Introduction
Most individuals over age 60 have progressively enlarging deposits of calcium mineral in their major arteries.1 This vascular calcification reduces aortic and arterial elastance, which impairs cardiovascular hemodynamics, resulting in substantial morbidity and mortality2-4 in the form of hypertension, aortic stenosis, cardiac hypertrophy, myocardial and lower limb ischemia, congestive heart failure, and compromised structural integrity.5-7 The severity and extent of mineralization reflect atherosclerotic plaque burden8 and strongly and independently predict cardiovascular morbidity and mortality.9
Previously considered passive and degenerative, vascular calcification is now recognized as a pathobiological process sharing many features with embryonic bone formation. As evidence of this change in paradigm, research on vascular calcification has dramatically accelerated in the past decade. A search of PubMed (www.ncbi.nlm.nih.gov; U.S. National Library of Medicine) under the key words “vascular calcification” returned approximately 16 articles in 1982, 100 in 1994, and 250 in 2004. This year, 400 new publications are expected, for a total exceeding 3,500.
A breakthrough in this field was the recognition of its similarity to bone development and metabolism, where endothelial, mesenchymal, and hematopoietic cells interact and respond to mechanical, inflammatory, metabolic, and morphogenetic signals governing skeletal mineralization; their counterparts in the artery wall govern arterial mineralization. With increasing age and dysmetabolic conditions in our population, the clinical burden of vascular calcification will continue to increase.
A. Clinical impact of arterial calcification
Aortic calcification promotes congestive heart failure by eroding compliance and elastance. The hemodynamic demands of the cardiovascular system require that the aorta store energy in its elastance during systole and release it during diastole, which minimizes cardiac work and is the basis for balloon counter-pulsation. This function, known as Windkessel physiology, is reflected in the high density of elastin in the arch, where mechanical energy is highest. Its loss is detectable as increased arterial pulse wave velocity in calcified arteries, resulting in thoracic summation of reflected and orthograde pressure waves – increasing systolic and pulse pressures. It also increases cardiac work, promoting heart failure, left ventricular hypertrophy, and diastolic dysfunction, independently of atherosclerosis, aging or diabetes. The link between aortic rigidity and heart failure is most evident in the hypertensive cardiomyopathy observed in patients with idiopathic infantile arterial calcification and in animal models with aortic banding.
In the aortic valve, calcification produces life-threatening aortic stenosis. Though previously considered a passive, degenerative, untreatable disorder of “wear-and-tear” unrelated to atherosclerosis, recent findings now show that valvular calcification is regulated similar to atherosclerotic calcification and promoted by the systemic, inflammatory milieu characteristic of metabolic syndrome and type II diabetes – and the molecular “fingerprints” of activated Wnt signaling identified in diabetic medial artery calcification can also be detected in calcifying aortic valves.
In coronary arteries, calcium deposits weaken vasomotor responses10 and alter atherosclerotic plaque stability, depending on the size and distribution of deposits. Lesions associated with unstable angina or infarction tend to have multiple, small calcium deposits, in “spotty” or “speckled” patterns, whereas those in stable angina are associated with few, large calcium deposits.11,12 These clinical findings are in excellent agreement with finite element analyses showing that large deposits reduce circumferential stress in adjacent plaque13 and that small deposits increase stress at their edges.14 These are in further agreement with the frequent arterial dissection and rupture in mouse models with aortic vascular calcification.15,16 Thus, vascular calcification introduces compliance mismatch that can promote mechanical failure due to stress concentration at the interfaces of calcium deposits with softer plaque components.
B. Vascular calcification in comparison with skeletal calcification
Eukaryotic life forms evolved in calcium-rich seas, requiring them to evolve mechanisms to prevent widespread calcium crystallization in tissues. In mollusks, mucoproteins control deposition of the calcium carbonate shell. In vertebrates, lipid vesicles and regulatory proteins control crystal formation in skeletal bone, a biocomposite of structured cellular tissue impregnated with calcium phosphate mineral that aligns with the matrix and with the periodicity of negative charges on collagen. Crystals initially form octacalcium phosphate (Ca8H2(PO4)6•5H20) that re-organizes and seeds epitaxial growth of hydroxyapatite (Ca10(OH)2(PO4)6), the characteristic mineral of bone. As in bone, vascular apatitic mineral contains carbonate and magnesium impurities. In both bone and arteries, amorphous mineralization precedes mineralized tissue biogenesis, which follows vascular ingrowth and remodeling.
The vertebrate extracellular milieu – an internal sea – has calcium concentrations approaching the solubility product for several salts. Thus, mechanisms evolved to limit nucleation and propagation of calcium deposits in vertebrate soft tissues. Perhaps, then, it is not surprising that actively regulated osteogenic processes concomitantly evolved to locally and judiciously compromise these inhibitory mechanisms. Chief amongst these is the induction of bone alkaline phosphatase, an ectoenzyme required for vertebrate tissue biomineralization (vide infra).
In bone hydroxyapatite mineralization, crystals nucleate and propagate as they do in inorganic crystal formation from supersaturated solutions. But in biomineralization, organic constituents finely tune the rate of progression, (Fig. 1) limiting nucleation to sites on collagen fibers and matrix vesicles (MV). These MV are phospholipid-bound nanoparticles in which crystals are organized by phosphatidyl serine, annexins, bone sialoproteins and crucial ectoenzymes. These ectoenzymes act on inorganic pyrophosphate (PPi) which inhibits crystal propagation and stimulates production of osteopontin, an inhibitor of nucleation.17,18 These osteogenic activities distinguish matrix vesicles from the apoptotic bodies that nucleate dystrophic calcification during tissue necrosis. Alkaline phosphatase, a prominent constituent of matrix vesicles, locally degrades PPi as a necessary step to permit vertebrate biomineralization. Thus, ALP activity is a key factor and useful marker for active osteochondrogenesis.
Fig. 1.
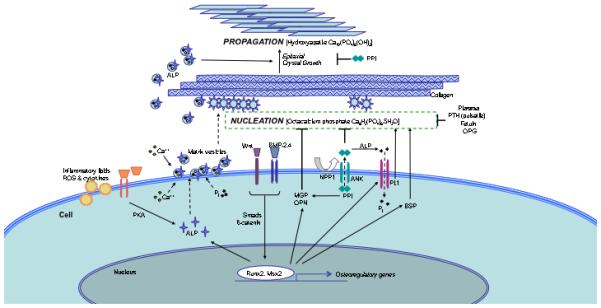
Schematic representation of selected regulatory factors and their possible roles in vascular biomineralization. Inorganic phosphate (Pi) translocates via Pit-1 to the cytoplasm. Cytoplasmic Ca++ and Pi incorporate with ALP into matrix vesicles, which bud off the plasma membrane and associate with extracellular proteins, such as collagen. NPP1 generates the mineralization inhibitor, pyrophosphate (PPi), which is inhibited by alkaline phosphatase. Some important factors have not been shown for clarity. Dashed arrows indicate translocation, solid-line arrows indicate induction, solid line bars indicate inhibition. Abbreviations: ALP, alkaline phosphatase; BMP, bone morphogenetic protein; BSP, bone sialoprotein; MGP, matrix Gla protein; NPP1, ectonucleotide pyrophosphatasephosphodiesterease1; OPG, osteoprotegerin; OPN, osteopontin; OCN, osteocalcin; Pi, inorganic phosphate; Pit-1, type III sodium-dependent phosphate cotransporter; PPi, pyrophosphate; PKA, protein kinase A; PTH, parathyroid hormone; ROS, reactive oxygen species.
In arterial calcification, these same processes are called to action. Matrix vesicles are found in both medial and intimal calcium deposits.19 Cultured VSMC elaborate MV, which can promote or inhibit mineralization depending on their content of inhibitory factors, matrix gamma-carboxyglutamic acid protein (MGP) and fetuin.20 Calcified arteries and cultured vascular cells also express bone matrix proteins and regulatory factors including bone morphogenetic protein-2 (BMP-2), osteopontin (OPN), MGP, bone sialoprotein, osteonectin, collagen I, and osteocalcin.21-25
Cells that spontaneously produce mineralized matrix and undergo osteoblastic differentiation have been isolated from vascular tissue and identified as i) pericytes in microvessels, ii) pericyte-like, calcifying vascular cells in the aortic intima, iii) smooth muscle cells in the media, iv) myofibroblasts in the adventitia.21,26,27 All these cell types are closely related and may be variant phenotypes of one another 28. Three have multilineage potential including osteogenic, chondrogenic, adipogenic, leiomyogenic, and marrow stromogenic potential.29-32 It is not known whether these cells originate in the artery wall or immigrate from near (adventitial) or remote (marrow) sites; whether they result from osteogenic transdifferentiation of mature VSMCs, osteoblastic redifferentiation of dedifferentiated SMC, or primary osteochondrogenic differentiation of vascular mesenchymal stem cells or vascular pericytes. In vivo, tissues corresponding to these lineages form in the artery wall: bone, cartilage, fat, and even marrow.33,34 The predominant form of metaplasia in human vasculature is bone, which is found in 5-15% of specimens.35 Transitional stages between amorphous calcification and mature bone tissue are also seen, the latter apparently requiring microvascular invasion as in skeletal bone. Though less common than bone, ectopic cartilage also occurs in human vascular calcification,36 and it is the predominant form of ectopic mineralized tissue in mice,37 especially in the brachiocephalic arteries of apoE null mice.38 Thus, by structural, molecular, and cellular criteria, vascular calcification proceeds via active osteochondrogenic processes. It is important to note that this applies regardless of whether mature bone tissue is detectable, even when the deposits are amorphous such as in medial calcification of type II diabetes mellitus. This non-atherosclerotic process also follows osteochondrogenic mechanisms, but advances to ossification less often39 possibly due to slower angiogenic invasion or the greater abundance of elastin, which maintains SMC phenotype. Thus, both intimal and medial calcifications appear to be driven by osteochondrogenic molecular programs.
As in skeletal tissue, remodeling and regression may also occur in vascular calcium deposits, though not as rapidly as for other plaque components.40 By radiographic criteria, lipid-lowering treatment reduces progression of coronary and valvular calcification.41 In two rat models of medial artery calcification – one elicited by vitamin D (calcitriol) intoxication and the other by warfarin administration – regression has been observed.42,43. In the former, it was associated with monocyte / macrophages, which are closely related to bone-resorbing osteoclasts. This is in excellent agreement with an elegant study performed by Giachelli and colleagues showing mineral regression and matrix acidification and in ectopic valve leaflet allografts in mice.44. In the warfarin model, high dose vitamin K supplementation induced regression and restored vascular compliance.45 Whether ossified lesions regress is unclear; such a process may require osteoclast-like cells, the monocyte-derived cells responsible for skeletal bone resorption. Such cells have been identified in arterial ossification.46,47 The potential for cell-mediated regression therapy based on osteoclast induction targeted to the vasculature remains to be fully explored.
C. Major categories of arterial calcification
Arterial calcification has been usefully categorized by histoanatomic and etiological criteria (Table I). Histologically, the deposits may have osteomorphic, chondromorphic, or amorphic structure. Etiologically, they may be categorized as metastatic, in which diffuse tissue calcification arises from systemically high calcium-phosphate products, or dystrophic which is pathological but not metastatic. Anatomically, it may be intimal atherosclerotic calcification, which occurs in a patchy pattern or it may be arterial medial calcification, which is more diffuse and independent of atherosclerosis; in the arteriolar vessels, it is known as calcific uremic arteriolopathy or, previously, calciphylaxis. Since medial and intimal layers are in close proximity, noninvasive measures of vascular calcification generally do not distinguish them.
Table 1.
|
Types of Vascular
Calcification |
Location and features | Associated Condition(s) |
|---|---|---|
| Calcific atherosclerosis | Intimal; ossification | Atherosclerosis, hyperlipidemia; osteoporosis; hypertension; inflammation |
|
Calcific medial vasculopathy
(Monckeberg’s Medial Calcific Sclerosis) |
Tunica media | T2DM; ESRD; hyperphosphatemia; amputation |
| Elastocalcinosis | Internal elastic lamina | Pseudoxanthoma elasticum; Marfan’s Syndrome |
|
Calcific uremic
arterioloopathy |
Microvessels; amorphous | ESRD; warfarin? |
|
Calcific aortic valvular
stenosis |
Aortic face of the leaflets | Hyperlipidemia; congenital bicuspid valve; rheumatic heart disease |
| Portal vein calcification | Portal vein thrombus or venous wall |
Portal hypertension; liver disease |
C.1. Intimal Atherosclerotic Calcification
Atherosclerotic calcification, the most common form of calcific vasculopathy, appears to result from induction of osteogenic differentiation in subpopulations of vascular cells by inflammatory factors, such as modified lipoproteins and cytokines, that are found in atheromatous components of plaque. Most, but not all, clinical studies link dyslipidemia with the presence, severity and progression of vascular calcification48-50 particularly when the duration of exposure to cholesterol is taken into account as “cholesterol-years.”51 Given the relevance of exposure duration, the association may be masked in cross-sectional studies that include patients on lipid-lowering agents whose current lipid level may not reflect level of exposure over prior decades. Hyperlipidemia is known to promote calcification in mice.52 In vitro, HMG-CoA reductase inhibitors reduce vascular cell calcification via Gas-6/Axl signaling,53 and, in some clinical studies, they reduced progression of atherosclerotic calcification41,54 However, in recent randomized trials, statins did not affect severity or progression.55,56
The findings of BMP2 and OPN expression in human atherosclerotic plaque provided the first molecular evidence for an osteogenic signaling mechanism.21 Atherogenic stimuli, such as inflammatory cytokines, oxidized lipids, and monocyte-macrophage products, promote osteogenesis and matrix calcification in vascular cell culture.57-60 High glucose also activates osteogenic programs, reflected in Runx2/Cbfa1-dependent ALP expression.61 Both spontaneous and induced osteoblastic differentiation of vascular cells are regulated by the cAMP pathway, Msx-2, and the Wnt signaling pathway.62-64 Oxidant stress promotes65 -- and antioxidant factors, such as omega-3 fatty acids and HDL, inhibit – in vitro vascular cell calcification.66,67 In an important recent study, Aikawa and colleagues used near-infrared fluorescence imaging to show that atherosclerotic mineralization is linked with inflammation at its earliest stages. (Aikawa 2007 Osteogenesis associates…)
Given the central role of inflammation in atherogenesis, an exciting possibility is that vascular mineral itself may initiate, promote, or perpetuate atherosclerosis by inducing inflammatory cytokines in monocytes that encounter and ingest hydroxyapatite crystals.68
Murine models of atherosclerotic calcification have been developed. Several genetically distinct inbred mouse strains, including C57BL/6, Balb/C, C3H/HeJ, DBA/2J, SM/J and MRL-lpr/lpr, mice, develop spontaneous vascular calcification that increases with a high fat/high cholesterol diet.52,69 The occurrence of artery wall calcification differed among the strains, indicating for the first time a genetic component in the phenotype.
Vascular calcification occurs spontaneously in genetically modified mice such as apolipoprotein E (ApoE) deficient mice which were recently shown to have marked cartilaginous metaplasia in the brachiocephalic artery.38,70 In one of the first models, Towler and colleagues demonstrated that vascular calcification occurs in response to a high fat, diabetogenic diet in LDL receptor (Ldlr) deficient mice.71 In the apoE-deficient model, the calcification is accentuated by OPN deficiency,72,73 The Klotho mouse also develops atherosclerosis and calcification,74 but the calcification appears to be medial and is driven by hyperphosphatemia (vide infra).75
Calcifying vascular cells have served as a robust in vitro model for atherosclerotic calcification, developed based on the work of Canfield, Schor, and colleagues. They consist of bovine aortic SMC purified by dilutional cloning and selected for the capacity for form nodules.21 These cells have many features of microvascular pericytes and are distinguished from conventional SMC by a surface ganglioside found on pericytes. Unlike cultures of skeletal osteoblasts, which remain in monolayer, mineralize more diffusely, and require ascorbate and phosphate supplements, calcifying vascular cells spontaneously produce mineral, often in multicellular nodules that grossly and histologically resemble atherosclerotic plaque and aortic valve nodules. [FIG. 2] The patterns formed as these nodules form in cultures of calcifying vascular cells is determined by a specific molecular mechanism. Several days after uniform plating, calcified nodules arise in a pattern of spots or ridges approximately 500 µm in diameter. The spatial frequency increases with transforming growth factor-beta1 or 25-hydroxycholesterol treatments.76 The nodular pattern is abolished in the absence of apolipoprotein J (clusterin)77 or in the presence of forskolin.59 Importantly, the type of pattern formed by the nodules – diffuse, spotty, striped, or even labyrinthine -- is determined by a well-defined “reaction-diffusion” mechanism governed by interaction between the known morphogen, BMP2, and its inhibitor, MGP. A mathematical model of this mechanism successfully predicts that warfarin, an MGP inhibitor, would double the spatial frequency of the calcium deposits.78 Given the widespread use of warfarin in patients with atrial fibrillation and the critical importance of interface area of “spotty” vs. diffuse calcification in determining risk of unstable angina and myocardial infarction, this regulatory mechanism controlling calcification pattern may have substantial clinical importance.
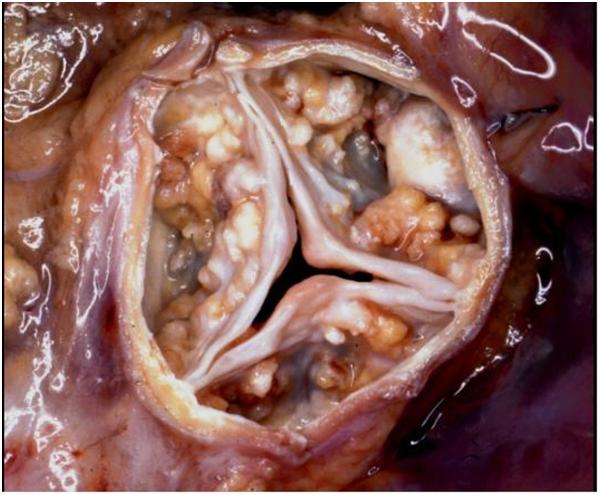
Fig. 2.
Calcified nodules in vivo and in vitro. (A) Calcific aortic stenosis (Image kindly provided by Dr. Michael Fishbein, UCLA Pathology; horizontal dimension ~4 cm) and (B) Dilutionally-cloned vascular smooth muscle cells (horizontal dimension ~1 cm). The nodules correspond in shape, size, and content.
C2. Valvular calcific aortic stenosis
Calcification of the aortic valve (recently reviewed79) is increasingly common and carries a high mortality in the setting of advanced age, congestive heart failure, and end stage renal disease where mechanical stress interacts with metabolic (mineral, diabetic, dysplidemic) and inflammatory disturbances. In seminal studies of human sclerotic aortic valves, Otto and O’Brien demonstrated elastin displacement, lipid accumulation, chronic inflammation, stippled calcification, and high levels of OPN.80 The cellular basis for calcific aortic stenosis (CAS) was clarified by Mohler et al. by isolating calcifying valvular cells from aortic valve tissue81, and, in a survey of human valve specimens, demonstrating inflammation, expression of BMP, and, importantly, mature bone tissue in over 10% 47.
Active osteochondrogenic differentiation and signaling were demonstrated by Rajamannan and colleagues in human valves, cultured valvular cells, and in hypercholesterolemic rabbits82. Using the rabbit model to test the role of inflammatory lipids, her group found that lipid-lowering agents reduced the valvular calcification.83 As evidence for osteochondrogenic signaling, they identified expression of Wnt-3a and LDLR-related protein (LRP5)-dependent activation of the canonical Wnt signaling cascade, including beta-catenin accumulation in the nucleus, which serves a critical co-factor role for LEF1/TCF7 and Smad transcription.84 Indeed, beta-catenin is indispensable for osteoblast development – genetically epistatic to the better-appreciated osteoblast transcriptional regulators, Runx2 and Osx.
The first robust murine models of CAS are making their debut. In apoE−/− and ldlr−/− mice, as well as mice on high-fat/high-carbohydrate diets, aortic valve leaflets have osteogenic activity.85,86 In an important development, ldlr−/− mice engineered to express only apoB100 were shown to develop hemodynamically significant transvalvular flow gradients, and thus true aortic stenosis.87
Calcium deposits are also associated with the mitral valve, but primarily as cartilaginous metaplasia in the annulus. This mitral annular calcification is often detected echocardiographically, and it correlates inversely with fetuin A levels88 and positively with atherosclerosis and cardiovascular mortality, independently of ejection fraction and CAD severity.89 It may have value as a clinical marker of disease. The reason one finds osteogenesis in the aortic valve leaflets, chondrogenesis in the mitral annulus, and neither in the mitral leaflets is unknown, but possibly due to differences in mechanical stress or in embryonic derivation. The aortic valve derives from embryonic neural crest cells, which are regulated by Wnt signaling. This ontogenetic history of aortic valve cells may influence their responses to mechanical and metabolic stress and account for differences between the two valves.
C3. Arterial medial calcification (AMC)
A comprehensive and insightful review of this topic has been provided by Dwight Towler (Towler DA. Vascular Calcification: A Perspective On An Imminent Disease Epidemic. IBMS BoneKEy. 2008 February;5(2):41-58). The most extensive vascular calcification is found in patients with arterial medial calcification (AMC), a highly characteristic feature of type II diabetes (T2DM)90 and chronic kidney disease (CKD).91 AMC was once considered benign because it was neither stenotic nor thrombogenic. It is now recognized that AMC associates with higher cardiovascular mortality and risk of amputation in T2DM92,93 and in end stage kidney disease.94 There is growing evidence for heterogeneous mechanisms within the category of medial calcification. For example, hydroxyapatite is the predominant mineral in diabetic AMC, but in vitamin D toxicity, it is whitlockite.95
In the setting of renal insufficiency, (reviewed recently96) Runx2/Cbfa1-dendendent osteochondrogenic processes figure prominently, enhanced by episodic excesses in serum phosphate and calcium. Recognizing that cultured osteoblasts mineralize in response to phosphate supplements, and the association of hyperphosphatemia and vascular calcification in patients with chronic renal failure, Giachelli and colleagues showed that inorganic phosphate promotes osteogenic differentiation in VSMC through induction of a sodium-dependent phosphate transporter (Pit-1) and subsequent induction of Runx2/Cbfa1 and downstream osteogenic programs,97 Pit-1 is induced by BMP-298 and suppressed by phosphonoformic acid, which also significantly reduces SMC calcification.99 In rat models of renal failure, mature cartilage tissue and major chondrogenic factors were found in the vessels.100 In such models, phosphate sequestration can ameliorate vascular calcification without suppressing parathyroid hormone (PTH) levels and bone formation.101 Thus, interventions reducing phosphate and Pit-1 phosphate transport may help retard the progressive arterial calcium burden in patients with chronic renal failure.
In a body of work addressing the context of type II diabetes, the Towler group has established that BMP2-Mxs2-Wnt signaling, which is entrained to inflammatory redox status, figures prominently in early stages of medial calcification.102 This was recently shown to occur independently of Runx2/Cbfa1.103 In a seminal report, Towler et al. showed that male ldlr−/− mice on a Western diet develop hypercholesterolemia, type II diabetes, and hypertriglyceridemia --- features characteristic of the metabolic syndrome-diabetes continuum.71 In these mice, circulating markers of inflammation such as TNF-alpha, haptoglobin, and hemopexin are increased, with concomitant valvular and medial calcification.71 Calcification was also demonstrated in ldlr−/−-ApoB100/100 mice rendered diabetic by overexpression of IGF-II.104 Procalcific BMP2-Msx2-Wnt signaling processes characteristic of intramembranous bone formation are upregulated in response to diet-induced obesity, inflammation, and dysmetabolic milieu.103 In concert with signals arising from the macrophage, TNF-alpha has emerged as a key stimulus for osteogenic differentiation in vascular cells.62 As further evidence of biological heterogeneity in medial calcification, patients with diabetes exhibit more severe vascular calcification at every stage of declining renal function105 and the association with hyperphosphatemia is not significant in diabetic patients,106 suggesting the presence of non-redundant mechanisms. Altogether, it appears that different but overlapping mechanisms guide medial calcification in vitamin D toxicity, chronic kidney disease, and diabetes.
Elastin degradation appears early in many forms of medial calcification. Several investigators 107-112 have shown that elastin metabolites can activate, and even nucleate, cell-dependent calcium deposition.113 Matrix metalloproteinase 9 (MMP9), an elastase expressed by the injured vessel wall, appears to promote arterial calcium deposition in warfarin/vitamin K models of medial calcification.114 Elastin degradation is a prominent feature of aortic calcification in Marfan’s syndrome.115. Since an intact elastin matrix stabilizes the vascular SMC phenotype in vivo, changes in osteopontin expression and MMP9-dependent elastin degradation may contribute significantly to medial calcification in diabetes.
In aging, medial calcification may develop by a distinct process of unknown etiology or result from a confluence of specific processes. Aging is associated with mild degrees of several processes believed to affect vascular calcification, including chronic renal insufficiency, insulin resistance, atherosclerosis, hormonal depletion, elastolysis, and slowly manifesting genetic vulnerabilities. Any one or a combination of these may contribute to medial calcification in aging.
C4. Calcific uremic arteriolopathy (CUA)
Calcific uremic arteriolopathy (CUA), formerly known as calciphylaxis, is a severely morbid and life-threatening form of medial vascular calcification that leads to cutaneous necrosis and panniculitis. CUA afflicts patients with advanced chronic kidney disease especially those receiving warfarin. Skin nodules and painful ulcers progress to black eschar and demarcating cutaneous necrosis. CUA is an active vasculopathy characterized by patchy medial calcification of arterioles (≤ 0. 6 mm diameter), with intimal proliferation, thrombotic occlusion, fibrosis, and adipose inflammation and necrosis. In the dermis, adjacent subcutaneous fat necrosis also seeds calcium deposition. Mesenteric and pulmonary tissues may be involved, and mortality approaches 100% within 2 years of disease initiation. Immunohistochemistry has revealed the expression of BMP4 in these lesions.116 Serum markers of systemic inflammation, including erythrocyte sedimentation rate and C-reactive protein are markedly elevated.
An important consideration for clinical cardiology and nephrology is the possible link between warfarin and vascular calcification. In patients, warfarin is associated with vascular calcification and calcific aortic stenosis.117 Warfarin may affect calcification by blocking MGP and other gamma-carboxylated proteins that regulate mineralization, such as osteocalcin and, Gas-6.118 MGP is believed to inhibit mineralization by two mechanisms: directly as part of a complex with fetuin119 and indirectly by inhibiting BMP2 induced osteogenic differentiation.120 To be fully functional, MGP requires post-translational modification by gamma-carboxylation, a vitamin K dependent process that is inhibited by warfarin. Thus, a high dose of vitamin K reverses warfarin-induced vascular calcium deposition in animal models. 107 The contributions of warfarin and Gla protein deficiency to pathogenesis of CUA have yet to be determined.
For this devastating, but fortunately uncommon, disease, only anecdotal reports are available to guide therapy. Parathyroidectomy has been used based on the potentially false impression that CUA pathobiology follows that of medial artery calcification of uremia. Traditional strategies for non-healing ulcers, including hyperbaric oxygen, have only modest success. Sodium thiosulfate infusion – a reducing agent that restores cellular glutathione and mobilizes amorphous calcium phosphate and brushite – has shown promise. Further research is needed in order to devise strategies to address this tragic disorder.
D. The bone-vascular axis and mineral regulators
Epidemiologic and preclinical studies have shown both parallel and reciprocal changes in arterial vs. skeletal mineralization. Whereas inflammatory lipids and cytokines appear to promote vascular calcification but inhibit bone mineralization,57,121 some osteoanabolic agents, such as parathyroid hormone (PTH) and BMP7, promote mineralization in the skeleton, but suppress it in arteries. 63,101,122
The clinical association of aortic calcification with osteoporosis, often age-independent, suggests a link between vascular and bone metabolism.123-125 Three causality vectors may apply: 1) vascular calcification promoting bone loss, 2) bone loss promoting vascular calcification, or 3) a common etiology. The first possibility is largely unexplored, though bone loss may be promoted by stenoses of bone supply arteries or by systemic inflammation associated with atherosclerosis. The second possibility has more supportive evidence. Bone matrix is rich in regulatory factors that are also active in the vasculature, such as osteopontin, MGP, and products of the TGF-beta gene superfamily. During resorption, these are released into the circulation. As evidence for their role in vascular calcification, agents that block bone resorption in animal models also block vascular calcification;126,127 however, high resorptive activity is not always required, since vascular calcification also occurs in conditions of low bone-turnover.128 The third potential mechanism, a causal factor shared by vascular calcification and osteoporosis, is supported by the many risk factors common to the two disorders.124,125 including aging, estrogen deficiency, vitamin D and K abnormalities, dyslipidemia, hyperparathyroidism, chronic inflammation, hyperhomocysteinemia, and oxidative stress.
In renal insufficiency, synergism between hyperphosphatemia, hyperparathyroidism, calcitriol, calcium carbonate, warfarin, low fetuin, hypertension, and atherosclerosis, have given it recognition as the “perfect storm” for vascular calcification and osteopenia. Hyperphosphatemia is associated with increased aortic calcification through Pit-1 osteoinduction. Secondary hyperparathyroidism often accompanies renal failure but it can induce aortic medial calcification independently of uremia and renal function.129 Importantly, however, Shao et al. showed that intermittent PTH treatment has the opposite effect of continuous hyperparathyroidism, and it suppresses vascular calcification without changes in serum phosphorus.130 Calcitriol (1,25-dihydroxyvitamin D) has historically been used to control secondary hyperparathyroidism in these patients. Calcium carbonate is used to bind enteric phosphorus and reduce hyperphosphatemia, but it also increases serum calcium and suppresses PTH levels. In the setting of CKD, oral calcium carbonate “paradoxically” suppresses bone formation and promotes vascular calcification, and low PTH levels correspond with low turnover osteoporosis and severe arterial calcification.128
Some possible contributors to bone-vascular interactions include osteopontin, FGF-23, phosphate/PTH, and vitamin D. Osteopontin release from bone may represent a major component of the bone-vascular axis. PTH-induced bone formation increases circulating levels of intact OPN130 a potent inducible inhibitor of vascular calcification.131 Fibroblast growth factor-23 (FGF-23) was recently identified as a circulating factor released by bone in response to high phosphate levels.132. It acts on the kidney reduce phosphate resorption. Inappropriately low FGF-23 levels associate with hyperphosphatemia and, in hemodialysis patients, with vascular calcification.133 Interestingly, a coreceptor for FGF-23 receptor binding is Klotho. For years, the vascular calcification and osteoporosis phenotypes of the Klotho mouse were attributed to a premature aging syndrome, but the Klotho phenotype has been reproduced in the FGF-23−/− mouse, suggesting that the vascular phenotype may be mediated by hyperphosphatemia instead.132 Thus, perturbations of the FGF23/Klotho/FGF receptor-signaling axis represent another critical component of the bone-vascular axis.
Not all regulatory systems active in both bone and artery are necessarily part of the bone-vascular axis. For example, members of the OPG/RANKL/RANK system may operate simultaneously, but independently, in the two tissues. Osteoprotegerin (OPG) is protective against osteoporosis because acts as a decoy receptor for the pro-osteoclastic factor, receptor activator of nuclear factor-kappaB (RANKL). In humans, low OPG levels are associated with lower vascular calcification.134,135 However, in mice, OPG deficiency is positively associated with vascular calcification.136,137 The possibility that the clinical correlation represents an incomplete compensatory response is supported by findings that direct OPG treatment reduces vascular calcification in a rat model of vitamin D toxicity 126 and in a mouse model of hyperlipidemia.138 Given the known immunomodulatory function of RANKL, a working model emerges. In bone, OPG may hold in check the pro-resorptive effects of RANKL, whereas, in the artery, OPG may hold in check inflammatory effects of RANKL 139-142 In support of this, OPG deficient mice have T lymphocyte infiltration in their calcified arteries,143 T cells are associated with valvular calcification in humans.144 and RANKL has been found in CD3-positive and F4/80-positive cells at the adventitial - medial junction in an atherosclerotic mouse model.138 Furthermore, ectopic mineralization induced by BMP2 treatment is not accelerated by the high-turnover state of OPG deficiency.145 The importance of local RANKL-OPG signaling was highlighted by the finding that postnatal treatment with OPG failed to reverse the vascular calcification phenotype.146 Thus, rather than serving as the foundation of the bone-vascular axis, RANKL-OPG interactions may reflect tissue-specific immunomodulation of OPG expressed in response to mechanical, endocrine and inflammatory cues. The roles of other vascular cells that produce OPG and RANKL, such as endothelial cells, have yet to be explored.
Similarly, bisphosphonates may also act directly on arteries, independently of their effects on bone resorption. These agents, used widely for osteoporosis treatment, are analogs of pyrophosphate, the critical endogenous inhibitor of extracellular mineralization. They inhibit vascular calcification in the vitamin D model,127 and whether this is a direct effect on the arteries or an indirect effect through blocking bone resorption is not clear. Pyrophosphate is produced from ATP by an enzyme at the cell membrane, ectonucleotide pyrophosphatasephosphodiesterease 1 (NPP1), and it is transported across the cell membrane, by the protein ANK, to its site of action in the extracellular matrix. This inhibitor is broken down by tissue nonspecific alkaline phosphatase (ALP), secreted from osteogenic cells in matrix vesicles. Conditions that disrupt extracellular pyrophosphate levels dramatically alter vascular calcification. Terkeltaub and colleagues showed that mice deficient in NPP1 have pyrophosphate deficiency and aortic calcification.147 A defect in the human gene encoding NPP1 is responsible for the often-fatal disorder, idiopathic infantile arterial calcification,148 which is now no longer “idiopathic.” Mice with mutant genes for the transport protein (ank/ank mice) are also depleted of extracellular pyrophosphate and also develop medial calcification.149. Millan and colleagues showed that blocking the pyrophosphate-degrading action of alkaline phosphatase reduced the calcification in VSMC from NPP1 and ANK deficient mice,150 offering a new therapeutic possibility. Therefore, while extremely relevant to potential treatments, the beneficial effects of OPG and bisphosphates in the vitamin D model may reflect, in part, direct local actions.
Thus, the bone-vascular axis may operate, directly or indirectly, through a variety of hormonal and physiological systems that could not all be covered in this limited review. Other organs and tissues besides bone and arteries also secrete hormonal factors that regulate both vascular calcification and bone including leptin, dexamethasone, aldosterone, vasopressin, adiponectin, and IGF-1. Bone also secretes hormonal factors independently of resorption. The diversity of molecular mechanisms are reflected in the large number of genetically modified mice with vascular calcification phenotypes, such as those deficient in fibrillin, betaglucosidase, carbonic anhydrase II, desmin, klotho, fetuin-A, ectonucleotide pyrophosphatase, MGP, Smad6, and Abcc6.
Summary
Clinically, vascular calcification is now accepted as a valuable predictor of coronary heart disease.151 Achieving control over this process requires understanding mechanisms in the context of a tightly-controlled regulatory network, with multiple, nested feedback loops and cross-talk between organ systems, in the realm of control theory. Thus, treatments for osteoporosis such as calcitriol, estradiol, bisphosphonates, calcium supplements, and intermittent parathyroid hormone are likely to affect vascular calcification, and, conversely, many treatments for cardiovascular disease such as statins, antioxidants, hormone replacement therapy, ACE inhibitors, fish oils, and calcium channel blockers may affect bone health. As we develop and use treatments for cardiovascular and skeletal diseases, we must give serious consideration to the implications for the organ at the other end of the bone-vascular axis.
Acknowledgements
The authors are especially grateful to Dr. Dwight Towler, Washington University for his insightful discussions and his critical review of this manuscript. We are also grateful for the contributions of Wendy Tseng, Jeff Hsu, Michael Huang, and Dr. Jin-Xiu Lu to their research program.
Funding Sources
This work was supported in part by NIH grants HL081202, DK076009, and American Heart Association grant GIA0555028Y.
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 2.Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–30. doi: 10.1016/s0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 3.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253–60. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 4.Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy IP, Peyser PA, Schwartz RS. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–7. doi: 10.1161/hc2901.093112. [DOI] [PubMed] [Google Scholar]
- 5.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res. 1992;71:490–502. doi: 10.1161/01.res.71.3.490. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka S, Kakihana M, Watanabe H, Sugishita Y. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol. 1994;24:1406–14. doi: 10.1016/0735-1097(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y. Decreased aortic compliance aggravates subendocardial ischaemia in dogs with stenosed coronary artery. Cardiovasc Res. 1992;26:1212–8. doi: 10.1093/cvr/26.12.1212. [DOI] [PubMed] [Google Scholar]
- 8.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–33. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 9.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–7. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Jerosch-Herold M, Jacobs DR, Jr., Shahar E, Detrano R, Folsom AR. Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2006;48:1018–26. doi: 10.1016/j.jacc.2006.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 12.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–6. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 14.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–83. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orita Y, Yamamoto H, Kohno N, Sugihara M, Honda H, Kawamata S, Mito S, Soe NN, Yoshizumi M. Role of osteoprotegerin in arterial calcification: development of new animal model. Arterioscler Thromb Vasc Biol. 2007;27:2058–64. doi: 10.1161/ATVBAHA.107.147868. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 17.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–78. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 18.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–55. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanimura A, McGregor DH, Anderson HC. Matrix vesicles in atherosclerotic calcification. Proc Soc Exp Biol Med. 1983;172:173–7. doi: 10.3181/00379727-172-41542. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 21.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giachelli C, Bae N, Lombardi D, Majesky M, Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR) Biochem Biophys Res Commun. 1991;177:867–73. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- 23.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993;92:2814–20. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirota S, Imakita M, Kohri K, Ito A, Morii E, Adachi S, Kim HM, Kitamura Y, Yutani C, Nomura S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993;143:1003–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Canfield AE, Sutton AB, Hoyland JA, Schor AM. Association of thrombospondin-1 with osteogenic differentiation of retinal pericytes in vitro. J Cell Sci. 1996;109:343–53. doi: 10.1242/jcs.109.2.343. Pt 2. [DOI] [PubMed] [Google Scholar]
- 27.Proudfoot D, Skepper JN, Shanahan CM, Weissberg PL. Calcification of human vascular cells in vitro is correlated with high levels of matrix Gla protein and low levels of osteopontin expression. Arterioscler Thromb Vasc Biol. 1998;18:379–88. doi: 10.1161/01.atv.18.3.379. [DOI] [PubMed] [Google Scholar]
- 28.Campbell GR, Campbell JH. Vascular smooth muscle and arterial calcification. Z Kardiol. 2000;89(Suppl 2):54–62. doi: 10.1007/s003920070100. [DOI] [PubMed] [Google Scholar]
- 29.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–83. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 30.Farrington Rock C, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenc and adipogenic potential of microvascular pericytes. Circulation. 2004 doi: 10.1161/01.CIR.0000144457.55518.E5. CN. in press. [DOI] [PubMed] [Google Scholar]
- 31.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–10. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 32.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–77. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 33.Seemayer TA, Thelmo WL, Morin J. Cartilaginous transformation of the aortic valve. Am J Clin Pathol. 1973;60:616–20. doi: 10.1093/ajcp/60.5.616. [DOI] [PubMed] [Google Scholar]
- 34.Bunting CH. The formation of true bone with cellular (red) marrow in a sclerotic aorta. Journal of Experimental Medicine. 1906;8:365–376. doi: 10.1084/jem.8.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B, Cusack A, Mohler ER., 3rd Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–9. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 36.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003;34:402–7. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 37.Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, DelVecchio V, Vergona R, Sullivan ME, Rubanyi GM. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 beta-estradiol. Atherosclerosis. 1999;144:303–13. doi: 10.1016/s0021-9150(98)00325-6. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–92. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 39.Shanahan CM, Proudfoot D, Farzaneh-Far A, Weissberg PL. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr. 1998;8:357–75. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 40.Nicholls SJ, Tuzcu EM, Wolski K, Sipahi I, Schoenhagen P, Crowe T, Kapadia SR, Hazen SL, Nissen SE. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll Cardiol. 2007;49:263–70. doi: 10.1016/j.jacc.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 41.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–8. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 42.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol. 2000;20:317–27. doi: 10.1161/01.atv.20.2.317. [DOI] [PubMed] [Google Scholar]
- 43.Bas A, Lopez I, Perez J, Rodriguez M, Aguilera-Tejero E. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res. 2006;21:484–90. doi: 10.1359/JBMR.051211. [DOI] [PubMed] [Google Scholar]
- 44.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–46. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–31. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- 46.Jeziorska M, McCollum C, Wooley DE. Observations on bone formation and remodelling in advanced atherosclerotic lesions of human carotid arteries. Virchows Arch. 1998;433:559–65. doi: 10.1007/s004280050289. [DOI] [PubMed] [Google Scholar]
- 47.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 48.Schmermund A, Baumgart D, Mohlenkamp S, Kriener P, Pump H, Gronemeyer D, Seibel R, Erbel R. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: An electron-beam CT study. Arterioscler Thromb Vasc Biol. 2001;21:421–6. doi: 10.1161/01.atv.21.3.421. [DOI] [PubMed] [Google Scholar]
- 49.Bild DE, Folsom AR, Lowe LP, Sidney S, Kiefe C, Westfall AO, Zheng ZJ, Rumberger J. Prevalence and correlates of coronary calcification in black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–7. doi: 10.1161/01.atv.21.5.852. [DOI] [PubMed] [Google Scholar]
- 50.Pohle K, Maffert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, Achenbach S. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001;104:1927–32. doi: 10.1161/hc4101.097527. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt HH, Hill S, Makariou EV, Feuerstein IM, Dugi KA, Hoeg JM. Relation of cholesterol-year score to severity of calcific atherosclerosis and tissue deposition in homozygous familial hypercholesterolemia. Am J Cardiol. 1996;77:575–80. doi: 10.1016/s0002-9149(97)89309-5. [DOI] [PubMed] [Google Scholar]
- 52.Qiao JH, Fishbein MC, Demer LL, Lusis AJ. Genetic determination of cartilaginous metaplasia in mouse aorta. Arterioscler Thromb Vasc Biol. 1995;15:2265–72. doi: 10.1161/01.atv.15.12.2265. [DOI] [PubMed] [Google Scholar]
- 53.Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, Ouchi Y. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–31. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 54.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–61. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 56.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, Henein M, Kerkhoff G, Knollmann F, Kukharchuk V, Lahiri A, Leischik R, Moshage W, Schartl M, Siffert W, Steinhagen-Thiessen E, Sinitsyn V, Vogt A, Wiedeking B, Erbel R. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation. 2006;113:427–37. doi: 10.1161/CIRCULATIONAHA.105.568147. [DOI] [PubMed] [Google Scholar]
- 57.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 58.Proudfoot D, Davies JD, Skepper JN, Weissberg PL, Shanahan CM. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106:3044–50. doi: 10.1161/01.cir.0000041429.83465.41. [DOI] [PubMed] [Google Scholar]
- 59.Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–53. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 60.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–5. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 61.Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–31. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 62.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–42. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 63.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–9. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 65.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–19. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 66.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98:727–9. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 67.Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–6. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- 68.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, Landis RC, Haskard DO. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–56. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 69.Qiao JH, Xie PZ, Fishbein MC, Kreuzer J, Drake TA, Demer LL, Lusis AJ. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994;14:1480–97. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- 70.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–5. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 71.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–34. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 72.Speer MY, Chien YC, Quan M, Yang HY, Vali H, McKee MD, Giachelli CM. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res. 2005;66:324–33. doi: 10.1016/j.cardiores.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–34. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 74.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 75.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–13. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Millis AJ, Luciani M, McCue HM, Rosenberg ME, Moulson CL. Clusterin regulates vascular smooth muscle cell nodule formation and migration. J Cell Physiol. 2001;186:210–219. doi: 10.1002/1097-4652(200102)186:2<210::AID-JCP1019>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 78.Garfinkel A, Tintut Y, Petrasek D, Bostrom K, Demer LL. Pattern formation by vascular mesenchymal cells. Proc Natl Acad Sci U S A. 2004;101:9247–50. doi: 10.1073/pnas.0308436101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O'Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–8. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 80.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–53. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 81.Mohler ER, 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–60. [PubMed] [Google Scholar]
- 82.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005;91:806–10. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–86. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 86.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol. 2006;47:850–5. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 87.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–9. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 88.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–9. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willens HJ, Chirinos JA, Schob A, Veerani A, Perez AJ, Chakko S. The relation between mitral annular calcification and mortality in patients undergoing diagnostic coronary angiography. Echocardiography. 2006;23:717–22. doi: 10.1111/j.1540-8175.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 90.Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–85. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 91.Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–25. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 92.Nelson RG, Gohdes DM, Everhart JE, Hartner JA, Zwemer FL, Pettitt DJ, Knowler WC. Lower-extremity amputations in NIDDM. 12-yr follow-up study in Pima Indians. Diabetes Care. 1988;11:8–16. doi: 10.2337/diacare.11.1.8. [DOI] [PubMed] [Google Scholar]
- 93.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 94.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 95.Verberckmoes SC, Persy V, Behets GJ, Neven E, Hufkens A, Zebger-Gong H, Muller D, Haffner D, Querfeld U, Bohic S, De Broe ME, D'Haese PC. Uremia-related vascular calcification: more than apatite deposition. Kidney Int. 2007;71:298–303. doi: 10.1038/sj.ki.5002028. [DOI] [PubMed] [Google Scholar]
- 96.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 98.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–12. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 100.Neven E, Dauwe S, De Broe ME, D'Haese PC, Persy V. Endochondral bone formation is involved in media calcification in rats and in men. Kidney Int. 2007;72:574–81. doi: 10.1038/sj.ki.5002353. [DOI] [PubMed] [Google Scholar]
- 101.Hruska KA, Mathew S, Davies MR, Lund RJ. Connections between vascular calcification and progression of chronic kidney disease: therapeutic alternatives. Kidney Int Suppl. 2005:S142–51. doi: 10.1111/j.1523-1755.2005.09926.x. [DOI] [PubMed] [Google Scholar]
- 102.Towler DA. Angiogenesis and marrow stromal cell fates: roles in bone strength. Osteoporos Int. 2003;14(Suppl 5):46–53. doi: 10.1007/s00198-003-1473-5. [DOI] [PubMed] [Google Scholar]
- 103.Chen NX, Duan D, O'Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21:3435–42. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- 104.Heinonen SE, Leppanen P, Kholova I, Lumivuori H, Hakkinen SK, Bosch F, Laakso M, Yla-Herttuala S. Increased Atherosclerotic Lesion Calcification in a Novel Mouse Model Combining Insulin Resistance, Hyperglycemia and Hypercholesterolemia. Circ Res. 2007;101(10):1058–67. doi: 10.1161/CIRCRESAHA.107.154401. [DOI] [PubMed] [Google Scholar]
- 105.Taki K, Takayama F, Tsuruta Y, Niwa T. Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int. 2006;70:218–24. doi: 10.1038/sj.ki.5000330. [DOI] [PubMed] [Google Scholar]
- 106.Taniwaki H, Ishimura E, Tabata T, Tsujimoto Y, Shioi A, Shoji T, Inaba M, Inoue T, Nishizawa Y. Aortic calcification in haemodialysis patients with diabetes mellitus. Nephrol Dial Transplant. 2005;20:2472–8. doi: 10.1093/ndt/gfi039. [DOI] [PubMed] [Google Scholar]
- 107.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–7. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 108.Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–17. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 109.Essalihi R, Dao HH, Gilbert LA, Bouvet C, Semerjian Y, McKee MD, Moreau P. Regression of medial elastocalcinosis in rat aorta: a new vascular function for carbonic anhydrase. Circulation. 2005;112:1628–35. doi: 10.1161/CIRCULATIONAHA.104.528984. [DOI] [PubMed] [Google Scholar]
- 110.Bailey M, Pillarisetti S, Jones P, Xiao H, Simionescu D, Vyavahare N. Involvement of matrix metalloproteinases and tenascin-C in elastin calcification. Cardiovasc Pathol. 2004;13:146–55. doi: 10.1016/S1054-8807(04)00009-2. [DOI] [PubMed] [Google Scholar]
- 111.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 112.O'Neill WC. Vascular calcification: Not so crystal clear. Kidney International. 2007;71:282–283. doi: 10.1038/sj.ki.5002119. [DOI] [PubMed] [Google Scholar]
- 113.Lee JS, Basalyga DM, Simionescu A, Isenburg JC, Simionescu DT, Vyavahare NR. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am J Pathol. 2006;168:490–8. doi: 10.2353/ajpath.2006.050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–6. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 115.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96:3819–23. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Griethe W, Schmitt R, Jurgensen JS, Bachmann S, Eckardt KU, Schindler R. Bone morphogenic protein-4 expression in vascular lesions of calciphylaxis. J Nephrol. 2003;16:728–32. [PubMed] [Google Scholar]
- 117.Schurgers LJ, Aebert H, Vermeer C, Bultmann B, Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–2. doi: 10.1182/blood-2004-04-1277. [DOI] [PubMed] [Google Scholar]
- 118.Collett GD, Sage AP, Kirton JP, Alexander MY, Gilmore AP, Canfield AE. Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res. 2007;100:502–9. doi: 10.1161/01.RES.0000258854.03388.02. [DOI] [PubMed] [Google Scholar]
- 119.Price PA, Williamson MK, Nguyen TM, Than TN. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem. 2004;279:1594–600. doi: 10.1074/jbc.M305199200. [DOI] [PubMed] [Google Scholar]
- 120.Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–52. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 121.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 122.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16:917–28. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 123.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–31. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 124.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–46. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 125.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 126.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–6. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 127.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–24. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- 128.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–51. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 129.Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Neves CL, Magalhaes AO, Custodio MR, Batista DG, Jorgetti V, Moyses RM. Vascular calcification: contribution of parathyroid hormone in renal failure. Kidney Int. 2007;71:1262–70. doi: 10.1038/sj.ki.5002241. [DOI] [PubMed] [Google Scholar]
- 130.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 131.Scatena M, Liaw L, Giachelli CM. Osteopontin. A Multifunctional Molecule Regulating Chronic Inflammation and Vascular Disease. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 132.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Inaba M, Okuno S, Imanishi Y, Yamada S, Shioi A, Yamakawa T, Ishimura E, Nishizawa Y. Role of fibroblast growth factor-23 in peripheral vascular calcification in non-diabetic and diabetic hemodialysis patients. Osteoporos Int. 2006;17:1506–13. doi: 10.1007/s00198-006-0154-6. [DOI] [PubMed] [Google Scholar]
- 134.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 135.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–8. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 136.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–24. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 138.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostuneuik PJ, Demer LL. Osteoprotegerin (OPG) inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.707380. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hofbauer LC, Schrader J, Niebergall U, Viereck V, Burchert A, Horsch D, Preissner KT, Schoppet M. Interleukin-4 differentially regulates osteoprotegerin expression and induces calcification in vascular smooth muscle cells. Thromb Haemost. 2006;95:708–14. [PubMed] [Google Scholar]
- 140.Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–72. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 141.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 142.Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Monckeberg's sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89:4104–12. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- 143.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–74. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wallby L, Janerot-Sjoberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88:348–51. doi: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, Saito N, Hiraoka BY, Kobayashi Y, Takaoka K, Ozawa H, Miyazawa H, Takahashi N. Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology. 2003;144:5441–9. doi: 10.1210/en.2003-0717. [DOI] [PubMed] [Google Scholar]
- 146.Kim N, Odgren PR, Kim DK, Marks SC, Jr., Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000;97:10905–10. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol. 2005;25:686–91. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 148.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nat Genet. 2003;34:379–81. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 149.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–70. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 150.Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL. Novel Inhibitors of Alkaline Phosphatase Suppress Vascular Smooth Muscle Cell Calcification. J Bone Miner Res. 2007 doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 151.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr., Stein JH, Tracy CM, Vogel RA, Wesley DJ. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–26. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]