Abstract
Astrocytes are now emerging as key participants in many aspects of brain development, function and disease. In particular, new evidence shows that astrocytes powerfully control the formation, maturation, function and elimination of synapses through various secreted and contact-mediated signals. Astrocytes are also increasingly being implicated in the pathophysiology of many psychiatric and neurological disorders that result from synaptic defects. A better understanding of how astrocytes regulate neural circuit development and function in the healthy and diseased brain might lead to the development of therapeutic agents to treat these diseases.
The function of astrocytes in neural circuits has long been mysterious. Initially thought to be passive support cells, astrocytes have been shown in the past decade to have crucial roles in controlling synapse formation1–6 and function7–9. In recent years, several families of astrocyte-secreted molecules have been identified1,2,4,10, and these molecules have been shown to regulate several aspects of synaptic development and function in vitro and in vivo (TABLE 1).
Table 1.
Synaptogenic molecules secreted by astrocytes
| Synaptogenic molecule | Key binding partner (or partners) | Effect in vitro | Effect in vivo |
|---|---|---|---|
| Brain-derived neurotrophic factor (BDNF) | Neuronal neuregulin 1 and glial ERBB receptor interactions. Activation of glial ERBB receptors in response to neuronally derived neuregulin 1 induced glia to produce trophic factors such as BDNF | Transgenic elimination of ERBB receptors in the glia of the vestibular sensory epithelium reduced the number of excitatory synapses between hair cells and sensory neurons by 82% and led to vestibular defects. Similarly, BDNF expression was reduced in the glia of the vestibular system of ERBB-deficient mice. Restoration of BDNF expression in these cells rescued synapse formation and the vestibular deficits116 | |
| Cholesterol | Apolipoprotein E delivers cholesterol to neurons | Addition of cholesterol to retinal ganglion cells (RGCs) increased the number of excitatory synapses by 69% and increased presynaptic vesicle release 12-fold. Cholesterol is thought to act as a building material to enhance dendrite and presynaptic terminal differentiation10,33 | |
| Ephrins | EPH receptor tyrosine kinase | Ephrin–EPH interactions modulate dendritic spine dynamics. Activation of the receptor EPH type A4 (EPHA4) by ephrin A3 induces spine retraction, whereas inhibition of ephrin–EPHA4 interactions distorted spine shape and organization69,70 | EPHA4- and ephrin A3-knockout mice showed irregularities in the morphology of their dendritic spines69,70 |
| Glypican | Unknown | Glypicans converted silent synapses to functional ones by increasing the amplitude and frequency of glutamatergic synaptic events in cultured RGCs. This conversion was achieved by increasing the surface level of AMPA receptors1 | Genetic deletion of glypicans in vivo led to defective synapse formation, with decreased amplitude of excitatory synaptic currents and reduced recruitment of AMPA receptors1 |
| Hevin | Neuroligins and neurexins (S. Singh, A. Pamukcu and C. Eroglu, personal communication) | Addition of hevin to astrocyte-free RGC cultures significantly increased the number of postsynaptically silent excitatory synapses4 | |
| SPARC | Integrins39 | SPARC is not synaptogenic but specifically antagonized the synaptogenic function of hevin4 | At P14, SPARC-null mice had around a 70% increase in the number of vesicular glutamate transporter 2- and postsynaptic density 95-positive synapses at the superior colliculus in comparison with wild-type mice4 |
| Thrombospondins (TSPs) |
|
In the TSP1 and TSP2 double-null cerebral cortex, synaptic puncta were decreased by 40% at P8 and by 25% at P21 compared with wild-type controls2 | |
| Transforming growth factor β1 (TGFβ1) | TGFβ receptor type II | Addition of TGFβ1 to cultured cortical neurons increased the number of excitatory synapses by 150%. TGFβ1 was reported to induce synapse formation by increasing the release of the NMDA receptor co-agonist D-serine. Inhibition of NMDA receptors or knocking down serine racemase to inhibit the conversion of L-serine to D-serine inhibited TGFβ1-induced synapse formation29 |
Establishment of the correct number and types of synapses is essential for the formation of neural circuits and for information processing in the brain. Neural circuit formation occurs in three distinct stages. First, immature synapses form between axons and dendrites. Second, synapses undergo maturation, which involves the conversion of silent synapses to active ones. Third, excess synapses are eliminated or pruned to refine the neuronal connections within the circuit. In the past, research has focused on identifying the neuron-derived molecules that regulate these intricate processes11. Despite the identification of a diverse range of neuronal mechanisms that regulate neural circuit formation, we are still far from fully understanding the processes involved. The surprising discovery that neurons rely on astrocytes to instruct the formation of their synapses leads to the possibility that astrocytes provide a layer of control that acts in parallel with, and interacts with, the neuronal processes that control circuit formation. A better understanding of the bidirectional signals between neurons and astrocytes should advance our knowledge of neuronal circuit development.
There is mounting evidence that many neurodevelopmental diseases, such as autism and schizophrenia, are characterized by defects in synapse formation and function12–19. Recent studies have also implicated astrocytes in the pathophysiology of many of these diseases20,21. This is not surprising, given that astrocytes have such a pivotal role in synapse formation and function. Thus, astrocytes might be a new target for the development of therapeutic agents to treat these diseases in the future.
In this Review, we discuss our rapidly evolving understanding of how astrocytes contribute to normal synapse formation and neural circuit development and highlight the effect of perturbation of these processes on human health and disease.
Astrocyte control of synapse formation
During the development of the mammalian nervous system, neural precursor cells initially generate neurons and then astrocytes (reviewed in REF. 22). Although the generation and expansion of astrocytes is largely completed by early postnatal stages, astrocytes continue to elaborate and refine their processes after birth during the active period of synaptogenesis6. A single rodent astrocyte can associate with multiple neurons and can contact up to 100,000 synapses23,24. Furthermore, like neurons, astrocytes express ion channels, receptors and cell surface molecules that allow them to respond to neurotransmitters and environmental cues25. These features place astrocytes in a crucial position to communicate actively with neurons and thereby to coordinate the development of neural circuits. Recent studies in rodents have shown that astrocytes can use both secreted and contact-mediated signals to control synapse formation, and both of these are discussed here.
Secreted signals control excitatory synapse formation
The first evidence that astrocytes instruct synapse formation came from a study that used purified rodent retinal ganglion cells (RGCs). This model has proven to be an extremely useful system in which synapse development can be studied, as RGCs can be immunopurified and cultured for several weeks in the absence of glia and other cell types26,27. In the complete absence of glia, RGCs form very few synapses5. Surprisingly, when cultured in the presence of astrocytes, or in a medium that had been conditioned by soluble signals released by astrocytes, these neurons could form tenfold more excitatory synapses, and synaptic functionality was increased6. Later in vitro studies confirmed that astrocytes can also instruct synapse formation in rodent hippocampal neurons3,28, cortical neurons29, spinal motor neurons30, cerebellar Purkinje cells31 and human neurons29,32.
What are the astrocyte-secreted signals that control synapse development? The first synaptogenic, astrocyte-secreted molecule to be identified was apolipoprotein E bound to cholesterol10,33. The authors showed that cholesterol enhances presynaptic differentiation in cultured RGCs by increasing the release capacity of individual synapses; it does this by promoting the synthesis and maturation of synaptic vesicles33.
In addition, the thrombospondins (TSPs; also known as the THBSs) were recently identified as one of the key astrocyte-secreted signals for promoting synapse formation2 (FIG. 1). The TSPs are large extracellular matrix proteins that are secreted by cultured astrocytes and are abundantly expressed by astrocytes in vivo. In particular, two TSP isoforms, TSP1 and TSP2, can promote the formation of structurally normal excitatory synapses2. In addition, immunodepletion of TSP1 and TSP2 from astrocyte-conditioned medium inhibited the synapse-inducing effect of astrocytes. During development, astrocytes express high levels of TSP1 and TSP2, and mice lacking both of these proteins have significantly fewer excitatory synapses than wild-type mice do2, highlighting the crucial role of the TSPs in synapse formation in vivo. Although the expression of TSP1 and TSP2 is downregulated after the peak of synaptogenesis, the closely related protein TSP4 is expressed in the adult brain2. This raises the question of whether astrocyte-secreted TSP4 is involved in synaptogenesis in the adult brain. Interestingly, comparative gene expression profiling has revealed that TSP4 is much more highly expressed in the human brain than in the non-human primate brain34, suggesting that perhaps higher levels of TSP expression enable humans to form more synapses, thus contributing to the greater capacity of the human brain to learn and adapt (BOX 1).
Figure 1. Astrocytes instruct structural synapse formation by the secretion of several molecules.
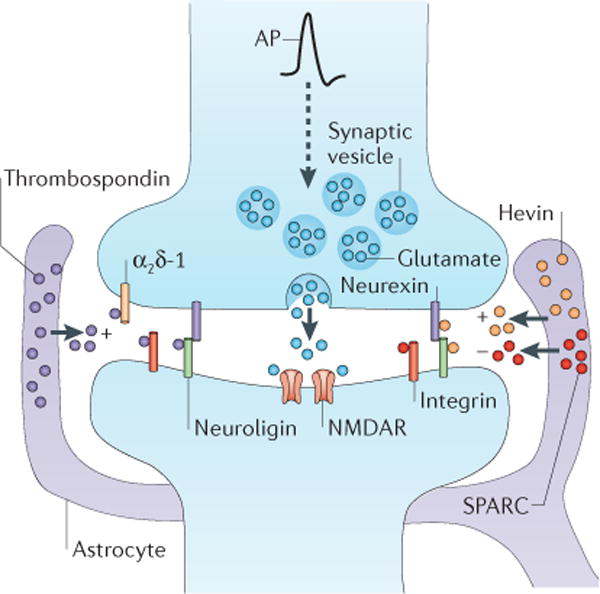
Astrocytes secrete the thrombospondins (purple circles), which are reported to interact with neuroligins, the α2δ-1 voltage-gated calcium channel subunit and integrins to induce excitatory structural synapse assembly. Astrocytes also secrete hevin (orange circles) and SPARC (red circles). Hevin promotes the formation of excitatory synapses, whereas SPARC antagonizes the synaptogenic function of hevin. Both neurexins and neuroligins have been identified as binding partners for hevin, and SPARC can bind integrins. It is thought that the thrombospondins and hevin bind to and cluster trans-synaptic adhesion molecules to induce the formation of structurally normal but postsynaptically silent excitatory synapses. The dashed arrow indicates action potential (AP) propagation, which induces glutamate release from synaptic vesicles. NMDAR, NMDA receptor.
Box 1. Astrocyte control of synapse development in humans.
The control of synapse development by astrocytes is highly conserved across all species, including humans. As discussed throughout this Review, many studies have shown that rodent astrocytes control every stage of synapse development. There are similar findings in humans, as recent studies show that human neurons generated from induced pluripotentstem cells or embryonic stem cells form few synapses unless astrocytes are present29,32. The superior cognitive abilities of the human brain have long been assumed to be the result of an evolutionary increase in brain size and the generation of new neural circuits114. However, the demonstration that astrocytes control the formation and function of synapses raises the question of whether human astrocytes have evolved a much greater ability to control the formation, function and plasticity of synapses. Consistent with this possibility, a gene-profiling study of mouse and human brains suggested that the glial transcriptomes might have evolutionarily diverged more than the neuronal transcriptomes between the two species115. In addition, a recent study comparing gene expression in the cerebral cortices of humans and non-human primates found only two genes that were much more highly expressed in the human brain. Interestingly, one of these was thrombospondin34, which encodes an astrocyte-secreted protein that strongly promotes synapse formation. Finally, a recent study compared the synaptogenic effects of rodent and human astrocytes and found that human astrocytes were able to induce the formation of a larger number of synapses in rodent neurons than could rodent astrocytes29. Future studies comparing the transcriptomes of human and mouse astrocytes and investigating the molecules released from human astrocytes might provide new insights into how human astrocytes control synapse formation and function, and whether differences between human astrocytes and those of other species contribute to the much greater capacity of the human brain to learn and adapt.
How do the TSPs promote the formation of neuronal synapses? One of the neuronal receptors for the TSPs is the α2δ-1 subunit of the voltage-gated calcium channel35. Binding of the TSPs through their type 2 epidermal growth factor-like repeats to the von Willebrand factor type A domain of the neuronal α2δ-1 receptor triggers a series of cellular events that lead to the recruitment of synaptic adhesion and scaffolding molecules to synaptic sites36. The TSPs can also interact with other synaptic molecules, including the integrins37 and neuroligins28, both of which are essential for synapse formation. Given that the TSPs can interact with many molecules at the synapse, it is possible that the TSPs might promote synapse formation by modulating several different signalling pathways. At present, the signalling pathways immediately downstream of TSP interactions with synaptic proteins remain undetermined, although protein kinase A seems to be important for these processes38.
In addition to the TSPs, astrocytes also influence excitatory synapse development by releasing hevin (also known as SPARCL1) and SPARC, which are members of the matricellular SPARC family4 (FIG. 1). Unlike TSP1 and TSP2, which are primarily expressed in the developing brain but are subsequently downregulated, astrocytes continue to express hevin and SPARC in the adult brain. Both in vitro and in vivo, hevin can promote the formation of excitatory synapses, whereas SPARC antagonizes the synaptogenic function of hevin4. As well as regulating synapse number, hevin also increases synapse size. The presynaptic and postsynaptic adhesion molecules, the neurexins and neuroligins, have been shown to interact with hevin, and knocking down neuroligin expression abolished hevin-induced synaptogenesis (S. Singh, A. Pamukcu and C. Eroglu, personal communication). These data suggest that hevin induces excitatory synapse formation by clustering trans-synaptic adhesion molecules.
Collectively, these findings indicate that the structural assembly of excitatory synapses can be mediated by different astrocyte-secreted proteins. Given the time course of the expression of the TSPs and hevin, it is possible that the TSPs have a primary role in the induction of synapses, whereas hevin might regulate the morphological maturation and maintenance of synapses.
The negative regulation of synapse formation by SPARC raises the question of whether SPARC can disassemble pre-existing synapses or cause them to revert to an immature state and thereby contribute to plasticity within neural circuits. Consistent with this idea, a recent study showed that neural activity can regulate SPARC expression by astrocytes and that SPARC-null mice have defects in synaptic plasticity39. The authors of this study proposed that SPARC interacts with integrin receptors at excitatory synapses to control the levels of AMPA receptor expression and thereby regulates the strength of excitatory synapses.
Despite the key roles of the TSPs and hevin in promoting excitatory synaptogenesis, additional astrocyte-secreted signals are required to induce synapse maturation and functionality, as synapses induced by the TSPs and hevin are structurally normal but postsynaptically silent2,4. Recent evidence suggests that astrocytes secrete additional signals to induce the insertion of AMPA receptors into the postsynaptic terminals in order to convert these immature synapses into functional mature synapses1. The roles of astrocytes in synapse maturation are discussed later.
Astrocytes control inhibitory synapse formation
During development, inhibitory synapses form before excitatory synapses, and they initially form an excitatory network that is thought to be decisive for neural circuit development (reviewed in REF. 40). As well as promoting excitatory synapse formation, astrocytes also promote the formation of inhibitory synapses. They do this by secreting molecules to regulate both presynaptic and postsynaptic differentiation. Culturing developing hippocampal neurons in the presence of astrocytes or in astrocyte-conditioned medium significantly increased the amplitude and density of type A GABA (GABAA) receptor currents41. Consistent with this finding, astrocyte-conditioned medium also increased the length and branching of GABAergic axons and the number of inhibitory synapses in hippocampal cultures3,42. Thus, soluble factors released by astrocytes can modulate presynaptic and postsynaptic differentiation and regulate the expression of GABAA receptors at developing inhibitory synapses. At present, the molecular identities of the astrocyte-secreted proteins that are responsible for promoting inhibitory synapse formation remain unknown. Interestingly, the TSPs, which strongly induce excitatory synaptogenesis, are not responsible for inducing inhibitory synaptogenesis. TSP depletion does not prevent inhibitory synaptogenesis3, suggesting that astrocytes release different molecules that independently promote excitatory or inhibitory synapse formation. Although further work is required to determine the exact mechanism by which astrocytes control inhibitory synapse formation, recent work43 has highlighted the importance of astrocyte-mediated regulation of inhibitory synaptogenesis. In this work, the authors genetically depleted specific developmental populations of astrocytes from the spinal cord and found that, in the absence of astrocytes, motor neurons had more inhibitory synapses and fewer excitatory synapses than when astrocytes were present43. This indicates that astrocytes are needed to support the appropriate formation and/or maintenance of excitatory and inhibitory synapses in the spinal cord.
Astrocyte contact controls synapse formation
So far we have focused on astrocyte-secreted signals, but other findings suggest that direct contact between astrocytes and synapses might also be important for synapse development. In one study, researchers44 investigated the effects of local astrocyte contact on synapse formation by time-lapse imaging of hippocampal neurons cultured on ‘micro-islands’ of extracellular matrix proteins with astrocytes overlaid. Local astrocyte contact increased presynaptic activity, the amplitude of excitatory postsynaptic currents and the overall number of excitatory synapses that formed among the neurons. This contact-dependent mechanism was found to be mediated by astrocytic activation of neuronal integrin receptors followed by activation of the protein kinase C signalling cascade44. In addition, contact-mediated signals from astrocytes enhanced the ability of neurons to receive synapses45. Neurons are not born with the ability to receive synapses but instead acquire this ability. Direct contact with astrocytes was sufficient to induce synaptic receptivity, and this effect was mediated by alterations in the localization of the synaptic adhesion molecule neurexin in dendrites42. However, in developing RGCs (unlike in hippocampal neurons), the protein kinase C signalling pathway was not sufficient or necessary for astrocyte-contact-mediated increases in synapse number45, suggesting that astrocytes might use different contact-mediated signals to induce synapse formation in different neurons. Future studies will be required to identify the astrocytic ligands that induce contact-mediated synapse formation. With the improvement of live imaging techniques in brain slices and in vivo, it will be particularly interesting to investigate the importance of astrocyte contact for synapse development in vivo.
Astrocyte control of synapse maturation
The TSPs and hevin control the structural formation of excitatory synapses between CNS neurons, but these synapses are postsynaptically silent because they lack AMPA receptors. Here, we review the evidence from rodent studies that astrocytes secrete specific molecules to promote synapse maturation and that astrocytic contact can promote dendritic spine maturation.
Astrocyte-secreted signals in synapse maturation
A biochemical approach has been used to identify a family of astrocyte-secreted molecules that are sufficient to induce the formation of functional excitatory synapses1. These molecules were shown to be the glypicans, which are a family of heparan sulphate proteoglycans. Glypicans are anchored to the extracellular side of the cell plasma membrane by glycosyl-phosphatidylinositol linkages, which can be cleaved by endogenous phospholipases, thus releasing the protein. The application of glypicans to purified RGCs was sufficient to increase the frequency and amplitude of glutamatergic synaptic events1. The glypican-mediated increase in synaptic activity was achieved by increasing the surface expression and clustering of the GluA1 subunit of the AMPA receptor1 (FIG. 2). Furthermore, astrocytes express glypican 4 and glypican 6 in vivo, with glypican 4 being highly expressed in the hippocampus and glypican 6 being highly expressed in the cerebellum1. The importance of the glypicans in synapse maturation has also been demonstrated in vivo. Glypican 4-deficient mice had defective synapse formation, with a marked decrease in the amplitude of excitatory postsynaptic currents in the developing hippocampus and a reduction in recruitment of AMPA receptors to synapses1. These data indicate that astrocytes release distinct factors, including the TSPs, hevin, SPARC and glypicans, with spatiotemporal specificity to control both the formation and the maturation of the neural circuitry.
Figure 2. Astrocytes secrete signals to induce synapse maturation.
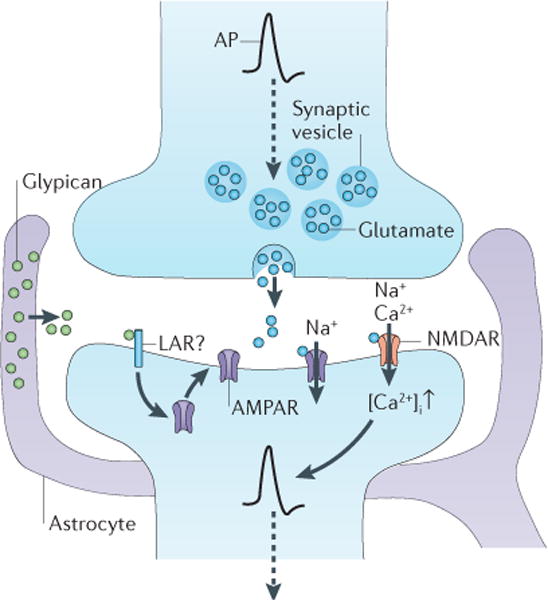
Astrocytes secrete molecules to convert silent synapses (synapses lacking AMPA receptors (AMPARs)) into functional synapses. At the resting potential, NMDA receptors (NMDARs) barely pass any current (not shown) in response to activation by glutamate (blue circles). This is because NMDARs are subject to a voltage-dependent magnesium block. Therefore, NMDARs rely on AMPAR activation to produce sufficient depolarization to allow cations to flow through the channel and to produce an excitatory postsynaptic current (which in turn generates an action potential (AP) that propagates along the axon, as indicated by the dashed arrow). Astrocytes secrete glypicans (green circles) to increase the surface levels and clustering of the GluA1 subunit of the AMPAR and thereby to induce excitatory synapse functionality. The neuronal receptor for the glypicans has yet to be identified, but studies in Drosophila melanogaster indicate that the protein tyrosine phosphatase receptor leukocyte antigen-related receptor (LAR) could be involved.
How do astrocyte-secreted glypicans convert silent synapses into functional synapses? An attractive neuronal receptor for translating the actions of the glypicans is the protein tyrosine phosphatase receptor leukocyte antigen-related receptor (LAR; also known as PTPRF). The glypicans are conserved across species, and glypican 4 is homologous to Dally-like in Drosophila melanogaster46. In the fly brain, Dally-like binds to and signals through LAR to promote the maturation of excitatory synapses47. Consistent with this idea, removal of LAR from mammalian hippocampal neurons reduced their ability to recruit AMPA receptors to synapses48. These studies provide interesting insights into how the glypicans induce synapse maturation, but further work will be required to identify the neuronal receptor for the glypicans.
Astrocytes in dendritic spine maturation
Synapse maturation involves both the recruitment of postsynaptic receptors and the stabilization of dendritic spines. Dendritic spines are highly specialized subcellular compartments that contain the excitatory postsynaptic machinery and are extremely dynamic49. Spine morphology changes markedly in response to changes in neuronal activity and environmental stimuli. For example, during long-term depression, spines shrink in size and AMPA receptors are removed from the plasma membrane, leading to a reduction in synaptic strength50. By contrast, during long-term potentiation, spines become larger and more stable and the number of AMPA receptors expressed at the synapse increases, leading to a strengthening of the synapse51. Moreover, highly motile dendritic spines are more frequently observed during development, whereas spines are thought to become more stable in the adult brain52,53. Many studies support a model in which highly motile dendritic protrusions are extended towards presynaptic boutons during synaptogenesis, and a subset of these are stabilized and converted to mature spines in the adult brain54–56.
Fine astrocyte processes wrap intimately around dendritic spines57, and this interaction is almost exclusive to spines as opposed to presynaptic terminals58. Electron microscopy studies in the hippocampus have found that approximately 60% of synapses are contacted by astrocytes59. Intriguingly, like dendritic spines, astrocytic processes display rapid remodelling behaviour60–62. In fact, fine astrocytic processes show cooperative motility with dendritic spines and can rapidly elaborate and retract their processes to change their proximity to the synapse61. Furthermore, larger and more stable spines were found to have more stable contacts with astrocytic processes61, suggesting that astrocytes might be important for the stabilization of spines.
Despite the ideal positioning of astrocytes close to dendritic spines, the detection of neuronal signals by astrocytes has been thought to be too slow to be involved in the local modulation of synaptic transmission63–65. Indeed, the calcium transients that have been reported in astrocyte processes occur on a slower timescale than fast synaptic transmission, and, typically, trains of sustained stimulation were necessary to induce these glial calcium transients63–65. However, a recent study using two-photon and confocal imaging techniques similar to those used to study synaptic activity in dendrites revealed that fine astrocyte processes can detect synaptic activity induced by single physiological synaptic stimulation in functional compartments along their processes66. Astrocytes responded to synaptic activity by local discrete calcium transients that occurred on a similar timescale to that of synaptic activity66. These data suggest that astrocytes are intimately involved with neuronal dendrites, can respond to synaptic activity and regulate synaptic transmission66. Perhaps these local calcium transients in astrocytes, which are evoked by synaptic activity, can also induce changes in the morphology of astrocytic processes to regulate their proximity to dendritic spines.
The positioning of astrocyte processes close to dendritic spines also has a crucial role in the buffering of the glutamate released at the synapse. During excitatory synaptic transmission, much of the glutamate that is released by the presynaptic terminal is taken up by astrocytic glutamate transporters67. Therefore, remodelling of astrocytic processes is likely to have a large influence on the glutamate concentration at the synaptic cleft and the size of the postsynaptic current. ‘Spillover’ of glutamate into the local area around the synapse has been reported to direct the remodelling of dendritic spines and to promote the extension of new spine heads towards neighbouring presynaptic terminals, resulting in the formation of new synapses68. Interestingly, astrocytes were found to reduce their contact with spines during spine head remodelling68. In addition, spine head remodelling was inhibited by glutamate transporter blockers, suggesting that glial control of extracellular glutamate is important for plastic changes in spine morphology68. Thus, the astrocytic control of extracellular glutamate provides another mechanism by which astrocytes can control neural circuit development.
Astrocytes might also regulate dendritic spine dynamics through receptor tyrosine kinase EPHs and their ligands, the ephrins. In the hippocampus, dendritic spines are enriched with EPH type A4 (EPHA4), and its ligand ephrin A3 is localized to the astrocytic processes that surround the spines. Activation of EPHA4 by ephrin A3 induced spine retraction, whereas inhibition of ephrin A3–EPHA4 interactions distorted spine shape and organization69. In accordance with these findings, EPHA4-knockout mice were found to have irregularities in the morphology of their dendritic spines69. Ephrin A3-knockout mice have spine irregularities similar to those observed in EPHA4-knockout mice70. Intriguingly, glutamate uptake by A3-knockout mice. Furthermore, some forms of hippocampus-dependent learning are impaired in ephrin A3-knockout mice70. This evidence suggests that the interaction between neuronal EPHA4 and glial ephrin A3 bidirectionally controls synapse morphology, glial glutamate transport and the functionality of neural circuits.
To summarize, these studies indicate that soluble and contact-mediated signals between astrocytes and neurons are important for the formation, maturation and maintenance of synaptic connections and ultimately for the development of neural circuits.
Astrocyte control of synapse pruning
To establish precise connectivity in neural networks, excessive synaptic connections are eliminated during brain development71–74. Synapse elimination is also important in the mature nervous system, in which sensory experience can drive the activity-dependent removal of synapses, and these changes might underlie the adaptive remodelling of neural circuits75. The cellular and molecular mechanisms that underlie synapse elimination are largely unknown, although neural activity has an important role73,76. Here, we review evidence that implicates astrocytes in the process of synapse elimination.
Astrocytes release synapse elimination signals
Recent studies have reported that astrocytes can control synapse elimination. Synapses in the retinogeniculate system are a well-established model system for studying synapse refinement and elimination. In this system, signals released from astrocytes induced the expression of the complement component C1q, the initiating protein in the classical complement cascade, in neuronal synapses throughout the postnatal brain77 (FIG. 3). In particular, immature astrocytes induced the expression of C1q, and C1q mRNA expression was highest in RGCs between postnatal day 5 (P5) and P10 and declined significantly by P30 (REF. 77). In accordance with this, most synapse refinement and elimination occurs before P25 in the retinogeniculate circuit78.
Figure 3. C1q-dependent elimination of synapses by glia.
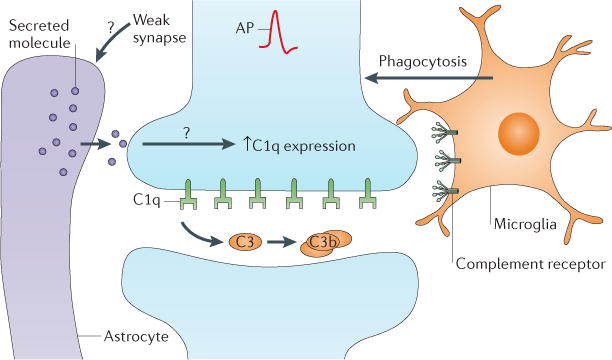
Astrocytes secrete an unidentified signal (purple) that upregulates the expression of the complement component C1q at synapses. Expression of C1q triggers the activation of downstream classical complement components including C3 and C3b. Microglia are the resident immune cells of the brain and express complement receptors. Activation of microglial complement receptors triggers the phagocytosis of synapses. Interestingly, C1q-dependent phagocytosis is regulated by neural activity and thus provides a mechanism by which weak synapses (as respresented by the red action potential (AP)) that generate weak synaptic signals can be targeted for removal during neural circuit remodelling.
In the innate immune system, the most well-described role of C1q is to opsonize unwanted debris or cells for removal by phagocytic immune cells79. Mice lacking either C1q or the downstream complement cascade protein C3 failed to execute synapse elimination, as determined by the absence of anatomical refinement of the retinogeniculate connections and by the excess of retinal innervations by lateral geniculate neurons77. Furthermore, the failure of synapse elimination in C1q-deficient mice led to enhanced synaptic connectivity and epileptic activity, indicating that this synapse elimination pathway has a crucial role in neural circuit development.
These findings raise the question of how complement-tagged synapses are eliminated from neural circuits. In the brain, microglia are the resident immune cells, and they express the receptors for C1q and C3 abundantly80. Consistent with this idea, microglia have been shown to phagocytose the synaptic terminals of motor neurons after injury81. Given that C1q functions as a molecular signal to identify cells for removal, it is possible that C1q tags weak synapses for removal by the same mechanism. This hypothesis was recently confirmed with the demonstration that microglia engulf presynaptic terminals and, in some cases, also engulf juxtaposed pre- and postsynaptic structures82. Interestingly, when neural activity was blocked, microglia preferentially engulfed the weakened inputs, suggesting that microglial pruning is regulated by neural activity82. Moreover, disruption of microglia-specific pruning resulted in sustained deficits in eye-specific segregation in vivo82. Consistent with this idea, abnormal microglia function and complement cascade activation have been associated with neurodegeneration of the CNS. In a mouse model of glaucoma, a neurodegenerative disease associated with loss of RGCs and gliosis, C1q and C3 are highly upregulated and deposited on retinal synapses, and C1q deficiency or microglial ‘inactivation’ provide significant neuroprotection77. Thus, dysfunction of microglia or complement (or both) might be directly involved in diseases associated with synapse loss, dysfunction and development. These studies suggest that astrocytes induce the expression of complement proteins to promote synaptic pruning and that this pathway has a crucial role in the development of neural circuits and in the progression of glia-mediated diseases. Future work will be required to identify the signal released from astrocytes that induces complement protein expression at synapses and to determine how complement protein expression is regulated by neural activity.
Synapse elimination by astrocytes
In addition to the elimination of unwanted synapses by microglia, there is growing evidence that astrocytes might also be involved in the phagocytosis of neuronal debris and synapses in the brain. Phagocytic activity by astrocytes has been reported in response to trauma83,84 or glioma85 and during developmental axon death86. For example, astrocytes actively collect and engulf whole dead cells in an in vitro model of brain injury87. Surprisingly, astrocytes also show phagocytic activity in the healthy, uninjured brain. In particular, astrocytes in the optic nerve head constitutively phagocytose axons and organelles88. The astrocytes in this region expressed the phagocytosis-related gene Mac-2 (also known as Lgals3). Moreover, expression of Mac-2 in astrocytes in the optic nerve head was upregulated in response to injury and in mouse models of glaucoma88, indicating that this pathway might be responsible for the removal of axons in neurodegenerative diseases such as glaucoma.
Gene-profiling studies indicate that astrocytes express a plethora of genes that have been implicated in the processes of engulfment and phagocytosis89–91. These genes fall into three redundant pathways that activate phagocytosis92. The first pathway includes the genes encoding CRKII, dedicator of cytokinesis protein 1 (DOCK1), engulfment and cell motility protein (ELMO) and RAC1, which control the rearrangement of the actin cytoskeleton that is required for a phagocytic cell to surround the cell debris93,94. A recent study has shown that astrocytes also express the brain-specific angiogenesis inhibitor 1 (BAI1) phagocytic receptor95, which acts upstream of the CRKII, DOCK1, ELMO and RAC1 components96. The second pathway includes the c-Mer tyrosine kinase receptor (MERTK), which interacts with the αVβ5 integrin pathway to regulate the CRKII, DOCK1 and RAC1 signalling molecules97. Astrocytes also express a separate RAC1-independent pathway consisting of multiple epidermal growth factor-like domains 10 (MEGF10; Caenorhabditis elegans homologue CED-1 and D. melanogaster homologue Draper), ATP-binding cassette A7 (ABCA7; C. elegans homologue CED-7) and GULP (also known as GULP1; C. elegans homologue CED-6), which is thought to participate in the recognition and engulfment of cellular debris87,98. The expression of these phagocytic proteins in astrocytes suggests that astrocytes could act as phagocytes in the brain. Thus, like microglia, astrocytes might participate in synapse elimination to sculpt neural circuits during development. However, further work will be required to investigate the function of these phagocytic pathways in astrocytes, the extent to which astrocytes are phagocytic in vivo and whether astrocytes eliminate synapses in the brain. Furthermore, it will be interesting to assess whether these different phagocytic pathways synergize or independently mediate the clearance of specific types of debris. It may be that these different pathways aid the coordination of the elimination process and provide molecular specificity in recognizing target synapses for elimination. These are important questions that need to be addressed in future studies.
Implications for human health and disease
A greater understanding of human astrocyte function might provide a foundation for investigations into pathological changes in astrocytes in human neurodevelopmental and psychiatric disorders such as autism, schizophrenia, anxiety disorder and depression. An emerging theme from recent research is that autism and schizophrenia are diseases of synapses12,18. Given that astrocytes are so intimately associated with synapses and can control their formation, function, elimination and plasticity, it is possible that the dysfunction of astrocytes could contribute to the pathophysiology of these diseases. Indeed, analyses of human neurodevelopmental diseases with known genetic mutations (in particular, Rett syndrome, fragile X syndrome and Down’s syndrome) are beginning to reveal that astrocyte dysfunction contributes to the disease pathology.
Rett syndrome is an X-linked neurodevelopmental disorder caused by the loss of the transcriptional repressor methyl-CpG-binding protein 2 (MECP2). The clinical features of Rett syndrome include autism, respiratory abnormalities, cognitive impairment and a decrease in brain weight and volume99. MECP2 expression has been detected in both neurons100,101 and astrocytes102. Interestingly, a recent study found that wild-type hippocampal neurons cultured with astrocytes from MECP2-deficient mice had abnormally stunted dendrites102, suggesting that MECP2-deficient astrocytes impair neuronal outgrowth and dendrite development. Moreover, conditional reactivation of MECP2 in astrocytes partially rescued dendrite development in MECP2-deficient mice in vivo21. These findings indicate that the loss of MECP2 function in astrocytes contributes to the developmental defects in neurons in the mouse model of Rett syndrome. Furthermore, they raise the question of whether MECP2 could control the transcription of genes that encode synaptogenic astrocyte-secreted molecules (such as the TSPs, hevin or glypicans). In this way, the loss of MECP2 could impair the expression of synaptogenic astrocyte-secreted molecules and lead to the deficits in dendrite development that is found in Rett syndrome.
Fragile X syndrome is a common cause of inherited mental retardation and is caused by a mutation in fragile X mental retardation 1 (FMR1). FMR1 encodes the RNA-binding protein fragile X mental retardation protein (FMRP), which is localized to synapses, where it associates with mRNA and polyribosomes. FMRP has been reported to have many physiological functions, including the regulation of local protein translation to control synapse development, plasticity and elimination (reviewed in REF. 103). More recently, FMRP has been shown to have translation-independent functions: for example, it has been shown to regulate neurotransmitter release by modulating the action potential duration104.
Patients with fragile X syndrome have cognitive impairment, autistic characteristics, epilepsy and motor abnormalities105. In most patients, polyglutamine repeats in FMR1 lead to the loss of FMRP, and this can be recapitulated by knocking out Fmr1 in mice. Hippocampal neurons grown in the presence of Fmr1-deficient astrocytes show abnormal dendritic morphology and reduced synapse density20. Interestingly, normal astrocytes could prevent the development of abnormal dendrite morphology and the reduction in synapses in neurons from the Fmr1-deficient mice20. Thus, astrocytes contribute to abnormal dendrite and synapse development in Fmr1-deficient mice.
Down’s syndrome, which is caused by triplication of chromosome 21 (trisomy 21), is the most common genetic cause of mental retardation. The cognitive deficits in patients with Down’s syndrome have been associated with structural changes in the architecture and alterations in the number of dendritic spines106. Hippocampal neurons grown in the presence of astrocytes from patients with Down’s syndrome have abnormal spine development and reduced synaptic density and activity107. This study identified TSP1 as the key astrocyte-secreted factor that modulates spine number and density. Furthermore, TSP1 levels were markedly reduced in astrocytes from patients with Down’s syndrome. The restoration of TSP1 levels rescued the spine and synaptic defects found in hippocampal neurons cultured in the presence of Down’s syndrome astrocytes107. These results indicate that astrocyte-secreted molecules contribute to the synaptic defects in Down’s syndrome and raise the question of whether TSP1 could be used to treat these synaptic defects.
It is notable that the clinical features of both Rett syndrome and fragile X syndrome often include epilepsy108 and autism105. Although the molecular mechanisms that underlie epilepsy and autism are poorly understood, it is becoming clear that both of these diseases are associated with abnormal synapse development, elimination and function and an impairment of the excitatory–inhibitory balance12,14,109. Taken with the evidence that astrocytes strongly control synapse formation and the in vitro evidence for the role of astrocytes in Rett syndrome and fragile X syndrome, this raises the question of whether abnormal astrocyte development could contribute to the pathogenesis of these common neurodevelopmental diseases. Consistent with this idea, the expression of some of the genes that are thought to be associated with autism, such as 4-aminobutyrate aminotransferase (ABAT)110, fatty acid-binding proten 7 (FABP7)111 and glutathione S-transferase μ1 (GSTM1)112, is highly enriched in astrocytes89. Thus, these results show that astrocytes might have an underappreciated role in many neurodevelopmental diseases and raise the question of whether targeting astrocytes might lead to the development of new therapeutic agents for these diseases.
Conclusions and future perspectives
Astrocytes can no longer be thought of as passive support cells. Over the past 10 years, many compelling lines of evidence have shown that astrocytes powerfully control every stage of synapse formation, maturation and elimination to support the development and maintenance of neural circuits.
One important area for future investigation is to determine how specific astrocyte-secreted signals interact with the neuronal signalling pathways that regulate synapse development and plasticity. Furthermore, advances in our understanding of how astrocytes respond to neuronal activity to regulate the release of secreted signals should improve our understanding of the molecular mechanisms that control synapse formation and function.
Another exciting area of research will be the study of human astrocytes to determine how they differ from their rodent counterparts. An understanding of the unique characteristics of human astrocytes might provide a new insight into the basis of human cognition. This molecular insight might also further our understanding of the pathological changes in astrocytes that lead to several neurodevelopmental disorders.
As astrocytes are so essential to the normal structure and function of synapses, it has been difficult to dissect their role in synapse development and function in vivo. However, with the identification of astrocyte-specific markers89 and the molecules they release, the advent of optogenetics and improvements in imaging techniques, it might now be possible to manipulate astrocytes in specific brain regions and at different times during development in vivo. Furthermore, advances in cell culture techniques113 and induced pluripotent stem cell technology32 will allow researchers to purify astrocytes and neurons from patients with neurodevelopmental disorders. These studies might shed new light on the contributions made by astrocytes to the formation of neural circuits in both healthy and diseased human brains.
Glossary
- Silent synapses
Excitatory synapses whose postsynaptic membranes contain NMDA-type glutamate receptors but not AMPA-type glutamate receptors, rendering the synapses inactive under normal physiological conditions
- Type 2 epidermal growth factor-like repeats
Evolutionarily conserved protein sequences that are found in the extracellular domains of several membrane-bound or secreted proteins. Epidermal growth factor-like domains are frequently found in numerous tandem copies of proteins, and these repeats fold together to form a single functional unit
- Von Willebrand factor type A domain
A domain in the large human multimeric von Willebrand factor glycoprotein that is found in blood plasma. This domain is found in various plasma and extracellular proteins and allows these proteins to form multiprotein complexes to participate in numerous biological events (for example, cell adhesion, migration and signal transduction)
- Matricellular SPARC family
Matricellular glycoproteins that modulate the interaction of cells with the extracellular matrix primarily through regulation of cell adhesion, cell proliferation and matrix deposition
- Glycosyl-phosphatidylinositol linkages
Post-translational lipid modifications that are found in a diverse range of proteins. The glycosyl-phosphatidylinositol domain serves to anchor these proteins to the membrane. Glycosyl-phosphatidylinositol proteins can be released from the cell membrane upon cleavage by endogenous phospholipases
- Ephrins
Ephrins are ligands that bind to the EPH receptors. Both ephrins and EPH receptors are membrane-bound proteins, so activation of EPH–ephrin signalling pathways can only occur through direct cell–cell contact. Ephrin signalling regulates various biological processes, including the guidance of axon growth cones and cell migration
- Classical complement cascade
This system amplifies the immune response to aid the clearance of pathogens from an organism. The complement system consists of many small proteins found in the blood. When stimulated, proteases in this system cleave proteins to release cytokines and initiate a cascade of further cleavages to amplify the immune response
- Opsonize
To tag a pathogen by the binding of an antibody (or opsonin) to target it for removal by a phagocytic cell
- Polyglutamine repeats
Expanded runs of consecutive trinucleotide CAG repeats, which encode polyglutamine. These repeats are found in the genes of a large number of patients with neurodegenerative diseases such as Huntington’s disease, fragile X syndrome and ataxia. Polyglutamine repeats are thought to cause protein aggregation, which is a key feature of these diseases
Footnotes
Competing interests statement
B.A.B. declares competing financial interests. See Web version for details. L.E.C. declares no competing financial interests.
References
- 1.Allen NJ, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. This study identifies a novel family of astrocyte-secreted proteins that recruit glutamate receptors to excitatory synapses to induce synapse maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. This study identifies a novel family of astrocyte-secreted proteins that promotes the formation of excitatory synapses. [DOI] [PubMed] [Google Scholar]
- 3.Hughes EG, Elmariah SB, Balice-Gordon RJ. Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol Cell Neurosci. 2010;43:136–145. doi: 10.1016/j.mcn.2009.10.004. This study demonstrates that in addition to releasing molecules that regulate excitatory synaptogenesis, astrocytes release different molecules to control inhibitory synaptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucukdereli H, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA. 2011;108:e440–e449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. References 5 and 6 were the first studies to show that astrocytes can induce and control the number of excitatory synapses by secreting soluble signals. [DOI] [PubMed] [Google Scholar]
- 6.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 7.Pascual O, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 8.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, et al. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 11.Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett MR. Synapse formation and regression in the cortex during adolescence and in schizophrenia. Med J Aust. 2009;190:S14–S16. doi: 10.5694/j.1326-5377.2009.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 14.Berkel S, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nature Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 15.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 17.Irwin SA, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Penzes P, Cahill ME, Jones KA, Vanleeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nature Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs S, Doering LC. Astrocytes prevent abnormal neuronal development in the fragile X mouse. J Neurosci. 2010;30:4508–4514. doi: 10.1523/JNEUROSCI.5027-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lioy DT, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. This study shows that astrocytes are integral components of Rett’s syndrome and that the restoration of MECP2 in astrocytes can rescue abnormal dendrite morphology and function in vivo, supporting the idea that targeting of glia might be a new strategy for treating neurodevelopmental diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiacco TA, McCarthy KD. Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia. 2006;54:676–690. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- 26.Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 27.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nature Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- 29.Diniz LP, et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem. 2012;287:41432–41445. doi: 10.1074/jbc.M112.380824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol Cell Neurosci. 2004;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Buard I, Steinmetz CC, Claudepierre T, Pfrieger FW. Glial cells promote dendrite formation and the reception of synaptic input in Purkinje cells from postnatal mice. Glia. 2010;58:538–545. doi: 10.1002/glia.20943. [DOI] [PubMed] [Google Scholar]
- 32.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nature Biotech. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Preuss TM, Caceres M, Oldham MC, Geschwind DH. Human brain evolution: insights from microarrays. Nature Rev Genet. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- 35.Eroglu C, et al. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigrist SJ, Plested AJ. How to button a bouton with α2δs. Nature Neurosci. 2009;12:1357–1358. doi: 10.1038/nn1109-1357. [DOI] [PubMed] [Google Scholar]
- 37.DeFreitas MF, et al. Identification of integrin α3β1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron. 1995;15:333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 38.Crawford DC, Jiang X, Taylor A, Mennerick S. Astrocyte-derived thrombospondins mediate the development of hippocampal presynaptic plasticity in vitro. J Neurosci. 2012;32:13100–13110. doi: 10.1523/JNEUROSCI.2604-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones EV, et al. Astrocytes control glutamate receptor levels at developing synapses through SPARC-β-integrin interactions. J Neurosci. 2011;31:4154–4165. doi: 10.1523/JNEUROSCI.4757-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 41.Liu QY, Schaffner AE, Li YX, Dunlap V, Barker JL. Upregulation of GABAA current by astrocytes in cultured embryonic rat hippocampal neurons. J Neurosci. 1996;16:2912–2923. doi: 10.1523/JNEUROSCI.16-09-02912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai HH, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. The authors use genetic approaches to demonstrate that astrocytes are allocated to distinct spatial domains and that the ablation of specific astrocyte populations results in abnormal synaptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- 45.Barker AJ, Koch SM, Reed J, Barres BA, Ullian EM. Developmental control of synaptic receptivity. J Neurosci. 2008;28:8150–8160. doi: 10.1523/JNEUROSCI.1744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ybot-Gonzalez P, Copp AJ, Greene ND. Expression pattern of glypican-4 suggests multiple roles during mouse development. Dev Dyn. 2005;233:1013–1017. doi: 10.1002/dvdy.20383. [DOI] [PubMed] [Google Scholar]
- 47.Johnson KG, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 48.Dunah AW, et al. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nature Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunaevsky A, Blazeski R, Yuste R, Mason C. Spine motility with synaptic contact. Nature Neurosci. 2001;4:685–686. doi: 10.1038/89460. [DOI] [PubMed] [Google Scholar]
- 53.Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 54.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 57.Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J Neurosci. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehre KP, Rusakov DA. Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J. 2002;83:125–134. doi: 10.1016/S0006-3495(02)75154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 63.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 64.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 66.Panatier A, et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. This study uses elegant imaging techniques to demonstrate that astrocytes can detect and respond to physiological synaptic stimuli. [DOI] [PubMed] [Google Scholar]
- 67.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 68.Verbich D, Prenosil GA, Chang PK, Murai KK, McKinney RA. Glial glutamate transport modulates dendritic spine head protrusions in the hippocampus. Glia. 2012;60:1067–1077. doi: 10.1002/glia.22335. [DOI] [PubMed] [Google Scholar]
- 69.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nature Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 70.Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci USA. 2009;106:12524–12529. doi: 10.1073/pnas.0903328106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 72.Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 74.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 75.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 76.Fuentes-Medel Y, et al. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 78.Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21:228–237. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perry VH, O’Connor V. C1q: the perfect complement for a synaptic feast? Nature Rev Neurosci. 2008;9:807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- 80.Gasque P, et al. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543–3554. [PubMed] [Google Scholar]
- 81.Schiefer J, Kampe K, Dodt HU, Zieglgansberger W, Kreutzberg GW. Microglial motility in the rat facial nucleus following peripheral axotomy. J Neurocytol. 1999;28:439–453. doi: 10.1023/a:1007048903862. [DOI] [PubMed] [Google Scholar]
- 82.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.al-Ali SY, al-Hussain SM. An ultrastructural study of the phagocytic activity of astrocytes in adult rat brain. J Anat. 1996;188:257–262. [PMC free article] [PubMed] [Google Scholar]
- 84.Bechmann I, Nitsch R. Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia. 1997;20:145–154. doi: 10.1002/(sici)1098-1136(199706)20:2<145::aid-glia6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 85.Lantos PL. An electron microscope study of reacting astrocytes in gliomas induced by n-ethyl-n-nitrosourea in rats. Acta Neuropathol. 1974;30:175–181. doi: 10.1007/BF00685441. [DOI] [PubMed] [Google Scholar]
- 86.Berbel P, Innocenti GM. The development of the corpus callosum in cats: a light- and electron-microscopic study. J Comp Neurol. 1988;276:132–156. doi: 10.1002/cne.902760109. [DOI] [PubMed] [Google Scholar]
- 87.Loov C, Hillered L, Ebendal T, Erlandsson A. Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS ONE. 2012;7:e33090. doi: 10.1371/journal.pone.0033090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen JV, et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci USA. 2011;108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doyle JP, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovatt D, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Kinchen JM, et al. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- 94.Ziegenfuss JS, Doherty J, Freeman MR. Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy. Nature Neurosci. 2012;15:979–987. doi: 10.1038/nn.3135. This study identifies new components of the glial engulfment machinery and shows that glial activation and phagocytosis after injury are mediated by distinct signalling pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sokolowski JD, et al. Brain-specific angiogenesis inhibitor-1 expression in astrocytes and neurons: implications for its dual function as an apoptotic engulfment receptor. Brain Behav Immun. 2011;25:915–921. doi: 10.1016/j.bbi.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 97.Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in δvβ5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- 98.Yu X, Lu N, Zhou Z. Phagocytic receptor CED-1 initiates a signaling pathway for degrading engulfed apoptotic cells. PLoS Biol. 2008;6:e61. doi: 10.1371/journal.pbio.0060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 100.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 101.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci USA. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nature Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deng PY, et al. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beckel-Mitchener A, Greenough WT. Correlates across the structural, functional, and molecular phenotypes of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10:53–59. doi: 10.1002/mrdd.20009. [DOI] [PubMed] [Google Scholar]
- 106.Benavides-Piccione R, et al. On dendrites in Down syndrome and DS murine models: a spiny way to learn. Prog Neurobiol. 2004;74:111–126. doi: 10.1016/j.pneurobio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Garcia O, Torres M, Helguera P, Coskun P, Busciglio J. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down’s syndrome. PLoS ONE. 2010;5:e14200. doi: 10.1371/journal.pone.0014200. This study shows that astrocytic dysfunction can contribute to the synaptic defects that are found in Down’s syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hagerman PJ, Stafstrom CE. Origins of epilepsy in fragile X syndrome. Epilepsy Curr. 2009;9:108–112. doi: 10.1111/j.1535-7511.2009.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barnby G, et al. Candidate-gene screening and association analysis at the autism-susceptibility locus on chromosome 16p: evidence of association at GRIN2A and ABAT. Am J Hum Genet. 2005;76:950–966. doi: 10.1086/430454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maekawa M, et al. Polymorphism screening of brain-expressed FABP7, 5 and 3 genes and association studies in autism and schizophrenia in Japanese subjects. J Hum Genet. 2010;55:127–130. doi: 10.1038/jhg.2009.133. [DOI] [PubMed] [Google Scholar]
- 112.Ming X, et al. Genetic variant of glutathione peroxidase 1 in autism. Brain Dev. 2010;32:105–109. doi: 10.1016/j.braindev.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 113.Foo LC, et al. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller JA, Horvath S, Geschwind DH. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc Natl Acad Sci USA. 2010;107:12698–12703. doi: 10.1073/pnas.0914257107. This study compares human and mouse transciptomes and finds that glial transciptomes might have evolutionarily diverged more than neuronal transcriptomes between these two species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gomez-Casati ME, et al. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci USA. 2010;107:17005–17010. doi: 10.1073/pnas.1008938107. [DOI] [PMC free article] [PubMed] [Google Scholar]


