Abstract
Objective
This study aimed to evaluate the preliminary efficacy of Emotion Acceptance Behavior Therapy (EABT), an outpatient psychotherapeutic intervention for anorexia nervosa (AN) based on a disorder-specific model of symptom maintenance that emphasizes emotion avoidance. EABT combines standard behavioral interventions that are central to the clinical management of AN with evidence-supported strategies to increase emotion awareness, decrease emotion avoidance, and encourage resumption of valued activities and relationships outside the eating disorder.
Method
Twenty-four individuals aged ≥17 years with AN were treated using the EABT manual. EABT was delivered in 33–58 individual sessions provided over 38–53 weeks. Assessments were conducted before and after treatment, and at 3- and 6-month follow-ups.
Results
Thirteen patients (54.2%) completed EABT; 11 (45.8%) dropped out or were withdrawn. EABT was associated with significant improvements in weight, disordered eating symptoms, and emotion avoidance that were maintained over 6-month follow-up. The majority of EABT completers achieved a body mass index >18.5 (n=9/13) or had a normal Eating Disorder Examination Global score (n=10/13) at post-treatment.
Discussion
Preliminary data suggest that EABT may have utility for a subset of adults with AN. Future research will focus on improving outcomes in EABT non-responders and identifying of mechanisms that drive treatment response.
The development of acceptable and efficacious outpatient treatments for adults with anorexia nervosa (AN) is an important public health priority, given the morbidity and mortality associated with AN and the lack of evidence-supported interventions. Several recent trials have evaluated psychotherapeutic interventions for adults with AN [e.g., Refs. (1–3)], but no treatment has emerged as superior, and there is considerable room for improvement in patient outcomes.
This manuscript reports results from a pilot study of a novel psychotherapeutic intervention for older adolescents and adults with AN, Emotion Acceptance Behavior Therapy (EABT). EABT is based on an AN-specific model of symptom maintenance that emphasizes the role of eating disorder (ED) symptoms in facilitating avoidance of emotions (4). EABT combines standard behavioral interventions that are central to the clinical management of AN with psychotherapeutic techniques designed to increase emotion awareness, decrease emotion avoidance, and encourage resumption of valued activities and relationships outside the ED (5). Our preliminary studies have provided support for the EABT model (6, 7) and the acceptability of an abbreviated version of the EABT manual (4). Here, we report on the preliminary efficacy of EABT in a series of 24 AN patients.
Method
Study procedures were approved by the local Institutional Review Board. Participants provided informed consent or assent.
Participants
Participants were recruited from advertisements and individuals seeking treatment at an ED clinic. Inclusion criteria were age ≥ 17 years and meeting DSM-IV criteria for AN with the following modifications: 1) amenorrhea was not required; and 2) body mass index (BMI) was between 16.0 and 18.5. Exclusion criteria included: 1) major medical conditions that might interfere with treatment or require close monitoring; 2) alcohol or drug dependence in the last three months; 3) significant suicidal ideation; 4) previously identified developmental disability or special education; 5) psychosis; and 6) ongoing psychotherapy. Individuals taking stable doses of psychiatric medication (no dose adjustments in the previous four weeks) were included.
Twenty-four individuals met study eligibility criteria and initiated EABT (Figure 1). Participants were primarily female (n=23) and Caucasian (n=24). Mean age was 26.8 (SD=11.6) years, mean pre-treatment BMI was 17.5 (SD=0.8), and mean duration of illness was 7.8 (SD=9.3) years. Approximately half the sample (n=11; 45.8%) met criteria for AN-binge/purge type (n=4 had binge eating and purging; n=7 had purging). The majority of participants (n=23; 95.8%) had prior treatment: 66.7% (n=16) psychotherapy, 62.5% (n=15) nutrition counseling, 66.7% (n=16) medication, and 50% (n=12) hospitalization. Lifetime comorbities included mood (n=12; 50%), anxiety (n=11; 45.8%), and substance use (n=8; 33.3%) disorders.
Figure 1.
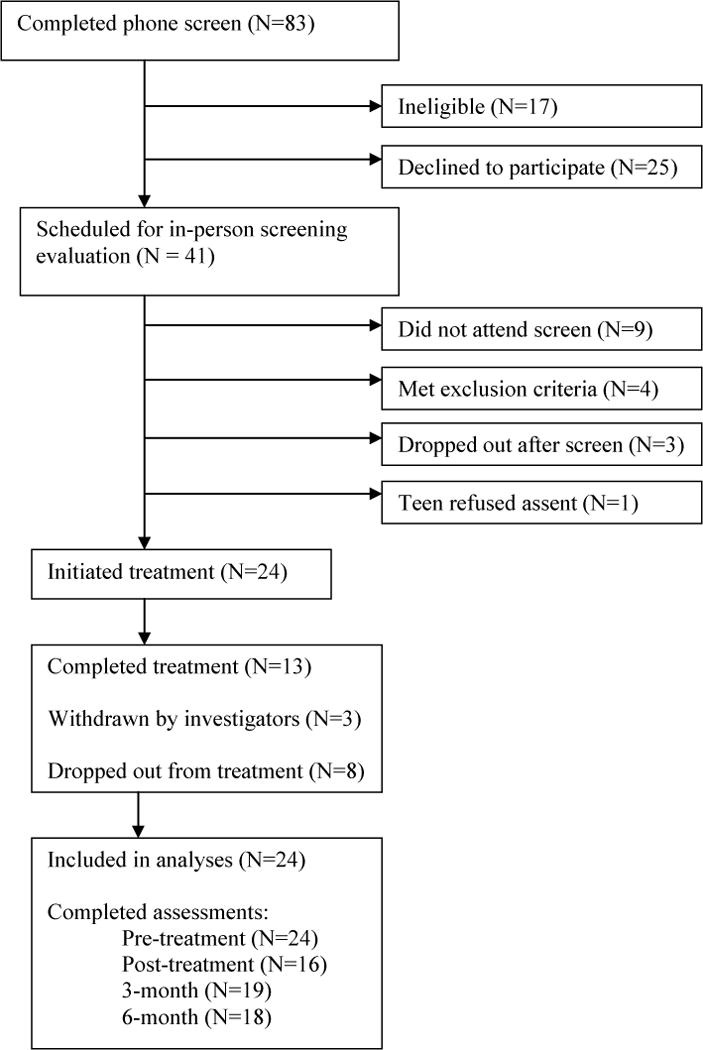
Participant flow through the study
Treatment
EABT has been described in detail (5). Treatment is divided into three overlapping phases. During phase 1, the patient and therapist work to develop a shared understanding of the patient’s illness, which is used to set goals for: 1) weight gain/reduction of ED symptoms, 2) acceptance of emotions and other avoided experiences, and 3) participation in other valued activities and relationships. Phase 2 focuses on helping the patient meet treatment goals using “contextual and experiential” (8) change strategies (e.g., mindfulness, acceptance, and contact with the present moment), as opposed to techniques focused on changing the form of problematic internal experiences (e.g., modifying dysfunctional thoughts about eating, weight, and shape). Phase 3 focuses on consolidation of gains, continued practice of behavioral strategies, and treatment termination.
EABT was delivered in 33–58 individual sessions over 38–53 weeks. All patients attended therapy twice weekly for the first four weeks of treatment. Patients that lost weight continued with twice weekly sessions until weight gain was achieved. If at any point during EABT a patient lost weight or increased other ED behaviors for two consecutive weeks, twice weekly sessions were resumed until symptomatic stability returned. Finally, participants attended therapy sessions every other week for the last eight weeks of treatment.
Therapists were master’s- or doctoral-level clinicians experienced in the treatment of AN. Therapists received training and weekly group supervision from the investigators (4, 5). Weight and vital signs were assessed by a nurse at each appointment, and participants met monthly with the study physician. Participants also received 1–3 sessions of nutrition counseling with a registered dietitian (M [SD] = 1.2 [0.8] sessions).
Assessments
One-time assessments were administered at screening and included the Structured Clinical Interview for DSM-IV-TR Axis I Disorders [SCID-I; (9)], and a questionnaire to document demographics and previous treatment. Participants also underwent a complete physical examination, electrocardiogram, and bloodwork to ensure medical stability and identify exclusionary medical conditions.
Repeated assessments were conducted before and after EABT, and at 3- and 6-month follow-ups, and included the: 1) Eating Disorder Examination, Edition 16.0D [EDE; (10)]; 2) Acceptance and Action Questionnaire [AAQ; (11)]; 3) Beck Depression Inventory-2 [BDI-2; (12)]; 4) Beck Anxiety Inventory [BAI; (13)]; 5) Eating Disorders Quality of Life Scale [EDQOL; (14)]; and 6) height and weight (assessed using a stationary stature board and digital scale).
Statistical Analyses
Mixed-effects models were used to examine the effect of treatment on continuous outcomes. Indicator variables for assessment time points were included in each model to allow estimation of a non-linear trajectory of change from baseline, and a random intercept was included to account for dependence between repeated measures on the same subject. Contrasts were used to test whether changes from baseline were significant at each subsequent time point; effect sizes were computed based on the average change over time.
Results
Participant Flow
Figure 1 shows participant flow through the study. EABT completers (n=13) attended an average of 43.6 (SD=7.1) sessions compared to 11.5 (SD=6.3) sessions among non-completers (n=11) (p<.001). There were no differences between patients who did and did not complete EABT in age, BMI, years of illness, AN subtype, or scores on the EDE and self-report questionnaires.
Continuous Clinical Outcomes
As shown in Table 1, EABT was associated with significant improvements on all clinical outcomes in the full sample and treatment completers. Effect sizes ranged from d=0.9 to d=2.9, indicating large effects. Outcomes were not significantly associated with age, duration of illness, AN subtype, or previous psychiatric hospitalization (data not shown).
Table 1.
Changes in Clinical Outcomes from Pre-Treatment to Six-Month Follow-Up in the Full Sample (N=24) and Treatment Completers (N=13)
| Pre-treatment | Post-treatment | 3 months | 6 months | ||
|---|---|---|---|---|---|
| Outcome | M (SE) | M (SE) | M (SE) | M (SE) | Effect Size† |
| Full sample | |||||
| Weight (lb) | 107.4 (2.7) | 112.7 (3.0)* | 112.9 (2.9)* | 115.1 (2.9)** | 0.9 |
| BMI | 17.5 (0.3) | 18.5 (0.4)* | 18.5 (0.4) | 18.9 (0.4)*** | 1.0 |
| EDE Global | 2.5 (0.3) | 1.7 (0.3)*** | 1.5 (0.3)*** | 1.8 (0.3)*** | 1.6 |
| AAQ total | 39.5 (2.1) | 31.7 (2.2)*** | 31.8 (2.2)*** | 35.9 (2.2)* | 1.4 |
| BDI total | 20.9 (2.6) | 11.3 (2.9)*** | 11.1 (2.7)*** | 15.7 (2.8)* | 1.2 |
| BAI total | 16.8 (2.0) | 7.8 (2.3)*** | 9.3 (2.2)*** | 10.6 (2.2)** | 1.5 |
| EDQOL total | 1.6 (0.1) | 1.0 (0.2)*** | 0.9 (0.1)*** | 1.0 (0.1)*** | 1.6 |
|
Completers | |||||
| Weight (lb) | 108.6 (2.8) | 117.4 (2.8)** | 116.1 (2.8)* | 118.0 (3.0)** | 1.2 |
| BMI | 17.7 (0.5) | 19.1 (0.5)** | 18.9 (0.5)* | 19.3 (0.5)** | 1.2 |
| EDE Global | 2.6 (0.4) | 1.5 (0.4)*** | 1.5 (0.4)*** | 1.5 (0.4)*** | 1.9 |
| AAQ total | 41.5 (2.8) | 30.9 (2.8)*** | 30.2 (2.8)*** | 33.1 (2.8)*** | 2.6 |
| BDI total | 21.8 (3.1) | 8.8 (3.1)*** | 9.9 (3.1)*** | 10.5 (3.2)*** | 2.3 |
| BAI total | 20.4 (2.4) | 7.6 (2.4)*** | 11.1 (2.4)*** | 8.1 (2.4)*** | 2.9 |
| EDQOL total | 1.6 (0.2) | 0.8 (0.2)*** | 0.7 (0.2)*** | 0.8 (0.2)*** | 2.3 |
Note: BMI = body mass index; EDE = Eating Disorder Examination; AAQ = Acceptance and Action Questionnaire; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; EDQOL = Eating Disorders Quality of Life Scale.
p < .05.
p < .01.
p < .001. Outcome versus pre-treatment.
Effect size (d) computed for mean (post-treatment, 3 months, 6 months) versus mean (pre-treatment). Per Cohen (18), d = 0.20 is a small effect, d = 0.50 is a medium effect, and d = 0.80 is a large effect.
Recovery Rates
We also examined rates of recovery at post-treatment and 3- and 6-month follow-ups using the following definitions from previous research (2): 1) BMI >18.5; 2) normal EDE Global score (i.e., score <1.74); and 3) BMI >18.5 and normal EDE Global score. The proportion of EABT completers with a BMI >18.5 was 69.2% (n=9) at post-treatment, and 61.5% (n=8) at 3- and 6-month follow-up. Seventy-seven percent (n=10) of completers had a normal EDE Global score at post-treatment and 3-month follow-up; this decreased to 46.2% (n=6) at 6-month follow-up. Finally, 46.2% (n=6) of completers had a BMI >18.5 and a normal EDE Global score at post-treatment and 3-month follow-up; this decreased to 30.8% (n=4) at 6-month follow-up.
Discussion
This pilot study aimed to evaluate the efficacy of EABT as an outpatient treatment for older adolescents and adults with AN. Our findings suggest that EABT produces improvements in weight and psychological symptoms that are comparable to other psychotherapeutic interventions for low-weight patients (1–3). Indeed, rates of recovery among EABT completers exceeded those reported for patients that completed the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) and Specialist Supportive Clinical Management (SSCM) in a recent randomized controlled trial (2). These preliminary data suggest that EABT may have utility for a subset of individuals with AN.
We do not know why some participants responded well to EABT, while others dropped or were withdrawn. It may be that some patients were not ready for the demands to increase emotional experiences while normalizing eating and gaining weight. Additionally, some participants reported that they were unwilling to continue weight gain after reaching a personally-defined threshold for health (e.g., BMI >18) and exited treatment at this point.
Identifying and intervening with treatment “non-responders” is a significant challenge in working with adults with AN, many of whom have a long duration of illness. If a clinician intervenes too early or aggressively, there is a risk that the patient might flee treatment altogether. Alternatively, benefits of watching a patient decline symptomatically even if s/he reports that therapy is helpful in other areas are questionable. Given that EABT shows promise for a subset of AN patients, we plan to examine the utility of providing adjunctive treatments, such as a short bout of day hospital care, to EABT non-responders.
The primary limitations of this preliminary study include the uncontrolled design and small sample size. Additionally, the utility of EABT for AN patients with BMI <16 is unknown, and because the SCID was administered at baseline only, changes in comorbid psychopathology were not assessed. Finally, the mechanisms by which EABT may lead to improvements in AN symptoms require elucidation. EABT is based on an AN-specific model of symptom maintenance that emphasizes the role of emotion avoidance in perpetuating anorexic behaviors. Although there is growing support for the salience of emotion avoidance and other emotion processing and regulation difficulties in AN (7, 15–17), future work is needed to determine whether improvements in these domains during EABT or other interventions help to facilitate remission of AN symptoms.
Acknowledgments
Research supported by National Institute of Mental Health grant R01 MH082685. We thank Lauren Carlson, Hillary Berglund, Alexis Fertig, Joanna Gould, Marcela Marin Dapelo, Eric Rickin, and Rebecca Ringham for their roles in assessing and treating the study patients.
Footnotes
Financial Disclosure
The authors have no financial conflicts of interest.
References
- 1.Fairburn CG, Cooper Z, Doll HA, O’Connor ME, Palmer RL, Dalle Grave R. Enhanced cognitive behaviour therapy for adults with anorexia nervosa: a UK-Italy study. Behav Res Ther. 2013;51:R2–R8. doi: 10.1016/j.brat.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt U, Oldershaw A, Jichi F, Sternheim L, Startup H, McIntosh V, et al. Out-patient psychological therapies for adults with anorexia nervosa: randomised controlled trial. Br J Psychiatry. 2012;201:392–399. doi: 10.1192/bjp.bp.112.112078. [DOI] [PubMed] [Google Scholar]
- 3.Touyz S, Le Grange D, Lacey H, Hay P, Smith R, Maguire S, et al. Treating severe and enduring anorexia nervosa: a randomized controlled trial. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713000949. [DOI] [PubMed] [Google Scholar]
- 4.Wildes JE, Marcus MD. Development of emotion acceptance behavior therapy for anorexia nervosa: a case series. Int J Eat Disord. 2011;44:421–427. doi: 10.1002/eat.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildes JE, Marcus MD, McCabe EB. Emotion acceptance behavior therapy for anorexia nervosa. In: Thompson-Brenner H, editor. Casebook of evidence-based treatments for eating disorders. In press. [Google Scholar]
- 6.Wildes JE, Marcus MD, Bright AC, Marin Dapelo M. Emotion and eating disorder symptoms in patients with anorexia nervosa: an experimental study. Int J Eat Disord. 2012;45:876–882. doi: 10.1002/eat.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildes JE, Ringham RM, Marcus MD. Emotion avoidance in patients with anorexia nervosa: initial test of a functional model. Int J Eat Disord. 2010;43(5):398–404. doi: 10.1002/eat.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes SC. Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies. Behav Ther. 2004;35:639–665. doi: 10.1016/j.beth.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 9.First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Patient Edition (With Psychotic Screen) (SCID-I/P (W/PSYCHOTIC SCREEN) New York: Biometrics Research Department; 2007. [Google Scholar]
- 10.Fairburn CG, Cooper Z, O’Connor ME. Eating Disorder Examination (Edition 16.0D) In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: The Guilford Press; 2008. pp. 265–308. [Google Scholar]
- 11.Hayes SC, Strosahl K, Wilson KG, Bissett RT, Pistorello J, Toarmino D, et al. Measuring experiential avoidance: a preliminary test of a working model. The Psychological Record. 2004;54:553–578. [Google Scholar]
- 12.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 13.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 14.Engel SG, Wittrock DA, Crosby RD, Wonderlich SA, Mitchell JE, Kolotkin RL. Development and psychometric validation of an eating disorder-specific health-related quality of life instrument. Int J Eat Disord. 2006;39:62–71. doi: 10.1002/eat.20200. [DOI] [PubMed] [Google Scholar]
- 15.Racine SE, Wildes JE. Emotion dysregulation and symptoms of anorexia nervosa: the unique roles of lack of emotional awareness and impulse control difficulties when upset. Int J Eat Disord. 2013;46:713–720. doi: 10.1002/eat.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldershaw A, Hambrook D, Stahl D, Tchanturia K, Treasure J, Schmidt U. The socio-emotional processing stream in anorexia nervosa. Neurosci Biobehav Rev. 2011;35:970–988. doi: 10.1016/j.neubiorev.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Harrison A, Tchanturia K, Treasure J. Attentional bias, emotion recognition, and emotion regulation in anorexia: state or trait? Biol Psychiatry. 2010;68:755–761. doi: 10.1016/j.biopsych.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]


