness, low BMIZ24 and future risk would be important given the continued high prevalence of enteric disease and rising rates of CVD and type 2 diabetes (T2DM) in developing countries[15, 22, 23].
We therefore evaluated the association of early childhood diarrhea burden with later prevalence of MetS in a cohort where enteric infections in childhood were common. Our hypothesis was that early childhood diarrhea burden would be associated with a higher prevalence of MetS and that this relationship would be stronger than that between BMIZ24 and MetS, potentially implicating enteric disease as an important contributor to developmental origins of adult disease.
Methods
Setting
The Institute of Nutrition of Central America and Panama (INCAP) Nutritional Supplementation Longitudinal Study was a study of growth and development conducted from 1969–1977 in four villages of mixed Spanish-Amerindian descent located near Guatemala City, Guatemala[24]. As described in detail elsewhere[25], the villages were randomized within pairs based on population size. Village residents were offered either atole, a dietary supplement made from maize, dry skim milk, and sugar (protein, 6.4 g/100 ml; energy, 3.80 MJ (900 kcal)/liter), or fresco, which contained no protein or fat and provided 1.35 MJ (330 kcal)/liter of energy, all from sugar. Both supplements were equally fortified by volume with micronutrients. All children <7 years old at study launch, and those born during the intervention period were eligible for study procedures.
Diarrhea Burden
Morbidity data were gathered through interviews of mothers in the home every 14 days by home visitors[26]. During the interview, the mother was asked to recall any symptoms that she and any of her children <7 years had in the previous 2 weeks. Beginning and ending dates of a symptom were noted. Diarrhea burden was defined as the total number of days of diarrhea divided by the total amount of time the child was followed with interviews during a defined period. A quality-control system was applied allowing the method to be standardized using a supervisor and validated, by a physician[27].
Anthropometry
Length and weight were obtained at specific ages by trained and standardized anthropometrists as described previously[28]. While length was obtained at 24 months and not height, the term HAZ24 is used here as a convention[11]. HAZ24 and BMIZ24 were computed based on 2006 WHO standards[29]. Stunting was defined as <-2 HAZ24.
The 2002–2004 follow-up study
Between 2002–2004, persons studied as children in 1969–1977 were resurveyed[30]. Of the 2,392 persons in the 1969–1977 sample, 1,855 (77%) were determined to be alive and living in Guatemala. Of these 1,855 persons, 1,570 (85%) completed at least one instrument during the 2002–2004 data collection. Overt refusal to participate was uncommon (<5% of those contacted). Data collection occurred at INCAP facilities in the study villages, at INCAP headquarters in Guatemala City, or at respondents’ homes. Data collection protocols were approved by the institutional review boards of Emory University (Atlanta, Georgia) and INCAP, and all participants gave written informed consent.
Measurements as adults
Trained field workers measured weight, height, and waist circumference (WC)[31]. Weight was measured using a digital scale (model 1582; Tanita Corporation, Tokyo, Japan) with a precision of 100 g while subjects were dressed in underclothes without shoes. Height was measured to the nearest 0.1 cm, with the participants standing barefoot with their backs to a stadiometer (GPM Anthropological Instruments, Zurich, Switzerland). WC was measured to the nearest 0.1 cm at the umbilicus, using a plastic inextensible measuring tape. All measurements were taken twice and the two values were averaged. If the difference exceeded 0.5 kg for body weight, 1.0 cm for height, or 1.5 cm for WC, a third measurement was taken and the average of the two closest measurements was used.
All clinical measurements were obtained by physicians. BP was measured using a digital sphygmomanometer (OMRON, model UA-767; A&D Medical, Milpitas, California) that was periodically checked for precision and accuracy. Participants were instructed to avoid tobacco products, alcohol, or caffeine in the 30 minutes preceding measurement. Participants sat quietly, with the left arm resting on a flat surface (at the level of the heart), for at least 5 minutes before the first measurement. Three measurements were taken at 3–5-minute intervals; readings from the second and third measurements were averaged. When the second and third measurements (of either systolic or diastolic pressure) differed by greater than 10 mmHg, a fourth measurement was taken and the two closest readings were averaged. A whole-blood sample was obtained by finger-prick after an overnight fast. Plasma glucose and lipid profiles were determined with an enzymatic/peroxidase dry chemistry method (LDX System; Cholestech Corporation, Hayward, California)[32]. Persons who had consumed food during the 5 hours and 8 hours prior to blood drawing were excluded from analysis of glucose levels and triglyceride levels, respectively.
Fasting glucose values were categorized using American Diabetes Association criteria[33]. BP was categorized according to the classification given by the US National High Blood Pressure Education Program[34]. MetS was defined on the basis of American Heart Association criteria using the ATP III cut-off values[35]. Participants had to meet ≥3 of the following 5 criteria: concentration of triglycerides ≥150 mg/dL, HDL-C <40 mg/dL for men and <50 mg/dL for women, WC ≥102 cm for males and 88 cm for females, glucose concentration ≥100 mg/dL (or diagnosis of diabetes), and systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg (or on anti-hypertensive medications). BMI was calculated as weight (kg) divided by the square of height (m). BMI was categorized as overweight for BMI 25–29.9 and obese for BMI ≥30. As a comparator, we also assessed MetS as defined by the International Diabetes Federation (IDF)[36], which requires central obesity (WC ≥90 cm for males and 80 cm for females) plus two of the following abnormalities: concentration of triglycerides ≥150 mg/dL, HDL-C <40 mg/dL for men and <50 mg/dL for women, glucose concentration ≥100 mg/dL (or diagnosis of diabetes), and systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg (or on anti-hypertensive medications).
Other variables
To assess socioeconomic status (SES) of participating households, a score was derived for 1975 household socioeconomic status, using an approach described elsewhere[37]. Data were collected regarding the number of years of schooling completed by the child’s mother.
Inclusion criteria
Inclusion criteria were having complete data for adult BMI and components of MetS (WC, triglycerides, HDL, BP and fasting glucose), as well as childhood anthropometry at 24 months and having data on diarrhea burden for at least 1 month within the 0–6 mo and 6–12 mo age periods or 2 months of observation within the 12–24 mo window. Subjects included in the analysis were compared to those who were followed at similar ages in childhood and years of the original study. This was performed by comparing to subjects who had any data on diarrhea burden between 0 and 24 months but who were excluded due to incomplete data as listed above (Supplementary Figure 1).
Statistics
We performed all statistical analyses using SAS software, Version 9.2 (SAS Institute Inc., Cary, NC, USA). We used t-tests to assess for potential differences between participants with complete data and with incomplete data. To assess for relationships between anthropometry (HAZ24 and BMIZ24) and adult MetS, we used logistic regression, adjusting for age, sex, and village of origin. In subsequent models we also adjusted for adult BMI, as has been done previously[10, 11].
To estimate potential associations between early childhood diarrhea and MetS status as young adults, we used logistic regression, with the independent variable being absolute diarrheal burden (expressed as percentage of days sick) within the given time interval and the dependent variables being classification of MetS or abnormalities in the individual components (elevated WC, high BP, etc.) based on ATP-III cut off values. We investigated multiple time periods (0–6 months, 6–12 months, 12–24 months) to assess the criticality of age-specific disease burdens, as such window periods have been considered critical in other studies of the early origins of adult disease [38]. We adjusted our models as described above. We tested for mediation by BMIZ24 and HAZ24 by entering these into the model and assessing the attenuation of the estimate for diarrheal disease burden. To assess for heterogeneity by adult BMI, we stratified the sample by adult weight category (underweight/normal weight vs. overweight/obese). Finally, we used linear regression of absolute diarrhea burden on the number of MetS component abnormalities in each time period.
Results
Participant characteristics
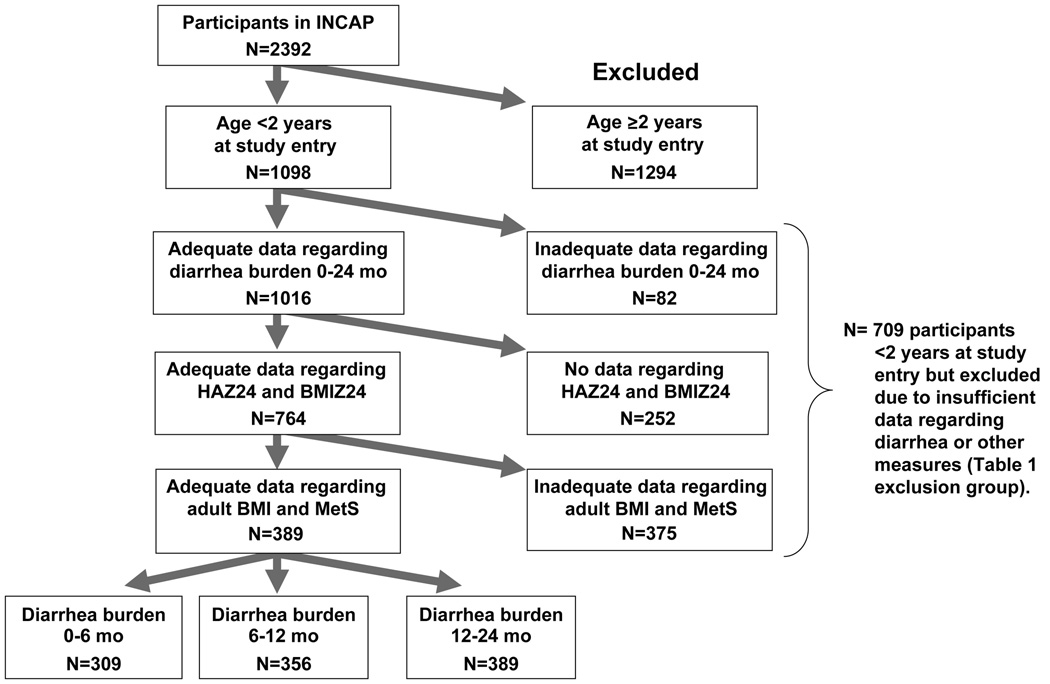
The initial study cohort consisted of 2392 participants, of whom 1098 were <2 years old at study entry, 1016 had adequate diarrhea data, 764 had adequate HAZ24 and BMIZ24 data and 389 had adequate adult BMI and MetS data (Figure 1). Of these, 309 had complete data on diarrhea burden at 0–6 mo, 356 had complete data for the period 6–12 mo and 389 had complete data for the period 12–24 mo (Supplementary Table 1). By contrast, the majority of cohort participants were excluded due to being too old (>24 mo) at study entry, loss tofollow-up, or incomplete data for the adult measures. Compared to participants with any data on childhood diarrhea burden 0–24 mo but not meeting the other inclusion criteria, included participants had a lower proportion of males (41.9% vs. 53%), older age when studied as adults (30.9 vs. 29.5), lower SES status (household belongings score −0.41 vs. −0.27) and more maternal schooling (1.37 vs. 1.22 years). They did not differ with respect to diarrhea burden (where available) or childhood growth measures. The analytic sample of children had a mean diarrhea burden of 6.9% of days during ages 0–6 months, 12.8% during 6–12 months and 10.6% during 12–24 months. The mean BMIZ24 was 0.44 and the mean HAZ24 was −3.09, with 82.8% of children being stunted at age 2 years. As adults the prevalence of MetS was 23% in males and 38% in females (Table 1).
Figure 1. Flow-chart of inclusion criteria for analytic sample from among all INCAP participants.
Participants were included based on being <2 years old at study entry, having adequate data regarding diarrhea burden (at least 1/6 of data for covered time period), having data regarding HAZ24 and BMIZ24 and having complete data on adult BMI and all metabolic syndrome (MetS) indicies. The comparator group (Table 1) consisted of participants who were <2 years old at study entry but lacked additional data as shown.
Table 1.
Prevalence of the metabolic syndrome (MetS) and its components among the analytic sample of participants of the INCAP Nutritional Supplementation Longitudinal Study, by gender.
| Males | Females | |
|---|---|---|
| Mean (std dev) or percent, as indicated |
Mean (std dev) or percent, as indicated |
|
| Data as adults | ||
| Age | 31.1 (2.0) | 30.8 (2.0) |
| MetS (percent) | 22.7 | 38.1 |
| BMI ‡ | 24.3 (3.6) | 26.6 (4.5) |
| Percent overweight (BMI 25–29.9) |
33.1 | 36.3 |
| Percent obese (BMI ≥30) |
6.1 | 21.7 |
| WC, cm | 85.9 (9.2) | 91.4 (11.3) |
| Percent elevated (males ≥102 cm; females ≥88 cm) |
5.5 | 58.4 |
| Blood pressure | ||
| SBP, mmHg | 116.9 (10.9) | 107.8 (11.4) |
| DBP, mmHg | 71.7 (9.4) | 69.0 (8.3) |
| Percent elevated (SBP ≥130 mmHg and/or DBP ≥85) |
17.2 | 5.3 |
| Triglycerides, mg/dL | 173.9 (98.5) | 168.8 (87.1) |
| Percent elevated (≥150 mg/dL) |
50.3 | 49.1 |
| HDL, mg/dL | 34.2 (8.8) | 39.8 (11.2) |
| Percent elevated (males <40 mg/dL; Females <50) |
76.1 | 84.1 |
| Glucose, mg/dL | 93.5 (10.4) | 91.2 (17.1) |
| Percent elevated (≥100 mg/dL) |
16.6 | 15.0 |
Abbreviations: BMI= body mass index; WC = waist circumference; BP = elevated blood pressure; TG = triglycerides; HDL = high density lipoprotein cholesterol.
Association of childhood growth parameters with adult MetS status
Supplementary Table 2 provides results of logistic regression analysis for our sample for associations of childhood growth (HAZ24 and BMIZ24) on adult overweight/obesity, MetS and elevations in MetS components as defined by ATP III cut-offs. In our initial model (adjusted for age, sex and village of origin) both HAZ24 and BMIZ24 were positively associated with overweight/obesity as adults (OR 1.22 [1.01–1.49] and 1.42 [1.09–1.84] per standard deviation increase in HAZ24 and BMIZ24, respectively), while HAZ24 was associated with elevated WC (OR 1.34 [1.04–1.73]) (Supplementary Table 2). Following adjustment for adult BMI, BMIZ24 became inversely associated with elevated WC (p<0.05).
Association of early childhood diarrhea burden with adult MetS status
When adjusted only for age, sex and village of origin, higher diarrhea burden in any age period was not associated with overall risk for MetS (Table 2). Each 1% increase in diarrhea burden during the 0–6 month period was associated with 3% increased prevalence of high BP in adulthood (p<0.05) while each 1% increase in diarrhea burden during 6–12 months was associated with a 4% increased prevalence of elevated WC (p<0.001). Further adjustment for HAZ24 and BMIZ24 did not significantly alter these associations (Table 2), nor did further adjustment for birthweight and measures of childhood SES, parental education level, and adult education level (data not shown).
Table 2. Relationship between diarrhea burden in early life (by age range) and metabolic syndrome (MetS) using logistic regression.
Diarrhea burden by age range was tested to examine for potential critical periods of exposure. In Models 2 and 3, BMIZ24, HAZ24 and adult BMI were adjusted for as performed in previous studies[10,11].
| Diarrhea burden 0–6 mo OR (CI)† |
p-value | Diarrhea burden 6–12 mo OR (CI)† |
p-value | Diarrhea burden 12–24 mo OR (CI) † |
p-value | |
|---|---|---|---|---|---|---|
| Model 1: adjusted for age, sex, village | ||||||
| Overweight/obesity | 0.99 (0.97–1.02) |
0.45 | 1.01 (0.99–1.03) |
0.18 | 0.99 (0.97–1.01) |
0.25 |
| MetS | 1.02 (0.99–1.04) |
0.06 | 1.01 (0.99–1.03) |
0.37 | 1.00 (0.98–1.02) |
0.72 |
| WC | 1.02 (0.99–1.04) |
0.14 |
1.04 (1.02–1.06) |
0.001 | 1.00 (0.98–1.02) |
0.95 |
| BP |
1.03 (1.00–1.06) |
0.05 | 1.02 (0.99–1.05) |
0.24 | 0.99 (0.96–1.03) |
0.65 |
| TG | 1.01 (0.99–1.03) |
0.27 | 0.99 (0.98–1.01) |
0.41 | 0.98 (0.7–1.00) |
0.09 |
| HDL | 1.00 (0.97–1.02) |
0.85 | 1.01 (0.99–1.03) |
0.42 | 1.00 (0.97–1.02) |
0.81 |
| Fasting glucose | 1.02 (1.00–1.05) |
0.10 | 1.01 (0.99–1.03) |
0.37 | 1.01 (0.98–1.03) |
0.58 |
| Model 2: Model 1 + BMIZ24 & HAZ24 | ||||||
| Overweight/ obesity | 0.99 (0.97–1.01) |
0.41 | 1.01 (0.99–1.03) |
0.18 | 0.99 (0.97–1.01) |
0.45 |
| MetS | 1.02 (1.00–1.04) |
0.07 | 1.01 (0.99–1.03) |
0.38 | 1.00 (0.98–1.02) |
0.81 |
| WC | 1.02 (0.99–1.04) |
0.18 |
1.04 (1.02–1.07) |
<0.001 | 1.00 (0.98–1.03) |
0.89 |
| BP |
1.03 (1.00–1.06) |
0.05 | 1.02 (0.99–1.05) |
0.23 | 1.00 (0.97–1.03) |
0.82 |
| TG | 1.01 (0.99–1.03) |
0.33 | 0.99 (0.98–1.01) |
0.38 | 0.99 (0.97–1.01) |
0.14 |
| HDL | 1.00 (0.97–1.02) |
0.86 | 1.01 (0.99–1.03) |
0.43 | 1.00 (0.97–1.02) |
0.76 |
| Fasting glucose | 1.02 (1.00–1.05) |
0.09 | 1.01 (0.99–1.03) |
0.36 | 1.01 (0.98–1.03) |
0.62 |
| Model 3: Model 2 + adult BMI | ||||||
| MetS |
1.03 (1.01–1.06) |
0.02 | 1.00 (0.98–1.03) |
0.71 | 1.00 (0.98–1.02) |
0.98 |
| WC |
1.03 (1.00–1.06) |
0.03 |
1.04 (1.01–1.07) |
0.02 | 1.00 (0.97–1.04) |
0.82 |
| BP |
1.03 (1.00–1.06) |
0.04 | 1.02 (0.99–1.05) |
0.21 | 1.00 (0.97–1.03) |
0.91 |
| TG | 1.01 (0.99–1.04) |
0.219 | 1.01 (0.98–1.03) |
0.20 | 0.99 (0.97–1.01) |
0.19 |
| HDL | 1.00 (0.98–1.03) |
0.947 | 1.01 (0.98–1.03) |
0.59 | 1.00 (0.98–1.03) |
0.98 |
| Fasting glucose | 1.02 (1.00–1.05) |
0.078 | 1.01 (0.99–1.03) |
0.43 | 1.01 (0.98–1.03) |
0.58 |
Abbreviations: BMI= body mass index; WC = waist circumference; BP = elevated blood pressure; TG = triglycerides; HDL = high density lipoprotein cholesterol.
Odds ratios are reported per 1% change in diarrhea.
Because of the potential influence that adult BMI has on the association between childhood growth parameters and future MetS we evaluated the interaction between adult weight status and early childhood diarrhea burden in its association with MetS. Among normal or under weight individuals there was a significant association between diarrhea burden in the 0–6 month period and adult MetS (OR 1.04 [1.00–1.08], p<0.05) and an increased OR for the association of diarrhea burden 0–6 months and high BP (OR 1.04 [1.00–1.09], p<0.05)(Table 3). The association between diarrhea burden at age 6–12 months with elevated WC was seen only among overweight/obese individuals but not among normal-weight individuals. We subsequently adjusted for adult overweight/obesity (Table 2). This revealed an association of diarrhea burden at age 0–6 months with adult MetS (OR 1.03 [1.01–1.06], p<0.05), elevated WC (OR 1.03 [1.00–1.06], p<0.05), and high BP (OR 1.03 [1.00–1.06], p<0.05), as well as persistence of the association of diarrhea burden at age 6–12 months on elevated WC (OR 1.04 [1.01–1.07]). There was no association between childhood diarrhea burden during either age period and either HAZ24 and BMIZ24 (data not shown). When the IDF MetS definition was assessed, there remained an association of diarrhea burden 0–6 mo with elevated BP in all models but not with other component abnormalities or with MetS itself (data not shown).
Table 3.
Relationship between diarrhea burden (age 0–6 months and 6–12 months) and metabolic syndrome (MetS) stratified by weight status, using logistic regression, adjusted for age, sex, village, BMIZ24 and HAZ24.
| Diarrhea burden during age 0–6 months | Diarrhea burden during age 6–12 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal weight only n=161 |
Overweight, obese only n=148 |
Normal weight only n=161 |
Overweight, obese only n=148 |
|||||
| Model 2 OR (CI) † |
Model 2 p-value |
Model 2 OR (CI) † |
Model 2 p-value |
Model 2 OR (CI) † |
Model 2 p-value |
Model 2 OR (CI) † |
Model 2 p-value |
|
| MetS * |
1.04 (1.00–1.08) |
0.04 | 1.03 (0.99–1.06) |
0.13 | 1.01 (0.97–1.04) |
0.57 | 1.00 (0.98–1.03) |
0.10 |
| WC | 1.04 (0.99–1.10) |
0.10 | 1.05 (0.99–1.10) |
0.11 | 1.01 (0.96–1.07) |
0.61 |
1.07 (1.02–1.13) |
0.009 |
| BP |
1.04 (1.00–1.09) |
0.04 | 1.01 (0.96–1.06) |
0.71 | 1.03 (1.00–1.08) |
0.09 | 0.99 (0.95–1.04) |
0.75 |
| TG | 1.01 (0.99–1.04) |
0.33 | 1.01 (0.98–1.05) |
0.50 | 1.00 (0.98–1.03) |
0.33 | 0.98 (0.95–1.00) |
0.09 |
| HDL | 0.99 (0.96–1.02) |
0.58 | 1.06 (0.97–1.16) |
0.20 | 1.00 (0.97–1.03) |
1.00 | 1.04 (0.98–1.11) |
0.15 |
| Fasting glucose | 1.01 (0.97–1.05) |
0.59 | 1.03 (1.00–1.07) |
0.06 | 1.00 (0.97–1.04) |
0.59 | 1.01 (0.97–1.04) |
0.76 |
Odds ratios are reported per 1 unit change in each z-score (one standard deviation).
Using linear regression of diarrhea burden on the number of MetS component abnormalities, we found a significant association between diarrhea burden and the number of MetS abnormalities in each of our 3 regression models during the 0–6 month time frame but not during the other time periods studied (Table 4).
Table 4. Relationship between diarrhea burden in early life (by age range) and number of metabolic syndrome (MetS) abnormalities using linear regression.
Diarrhea burden by age range was tested to examine for potential critical periods of exposure. In Models 2 and 3, BMIZ24, HAZ24 and adult BMI were adjusted for as performed in previous studies[10,11].
| Diarrhea burden 0– 6 mo, parameter estimate (std error) |
p- value |
Diarrhea burden 6–12 mo parameter estimate (std error) |
p- value |
Diarrhea burden 12–24 mo parameter estimate (std error) |
p- value |
|
|---|---|---|---|---|---|---|
| Model 1: adjusted for age, sex, village | 1.66 (1.17) | 0.03 | 0.0084 (0.0048) | 0.08 | −0.0045 (0.0052) | 0.39 |
| Model 2: Model 1 + BMIZ24 & HAZ24 | 0.012 (0.006) | 0.03 | 0.0086 (0.0048) | 0.08 | −0.0042 (0.0052) | 0.43 |
| Model 3: Model 2 + adult BMI | 0.014 (0.005) | 0.01 | 0.0053 (0.0042) | 0.21 | −0.0017 (0.0046) | 0.72 |
Discussion
In a prospective study in Guatemala we found an association between diarrhea burden in infancy and later risk for MetS and its components. Following adjustment for adult BMI, diarrhea burden during the 0–6 month period was associated with the prevalence of MetS as adults. Most of the association was attributable to the association of diarrheal disease burden with BP and WC. Our findings are consistent with the hypothesis that disruptions in homeostasis in early life can result in long-term effects on adult health.
These findings expand on prior reports that demonstrated associations of components of MetS in adulthood with poor weight gain by age 2 years in regions with high endemic rates of enteric infections[10, 11]. Victora et al, using pooled data from over 10,000 individuals from developing countries, showed that after adjustment for adult BMI, there was an inverse association between weight-for-age z-score at age 2 years and systolic BP and fasting glucose[11]. Fall et al found among their cohort from New Delhi that after adjustment for the effects of adult BMI on MetS, low childhood BMI at age 2 years was associated with higher levels of triglycerides, insulin resistance and a diagnosis of MetS[10]. In neither of these studies was diarrhea burden assessed, leaving unclear whether the increase in MetS risk was caused specifically by low childhood BMI or if low BMI represents only a marker for underlying exposures to diseases such as malnutrition or enteric disease that may have caused both low body weight at age 2 and an increase in MetS.
Our study represents an advance on those two studies insofar as we were able to test the hypothesis that both the low body weight and the increase in MetS risk from the prior studies may have been caused by diarrheal illness. In our cohort we were unable to confirm an association between diarrheal disease burden and either HAZ24. This may be due to using a cumulative indicator of linear growth to 24 months that includes intrauterine growth restriction instead of changes in Z-scores (i.e. growth rates) as in a prior study[27]; also, we require adult information and use only a subset of individuals with growth and diarrheal disease information. Adjusting for either HAZ24 or BMIZ24 did not alter the association between diarrhea burden and MetS. While we noted an inverse relationship between BMIZ24 and the odds of elevated WC in adulthood (following adjustment for adult BMI), we did not observe an association between childhood weight status and MetS risk per se. This leaves diarrhea burden and secondary malabsorption of macronutrients and micronutrients[15] as well as systemic inflammation[39–41] as possible explanations for MetS risk. Given the complexities of these relationships more detailed investigation is necessary in cohorts for which data regarding early childhood inflammation and later adult disease is available.
The relationship between diarrhea and elevations in the components of MetS was only apparent following adjustment for adult BMI. The need to adjust for adult BMI may reflect the strength and complexity of influences on MetS risk. Overall, our study and prior studies[10, 11] showed a positive correlation between BMIZ24 and overweight/obesity in adulthood. This positive association may reflect in part the early development of tracking of overweight and may reflect a genetic component. By adjusting for adult BMI we are controlling for this influence. This is supported by the association of diarrhea burden during 0–6 months and MetS being apparent among normal weight adults but not among overweight/obese adults, among whom other influences on MetS may predominate.
The association of childhood diarrhea with adult MetS was modest in magnitude, with every 1% of absolute diarrhea burden being associated with a 3% increased risk of MetS in the fully-adjusted model. While a high degree of diarrhea burden is necessary to produce any substantial increase in risk of MetS, high burdens of enteric infection are the norm in many low-income countries. Thus, the wide public health implications of our observations are substantial. The small effect size raises the possibility of residual confounding effect influencing our analysis, though we did not note differences after adjustment for multiple socioeconomic factors. We also acknowledge the small sample size in this analysis, which was limited by the requirement for adequate childhood and adult data, contributes to the potential for selection bias. The individuals who were included in the analysis were different in multiple ways that may have affected the analysis (Supplementary Table 1).
In our analysis, the relationship between early diarrhea burden and MetS itself was only significant for diarrhea during 0–6 months of life, though elevated WC was associated with diarrhea burden from 6–12 months. None of these relationships were apparent when diarrhea burden during 12–24 months of life was considered. If these relationships reflect some degree of epigenetic re-programming, this timing may indicate early infancy as a critical period of vulnerability to diarrheal illness. Most studies regarding the developmental origins of adult disease have focused on insults during gestation (often proxied by LBW), and far fewer studies have identified the impacts of events in early postnatal life, a period of elevated susceptibility for other processes, such as cognitive development[42]. While further research is needed to evaluate potential mechanisms behind these relationships, it is notable that early childhood diarrhea is associated with poor absorption of nutrients[15], poor weight gain and stunting[15–17, 19], and persistent systemic inflammation[39–41], providing plausible signals of homeostatic disruption[1]. Studies of prenatal nutritional deficiencies such as the Dutch famine have demonstrated that individuals exposed to in utero nutritional restrictions continued as adults to exhibit differences in the methylation pattern of genes involved in metabolism [43, 44], suggesting a potential mechanism for long-term changes from early triggers, as suggested previously[1]. In our study, associations of early childhood diarrhea with elevated blood pressure were the most consistently noted. This is intriguing given previously-recognized effects of inflammation on chronic remodeling of the intima media in arteries[45], as well as reports of epigenetic regulation of the angiotensin-converting enzyme[46, 47]—either of which could potentially contribute to elevated blood pressure over time. Further research will be needed to identify specific mechanisms.
In conclusion, we noted that following adjustment for adult overweight/obesity, childhood diarrhea burden in the first 6 months of life was associated with increased risk for future MetS and in particular for elevated WC and high BP. Our findings are independent of stunting and BMIZ24 arguing against childhood nutritional status as a mediating mechanism for these associations and consistent with a role for diarrhea as a trigger for the development of adult disease.
Supplementary Material
Acknowledgements
This work was funded by the following grant support: 5K08HD060739-04 (MDD). The Human Capital Study (2002–2004) was funded by grant R01 TW-05598 (RM). We would like to acknowledge Jessica O. Gonzalez, coordinator of the University of Virginia Guatemala Initiative for her continuing efforts related to this analysis.
List of Abbreviations and Acronyms
- BMIZ
body-mass-index-for-age z-score
- BP
blood pressure
- HAZ
height-for-age z-score
- HDL-C
high-density lipoprotein cholesterol
- INCAP
Institute of Nutrition of Central America and Panama
- MetS
metabolic syndrome
- T2DM
type 2 diabetes mellitus
- WC
waist circumference
Bibliography
- 1.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2011;21(4):199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13(9):364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. Bmj. 1993;307(6918):1524–1527. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab. 2002;87(10):4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 8.Williams S, St George IM, Silva PA. Intrauterine growth retardation and blood pressure at age seven and eighteen. J Clin Epidemiol. 1992;45(11):1257–1263. doi: 10.1016/0895-4356(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Bmj. 1989;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fall CH, Sachdev HS, Osmond C, Lakshmy R, Biswas SD, Prabhakaran D, et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care. 2008;31(12):2349–2356. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoer MD, Lima AA, Oria RB, Scharf RJ, Moore SR, Luna MA, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70(11):642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr. 2009;89(5):1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011;90(3):439–446. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66(9):487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30(6):1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 17.Moore SR, Lima NL, Soares AM, Oria RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139(4):1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Victora CG. Nutrition in early life: a global priority. Lancet. 2009;374(9696):1123–1125. doi: 10.1016/S0140-6736(09)61725-6. [DOI] [PubMed] [Google Scholar]
- 19.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37(4):816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastro Hepatol. 2012 doi: 10.1038/nrgastro.2012.239. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attig L, Gabory A, Junien C. Nutritional developmental epigenomics: immediate and long-lasting effects. Proc Nutr Soc. 2010;69(2):221–231. doi: 10.1017/S002966511000008X. [DOI] [PubMed] [Google Scholar]
- 22.Gupta N, Goel K, Shah P, Misra A. Childhood obesity in developing countries: epidemiology, determinants, and prevention. Endocr Rev. 2012;33(1):48–70. doi: 10.1210/er.2010-0028. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson JE, Mayosi B, Yusuf S, Reddy S, Hu S, Chen Z, et al. Global burden of cardiovascular disease. Heart. 2007;93(10):1175. doi: 10.1136/hrt.2007.131060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martorell R, Yarbrough C, Lechtig A, Habicht JP, Klein RE. Diarrheal diseases and growth retardation in preschool Guatemalan children. Am J Phys Anthropol. 1975;43(3):341–346. doi: 10.1002/ajpa.1330430307. [DOI] [PubMed] [Google Scholar]
- 25.Martorell R. Overview of long-term nutri- tion intervention studies carried out in Guatemala (1968–1988) Food Nutr Bull. 1992;14:270–277. [Google Scholar]
- 26.Martorell R. Results and implications of the INCAP follow-up study. J Nutr. 1995;125(4 Suppl):1127S–1138S. doi: 10.1093/jn/125.suppl_4.1127S. [DOI] [PubMed] [Google Scholar]
- 27.Martorell R, Habicht JP, Yarbrough C, Lechtig A, Klein RE, Western KA. Acute morbidity and physical growth in rural Guatemalan children. Am J Dis Child. 1975;129(11):1296–1301. doi: 10.1001/archpedi.1975.02120480022007. [DOI] [PubMed] [Google Scholar]
- 28.Martorell R, Habicht JP, Yarbrough C, Guzman G, Klein RE. The indentification and evaluation of measurement variability in the anthropometry of preschool children. Am J Phys Anthropol. 1975;43(3):347–352. doi: 10.1002/ajpa.1330430308. [DOI] [PubMed] [Google Scholar]
- 29.World_Health_Organizaion. Child Growth Standards. 2012 http://www.who.int/childgrowth/software/en/. In.
- 30.Martorell R, Behrman JR, Flores R, Stein AD. Rationale for a follow-up study focusing on economic productivity. Food Nutr Bull. 2005;26(2 Suppl 1):S5–S14. doi: 10.1177/15648265050262S102. [DOI] [PubMed] [Google Scholar]
- 31.Stein AD, Wang M, Ramirez-Zea M, Flores R, Grajeda R, Melgar P, et al. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: evidence from Guatemala. Am J Epidemiol. 2006;164(12):1160–1170. doi: 10.1093/aje/kwj328. [DOI] [PubMed] [Google Scholar]
- 32.Flores R, Grajeda RTB, Mendez H, Martorell R, Schroeder D. Evaluation of a dry chemistry method for blood lipids in field studies. Faseb J. 1998;12:S3061. (abs.) [Google Scholar]
- 33.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 34.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 36.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 37.Maluccio JA, Murphy A, Yount KM. Research note: A socioeconomic index for the INCAP longitudinal study 1969–77. Food Nutr Bull. 2005;26(2 Suppl 1):S120–S124. doi: 10.1177/15648265050262S112. [DOI] [PubMed] [Google Scholar]
- 38.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(1–2):93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 39.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133(5):1332–1338. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 40.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39(2):153–157. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Goto R, Mascie-Taylor CG, Lunn PG. Impact of intestinal permeability, inflammation status and parasitic infections on infant growth faltering in rural Bangladesh. Br J Nutr. 2009;101(10):1509–1516. doi: 10.1017/S0007114508083554. [DOI] [PubMed] [Google Scholar]
- 42.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18(21):4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsson PM. Elevated blood pressure predicts type 2 diabetes, but why? J Hypertens. 2008;26(9):1740–1741. doi: 10.1097/HJH.0b013e32830c6939. [DOI] [PubMed] [Google Scholar]
- 46.Lee HA, Cho HM, Lee DY, Kim KC, Han HS, Kim IK. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension. 2012;59(3):621–626. doi: 10.1161/HYPERTENSIONAHA.111.182428. [DOI] [PubMed] [Google Scholar]
- 47.Riviere G, Lienhard D, Andrieu T, Vieau D, Frey BM, Frey FJ. Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics. 2011;6(4):478–489. doi: 10.4161/epi.6.4.14961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.