Abstract
Background
Dual dependence on opiate and cocaine occurs in about 60% of patients admitted to methadone maintenance and negatively impacts prognosis (Kosten et al., 2003). Topiramate (TOP) is an antiepileptic drug that may have utility in the treatment of cocaine dependence because it enhances the GABAergic system, antagonizes the glutamatergic system, and has been identified by NIDA as one of only a few medications providing a “positive signal” warranting further clinical investigation. (Vocci and Ling, 2005).
Method
In this double-blind controlled clinical trial, cocaine dependent methadone maintenance patients (N=171) were randomly assigned to one of four groups. Under a factorial design, participants received either TOP or placebo, and monetary voucher incentives that were either contingent (CM) or non-contingent (Non-CM) on drug abstinence. TOP participants were inducted onto TOP over 7 weeks, stabilized for 8 weeks at 300 mg daily then tapered over 3 weeks. Voucher incentives were supplied for 12 weeks, starting during the fourth week of TOP induction. Primary outcome measures were cocaine abstinence (Y/N) as measured by thrice weekly urinalysis and analyzed using Generalized Estimating Equations (GEE) and treatment retention. All analyses were intent to treat and included the 12-week evaluation phase of combined TOP/P treatment and voucher intervention period.
Results
There was no significant difference in cocaine abstinence between the TOP vs P conditions nor between the CM vs Non-CM conditions. There was no significant TOP/CM interaction. Retention was not significantly different between the groups.
Conclusion
Topiramate is not efficacious for increasing cocaine abstinence in methadone patients.
Keywords: Cocaine Dependence, antiepileptic, topiramate, glutamate, GABA, contingency management
1. INTRODUCTION
Methadone is effective in the treatment of opioid dependence, but does not affect cocaine use, even at high doses (Castells et al., 2009). This is especially problematic in light of the fact that the majority of patients presenting for opioid maintenance treatment have concurrent cocaine dependence, which negatively impacts overall prognosis (Kosten et al., 2003). Nevertheless, effective adjunctive pharmacotherapies for cocaine dependence in this population are lacking. TOP has been identified as a strong candidate for this purpose (Vocci and Ling, 2005). In a key pilot study of relapse prevention in cocaine dependent men, TOP promoted abstinence at twice the rate of that achieved the placebo group (Kampman et al., 2004).
Topiramate [2.3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate] was FDA approved in 1996 for the treatment of adult seizures. TOP increases GABA central levels and potentiates its action at the GABAA receptor (Braga et al., 2009). TOP is an AMPA receptor antagonist at the glycine site, a selective GluK1 inhibitor (Braga et al., 2009) and decreases presynaptic glutamate release (Alves et al., 2003).
GABA antagonism and glutamate potentiation have each been identified as having potential value in the treatment of cocaine dependence. GABA agonists reduce the dopamine response to cocaine, response to conditioned cues, and cocaine self-administration (Barrett et al., 2005; Weerts et al., 2005). The presynaptic GABA reuptake inhibitor tiagabine appeared to reduce cocaine use in methadone maintenance (Gonzales et al., 2007) and vigabatrin (an irreversible inhibitor of intrasynaptic GABA transaminase) increased cocaine abstinence in parolees (Brodie et al., 2009), though a later clinical trial showed no effect (Samoza et al., 2013). Prefrontal glutamatergic neurons innervating the nucleus accumbens play a critical role in cocaine reinforcement (McFarland et al., 2003; Kalivas, 2004). Glutamate inhibition blocks cocaine-induced reinstatement of cocaine seeking (Cornish and Kalivas, 2000; McFarland et al., 2003). Glutamate is involved in memory and neuroplasticity and a disruption of glutamate homeostasis could be at the core of compulsive drug taking (Kalivas et al., 2009). In preclinical studies, antagonists at AMPA-receptors (a glutamate receptor subtype) decreased cue-induced cocaine seeking (Bäckström and Hyytiä, 2006). Drugs with potential to restore glutamate homeostasis show promise as potential treatments for cocaine addiction (Moran et al., 2005; Peters et al., 2008; Kalivas, 2009). Given the potential of medications targeting independently the GABA and glutamate systems to help patients abstain from cocaine use, targeting these systems simultaneously with a medication such as TOP is of particular interest.
In addition to its potential for directly reducing cocaine use, TOP may have broad beneficial effects that further enhance its utility in the treatment of cocaine dependent methadone patients by addressing a variety of other symptoms and conditions common among poly-substance users. For example, TOP has produced promising results in treating other substance use disorders, reducing drinking in alcohol dependence (Johnson et al., 2003, 2004; Johnson, 2005; Baltieri et al., 2008), reducing relapse to alcohol use after detoxification (Rubio et al., 2009), and promoting smoking cessation in men (Anthenelli et al., 2008). More broadly, TOP has been evaluated for the treatment of chronic pain (Khoromi et al., 2005), aggression (Nickel et al., 2004, 2005a, 2005b), compulsive disorders (Van Ameringen et al., 2006), and anxiety (Berlant, 2004; Khan and Liberzon, 2004).
The current study was designed to evaluate whether TOP would increase cocaine abstinence in cocaine dependent methadone patients. Participants were randomly assigned to one of four conditions in which they received TOP or placebo (P), and received vouchers contingent on providing cocaine negative urine samples (CM) or independently of their urine sample results (Non-CM). This design was planned as a means of comparing the effects of TOP against positive (CM) and negative (P) controls, as CM has been repeatedly demonstrated as successful in reducing cocaine use in methadone patients (Lussier et al., 2006; Prendergast et al., 2006). In all groups, voucher earnings were dependent on attendance and morning pills were consumed under observation. These procedures were designed to enhance attendance and TOP adherence across all participants. A secondary goal of the study was to evaluate the safety and acceptability of TOP in methadone patients, as there are no prior evaluations of TOP in this population.
2. METHODS
2.1 Trial Design and Study Flow
This randomized double-blind clinical trial featured a 2x2 factorial design. It was approved by the Johns Hopkins Medicine institutional review board and conducted from 2007 to 2011 at an outpatient methadone clinic on the Johns Hopkins Bayview Medical Campus, Baltimore, Maryland.
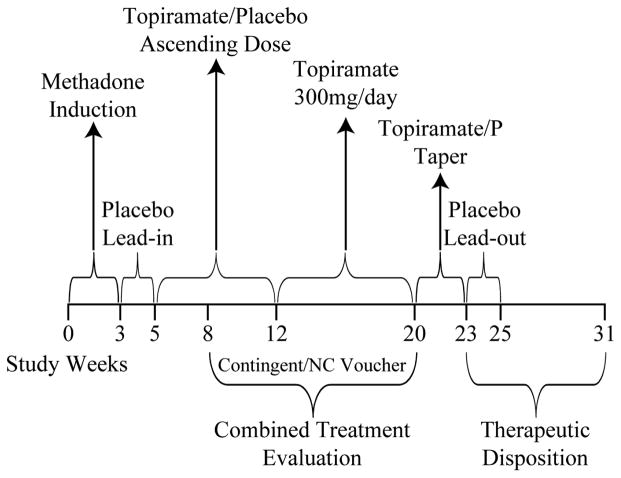
Figure 1 shows the study timeline. Opioid and cocaine dependent adults who passed a telephone screening interview were scheduled for a full interview to determine eligibility. Participants were induced and stabilized on methadone, then, during a placebo lead-in period, they received one capsule ingested with the methadone dose, followed by mouth check; a second capsule dispensed in a blister pack was given for evening ingestion, with request to return the empty blister pack the following day. At the end of week 5, participants were randomly assigned to one of 4 treatment conditions: TOP/CM, P/CM, TOP/Non-CM, and P/Non-CM. Participants and staff were blind to time of randomization and changes in medication doses. Randomization was stratified on gender, age (≤ 40 years old, Y/N), cocaine withdrawal severity (Cocaine Selective Severity Assessment [CSSA] ≤ 20, Y/N; Kampman et al., 2004), and current alcohol dependence (DSM-IV-R, Structured Clinical Inteview for DSM-IV [SCID]). Computerized stratified randomization with a 1:1:1:1 allocation ratio was implemented by staff members with no participant contact.
Figure 1.
Study Timeline
Contingency management procedures were implemented during weeks 9 through 20, which constituted the 12-week combined treatment evaluation phase. Capsules were discontinued after the topiramate taper and placebo lead-out phases. During the disposition phase, participants either continued methadone treatment at the clinic or were transferred to other community clinics of their choice.
2.2 Participants and recruitment
Participants were eligible if they were: 1) cocaine and opioid dependent and seeking treatment; 2) between 18 – 55 years old; 3) eligible for methadone maintenance; and 4) able to comply with study requirements. Participants were excluded for 1) sulfonamide or topiramate allergy; 2) diabetes, respiratory insufficiency, or other chronic risk factor for acidosis; 3) prior kidney stones, or unexplained blood in the urine; 4) current participation in Highly Active Antiretroviral Therapy; 5) glaucoma, family history of glaucoma, intraocular hypertension, or one-sided blindness; 6) seizure disorder or use of antiepileptic medications; 7) current benzodiazepine dependence; 8) serious psychiatric illness; 9) pregnancy, lactation, or sexual activity without effective contraception. Participants were recruited through flyers in local drug treatment, clinical care settings, and through advertisements in local print newspapers.
2.3 Intake and safety procedures
At intake, applicants were interviewed with the SCID and the Addiction Severity Index (ASI) to determine eligibility, urine samples were obtained for toxicology and urinalysis testing, and blood samples were tested for CBC, chemistry (liver and kidney function, amylase, lipase) and optional HIV testing. Medical evaluation was conducted, including EKG, visual acuity with corrected vision, and contact tonometry (Reichert Tonopen XL, Depew, NY) to measure intraocular pressure (IOP). Visual acuity and tonometry were repeated twice prior randomization and twice in the weeks after randomization to detect any change in IOP. Ongoing patient assessment was done with repeat chemistry, urinalysis, EKG, clinician-CSSA, and adverse event assessment, collected monthly.
2.4 Interventions
2.4.1 Methadone Maintenance
Methadone (methadose 10 mg/mL, Mallincrodt, Inc., Hobart, N.Y.) was administered daily by nursing staff via automated pump system. Doses started at 30 mg/day and inducted over three weeks to a maintenance dose (median 100 mg/day; range 20 – 140 mg/day). Doses for patients transferring from methadone maintenance were continued. At any time participants could request a dose decrease, and a dose increase could be requested after week seven. Take home doses were provided on holidays and for rare emergencies. Individual and group counseling was provided weekly, based on a manualized staged level of treatment. Subjects could be administratively discharged for violating clinic rules or missing three consecutive clinic visits.
2.4.2 Topiramate (TOP)
All study capsules were prepared at the on-site research pharmacy from bulk topiramate (LGM Pharmaceuticals, Inc., Boca Raton, FL), and lactose monohydrate powder, N.F. as filler (Ruger Chemical Co., Inc., Portland, OR). Lactose was premixed with 5 PPM denatonim benzoate (Bitrex®, Market Actives, LLC, Portland, OR) to give a similar bitter taste to all capsules. Each day’s study medication dose consisted of two size 0 opaque capsules of equal weight and appearance. Active capsules consisted TOP loose filled and supplemented with lactose powder to yield a total of 550 mg of ingredient and were prepared less than 60 days prior to ingestion. P capsules consisted of 550 mg lactose powder.
Placebo participants received P capsules until the end of week 25 placebo lead-out. Participants randomized to TOP received ascending TOP doses weeks 6 to 12, 300 mg/day weeks 13 to 20, then tapering doses weeks 21 to 23. The maintenance dose of TOP used in this study (300 mg/day) is considered to be on the low end of the normal efficacious range when used as an antiepileptic, and adverse events have been relatively rare in previous clinical trials for doses under 400 mg/day (LaRoche and Helmers, 2004). The schedule of TOP administration is in table 1. Patients, clinic and medical staff were blind to TOP/P conditions and to methadone doses.
Table 1.
TOPIRAMATE DOSE SCHEDULE (mg)
| Treatment Week | Topiramate Groups | |
|---|---|---|
| Morning dose (DOT) | Evening dose | |
| 4 – 5 | 0 | 0 |
| 6 | 25 | 0 |
| 7 | 25 | 25 |
| 8 | 50 | 25 |
| 9 | 50 | 50 |
| 10 | 75 | 75 |
| 11 | 100 | 100 |
| 12 | 125 | 125 |
| 13 – 20 | 150 | 150 |
| 21 | 100 | 100 |
| 22 | 50 | 50 |
| 23 | 25 | 25 |
| 24 – 25 | 0 | 0 |
DOT: Directly Observed Treatment
Participants in the placebo groups received 2 daily placebo capsules weeks 4 – 25.
2.4.3 Contingency Management
Vouchers of escalating monetary value could be earned in exchange for approved goods and services. Vouchers could be exchanged in minimum units of $25 and participants’ requests for exchanges were fulfilled within one week. Subjects in the CM groups received vouchers for recent cocaine abstinence determined by semi-quantitative urine toxicology testing (details below). The first cocaine negative urine earned a $2.50 voucher. The voucher value increased by $1.50 for each subsequent cocaine negative urine sample. A bonus of $10.00 was awarded for every three consecutive cocaine negative urine samples. A cocaine-positive or a missed scheduled urine sample resulted in no voucher. Two forfeited vouchers (for missing or cocaine positive) resulted in a resetting of the voucher value to $2.50 for the next cocaine negative sample. Perfect abstinence yielded a total payout of $1,155 in vouchers.
Vouchers for each non-CM participant were yoked to the values of a CM participant, and corresponded to the particular urine sample (e.g., urine #14 of 36). Non-CM participants were required to attend the clinic and provide a urine sample, but vouchers were delivered independent of cocaine toxicology results. Participants were instructed and quizzed on all aspects of the voucher procedures as appropriate for the CM and non-CM groups, respectively, but were not aware of the yoking procedure (Silverman et al., 1996b). The yoking procedures were designed to isolate the effects of the abstinence reinforcement contingency while promoting retention and adherence across all groups.
2.5 Assessments
2.5.1. Urine toxicology
Urine samples were collected under staff observation three times a week and tested onsite for cocaine metabolites (benzoylecgonine) using Syva 30R Chemical Batch Analyzer (Syva Corp., Palo Alto, CA) and with a threshold for a cocaine positive result of > 300 ng/ml. During the CM intervention (weeks 9–20), serial dilutions of qualitative positive tests were conducted to derive quantitative estimations of benzoylecgonine concentrations. Samples were coded as negative if benzoylecgonine concentration was below the 300 ng/ml cut-off or ≤50% that of the preceding sample (a modified Preston rule for abstinence, Silverman et al., 1996a). Urine samples were also tested qualitatively for opiates three times weekly and benzodiazepines once weekly.
Breath analysis for alcohol ([BAL; Alco-Sensor III, Intoximeters, Inc., Saint Louis, MO) was collected prior to methadone dosing on urine collection days. Breath was also analyzed for exhaled CO once a week (C50 Micro III Smokerlyzer, Bedfont Scientific Ltd., Kent, UK) to assess smoking status.
2.5.2 Self-Report Measures
Participants completed computerized assessments once a week: the CSSA, a measure of cocaine craving (score 0 – 112; Mulvaney et al., 1999), daily illegal drug and alcohol use, smoking behaviors (TLFB), dose adequacy for methadone and medications, Visual Analog Pain scale (VAS 1–10), the Wong-Baker Pain scale, and a medication symptom checklist (listing common side effects). Participants also completed the Beck Depression Inventory (BDI 1A; Beck et al., 1961) and State Trait Anxiety Inventory (STAI; Spielberger, 1983) on a biweekly basis. On week 4, patients were assessed for traumatic events using the Traumatic Life Event Questionnaire (TLEQ; Kubany et al., 2000) and monthly with the Modified PTSD Symptom scale revised (MPSS-R; Coffey et al., 1998).
2.6 Outcome measures
The primary outcome measures were cocaine abstinence, a dichotomous variable (Y/N) for each or the 36 samples collected between weeks 9 and 20, and coded as specified in section 2.8.1., and treatment retention, operationalized as the number of weeks between study admission and discharge.
Secondary outcome measures included the percentage of participants achieving three consecutive weeks of cocaine abstinence, the longest duration of cocaine abstinence (LDCA), percent of participants with consecutive abstinence based on combination of self-report, urine toxicology and no missing data; adverse events; opioid use based on urine toxicology testing; weekly self-reports of cocaine, alcohol, cigarette and other drug use (TLFB); cocaine craving (CSSA); depression, anxiety and pain symptoms; measures of cigarette smoking (CO in exhaled breath, Fagerstrom, smoking urges).
2.7 Data analysis
The statistical analyses were conducted in SAS 9.3 (SAS Institute, Inc., Cary, NC). All analyses were intent-to-treat (N = 171). Statistical significance was defined as p < .05. The four groups were compared for differences on baseline characteristics and baseline cocaine use using ANOVA for continuous measures and Chi-square tests for dichotomous characteristics. The four stratification variables used in the randomization process (age, sex, CSSA, alcohol dependence) were also used as covariates in all analyses. Pearson correlations were calculated between baseline proportion of cocaine negative urine samples during weeks 1 – 5 and cocaine abstinence during the combined treatment phase (weeks 9 – 20). As baseline cocaine use was found to be a predictor of treatment outcome, the analyses were repeated using baseline cocaine use as covariate.
Toxicology results were analyzed as repeated measures of binary outcomes (e.g., Y/N) by using Generalized Estimating Equations (GEE) with an autoregressive correlation structure (Zeger and Liang, 1992). Separate analyses were conducted in which missing samples were counted as positive or were ignored, respectively. A sensitivity analysis was also conducted in which we imputed data for all drop-outs as relapse to each participant’s baseline cocaine use (Johnson et al., 2013), but the results of these analyses were nearly identical to the results of the other analyses and are not presented here.
GEE was also used to analyze secondary measures such as CSSA composite scores, BDI-II scores, STAI, MPSS-R and longest duration of continuous cocaine abstinence. Retention in treatment was analyzed using a Cox proportional hazards model. For all of these tests, we evaluated the main effects for TOP and CM respectively. We also made planned post-hoc comparisons of the following study groups: TOP/CM versus P/CM; TOP/CM versus TOP/Non-CM; TOP/Non-CM versus P/Non-CM, and P/CM versus P/Non-CM.
3. RESULTS
3.1 Baseline demographic, treatment and drug use characteristics
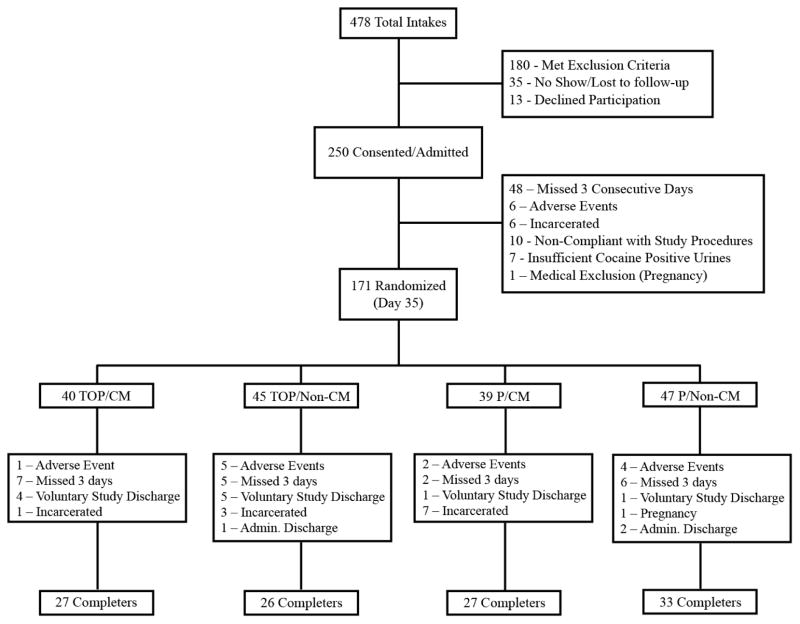
Figure 2 displays subject disposition through the end of the evaluation phase of the trial. No demographic differences were significant under a one factor analysis or for any post-hoc comparison between groups. A two-factor analysis showed that participants who received P were more likely to be alcohol dependent that those who received TOP (p=.03), but revealed no other significant differences. Of 250 participants admitted into the study, 79 (32%) dropped out prior to randomization. Treatment groups were balanced: mean age (± SD) was 42 ± 7 years old, 60% were African American, 48% were females, 58% completed 12th grade, 26% were employed, and 13% were married. Median CSSA score was 28, suggesting a high degree of severity in cocaine dependence among study participants, with no differences in baseline cocaine craving (CSSA > 20) scores between the groups. Twenty-one percent of participants were already engaged in methadone maintenance prior to study entry: 33% T/CM, 20% T/Non-CM, 18% P/CM, 15% P/Non-CM (p = .45).
Figure 2.
Consort Diagram
3.2 Primary Outcomes
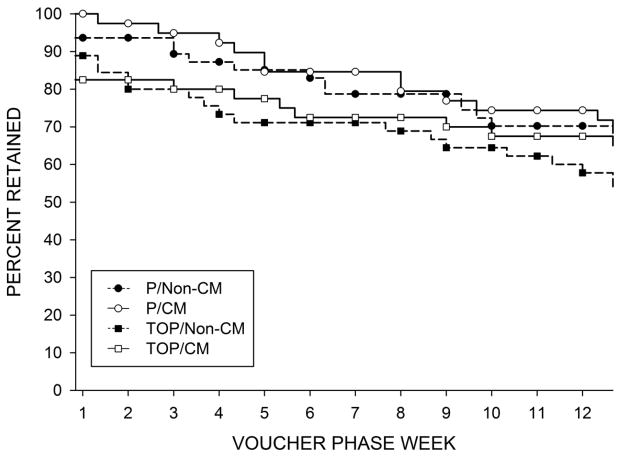
3.2.1. Treatment Retention (Figures 2 and 3)
Figure 3.
Retention during the combined treatment evaluation phase
Fifteen participants (9%, 12 in the TOP groups, and three in the P/Non-CM group) dropped out before starting the combined treatment evaluation phase. Of the subjects who initiated the combined treatment evaluation phase of the study, 113/156 (72%) completed with no significant group difference: TOP/CM 68%, TOP/Non-CM 58%, P/CM 69%, P/Non-CM 70%, p = .44. Length of treatment (weeks ± SE) was 16.9 ± 0.8 for the TOP/CM group, 16.4 ± 0.7 for the TOP/Non-CM group, 18.1 ± 0.6 for the P/CM group and 17.7 ± 0.6 for the P/Non-CM group.
3.2.2 Cocaine Abstinence
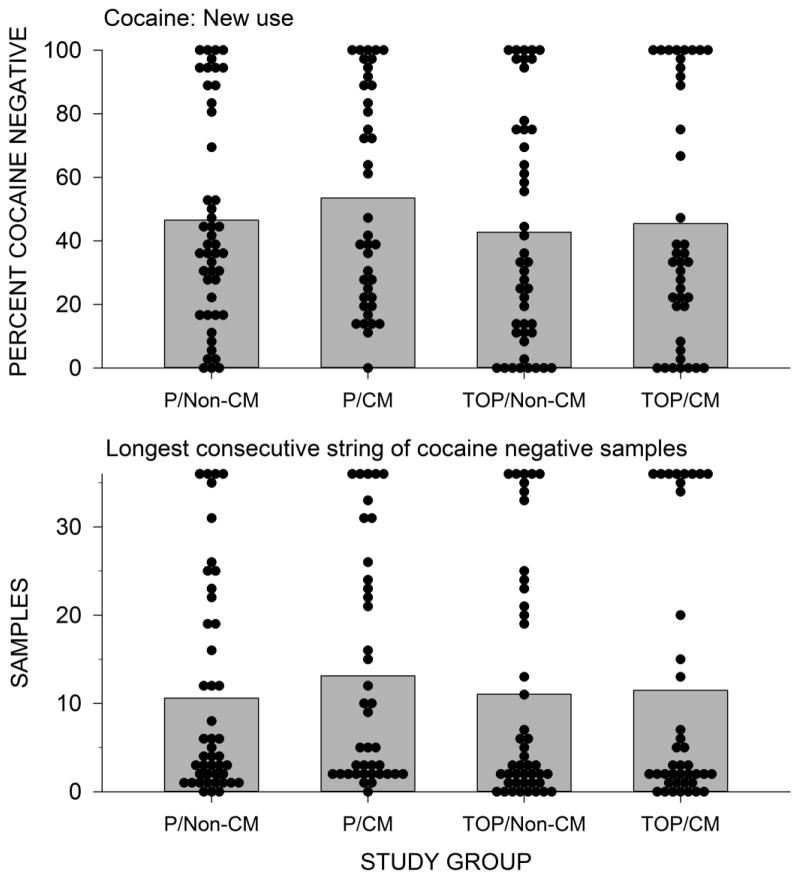
Figure 4 shows the percentage of cocaine negative urine samples (Preston rule) per treatment condition (mean % ± SE): 45 ± 6.2 for TOP/CM, 43 ± 5.5 for TOP/Non-CM, 54 ± 5.4 for P/CM, 47 ± 4.8 for P/Non-CM (p=.54, OR 0.87, 95%CI: 0.58 – 1.34). Using a benzoylecgonine cut-off ≤ 300 ng/mL, the percentages of cocaine negative urine samples were 34 ± 7 for TOP/CM, 34 ± 6 for TOP/Non-CM, 41 ± 6.6 for P/CM and 34 ± 5.7 for P/Non-CM (p = 0.86, OR: 1.051, 95%CI: 0.6 – 1.84). Excluding missing samples from the analysis, the percentage of cocaine negative urine samples were 59 ± 5.2, 59 ± 5.4, 63 ±: 5.1, 58 ± 4.7, respectively (p = 0.78, OR: 1.06, 95% CI: 0.7 – 1.6). Repeating the analysis imputing relapse to baseline cocaine use for drop offs did not change the results of the analysis. Including stratification variables and baseline cocaine use as covariates did not change the results. The LDCA in weeks was (mean ± SE) 3.8 + 0.8 for TOP/CM, 3.7 ± 0.7 for TOP/Non-CM, 4.4 ± 0.7 for P/CM, for 3.5 ± 0.6 P/Non-CM. Statistical analyses revealed no main effect of TOP or CM on LDCA, and no post-hoc comparisons were significant. Figure 5 shows the weekly cumulative percent of patients achieving biochemically confirmed self-reported abstinence with no missing samples, which is the definition of abstinence endorsed by the FDA, with no significant differences between the groups.
Figure 4.
Upper panel: Percent cocaine negative urine samples per participant by treatment groups (modified Preston rule). Dots represent the percentage of cocaine negative urine samples for individual participants. The bars represent the mean proportion of cocaine negative urine samples for the group. Lower panel: Longest duration of cocaine abstinence by treatment groups as shown by the number of consecutive cocaine negative urine samples
Figure 5.
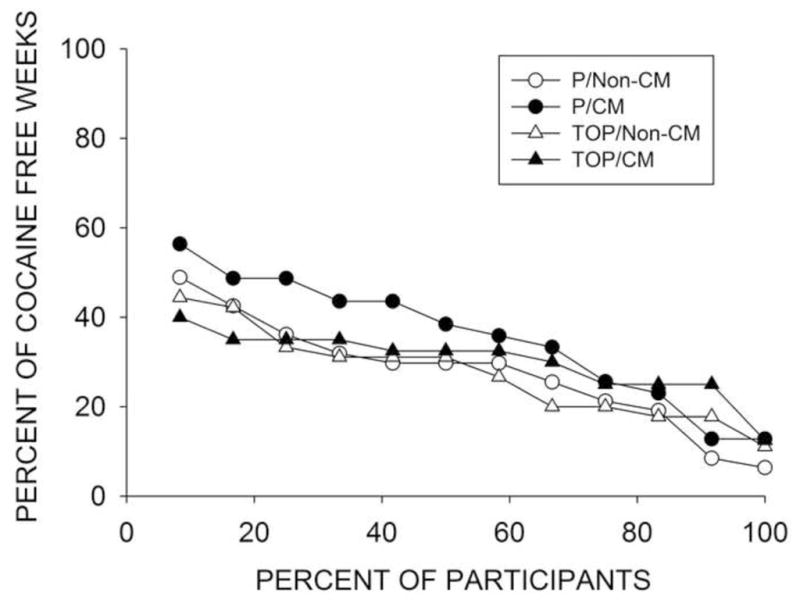
Combination of self-reported abstinence, biochemical confirmation of abstinence, and no missing samples across the 12-week combined treatment evaluation phase
None of the groups showed a significant change in cocaine abstinence during the voucher phase relative to baseline or in any voucher phase analysis that included time as a factor in the model, but descriptive data show that the proportion of participants abstaining from cocaine over time during the voucher phase increased slightly for the CM groups, and decreased slightly in the non-CM groups. A scatter plot of all participants’ cocaine toxicology results throughout the evaluation period is shown in the Supplementary Material, figure 1.1
3.3 Secondary outcomes
Baseline cocaine abstinence (weeks 1–5; < 300 ng/mL) was correlated with overall percent of cocaine negative urine samples, three consecutive weeks of cocaine abstinence, and the longest duration of cocaine abstinence during treatment, as shown in Table 3. This correlation was the strongest for the two TOP groups, and not significant for the control group (P/Non-CM). However, including baseline cocaine use as covariate did not change the results of cocaine abstinence between the treatment groups.
Table 3.
Pearson correlation between baseline percentage of cocaine negative urine samples (weeks 1–5) and cocaine abstinence during the combined treatment phase (weeks 9 – 20).
| At baseline (weeks 1–5) | TOP/CM N = 40 |
TOP/Non-CM N = 45 |
P/CM N = 39 |
P/Non-CM N = 47 |
|
|---|---|---|---|---|---|
| % cocaine negative urine | r | 0.45 | 0.55 | 0.34 | 0.21 |
| p | 0.004 | <.0001 | 0.033 | 0.168 | |
| 3 weeks consecutive abstinence | r | 0.46 | 0.50 | 0.45 | 0.21 |
| p | 0.003 | 0.000 | 0.004 | 0.16 | |
| Longest Duration of Abstinence | r | 0.57 | 0.60 | 0.51 | 0.312 |
| p | 0.000 | <.0001 | 0.001 | 0.033 |
Cocaine craving was measured with the CSSA. The median intake CSSA was 28, higher than in the studies by Kampman et al (1998), and treatment groups were well balanced on Intake CSSA (> 20, Y/N), but intake CSSA did not predict cocaine abstinence. There was no effect of TOP on CSSA scores over time.
Gender: women provided a lower percentage of cocaine negative urine samples at the 300 ng/mL cut off: 30 ± 4.2 vs 41 ± 4.6 for males (p = .05), but the difference (43 ± 3.8 versus 51 ± 3.9) was not significant when samples were dichotomized using the Preston rule.
Other drugs and alcohol use: there was no effect of TOP on the rate of opiate negative urine samples (%, M ± SE): 53 ± 4.3 vs 59 ± 3.9 for the TOP vs P groups respectively (p = .27). The percent of benzodiazepine positive urine samples, overall low, was significantly lower in the TOP groups (5 ± 1.8 versus 10.4 ± 2.7, p = .013). The rate of positive BAL was too low for any meaningful analysis: during the combined treatment phase 30 of 4630 (0.7%) BAL produced a positive reading. Regarding smoking behaviors, there were no significant differences in CO levels between the groups, nor any time effect.
Psychiatric symptoms: overall, psychiatric symptoms scores were very low. There was no effect of treatment group on depression, anxiety or PTSD symptomatology (results in supplementary material2).
Voucher earnings: The non-CM groups received vouchers of similar value to the CM group, as expected given the yoked procedure. The average earnings were $ (SE) 421.25 ± 79.58 for TOP/CM, 394.69 ± 72.06 for P/CM, 343.26 ± 65.34 for TOP/Non-CM, and 203.51 ± 50.89 for P/Non-CM (NS). CM and non-CM participant’s earnings were positively correlated with their longest duration of cocaine abstinence (rho = .969 p < .001 vs rho .494 p< .001 respectively).
Medication adherence: clinic attendance was high, as 91% to 93% of scheduled methadone doses were ingested by participants remaining in treatment, with no difference between the groups. This also reflects the high rate of observed ingestion of TOP or P capsules in the morning. No objective data is available to confirm medication adherence for the evening study medication doses.
3.4 Adverse events
In the TOP groups, 6 participants were discharged for medical reasons: 2 abnormal EKGs; 1 transient creatinine elevation; and 3 hospitalizations (1 accidental CO poisoning; 1 paranoid psychosis; 1 pneumonia). In the P groups, 7 participants were discontinued for medical reasons: 2 transaminases elevation > 3x ULN; 1 hematuria and long QTc.; 1 increase in intraocular pressure, 1 pregnancy; 2 hospitalizations: 1 suicidal ideation, 1 pancreatitis. A table of adverse events is in Supplementary Material3.
4. DISCUSSION
The results from this study show that topiramate was not different from placebo in reducing cocaine use in the treatment of dually cocaine and opioid dependent methadone patients. Although this finding is disappointing, it is not necessarily in conflict with the current evidence base for topiramate in the treatment of stimulant dependence. For example, a large double-blind randomized controlled trial of cocaine and alcohol dependent subjects who had achieved initial abstinence showed that 20% of participants who received topiramate (300 mg daily) achieved sustained cocaine abstinence the last 3 weeks of the trial compared to 7% in the placebo group (p = 0.01), but topiramate was not better than placebo in promoting cocaine or alcohol abstinence overall (Kampman et al., 2013). Similarly, in a multi-center trial, topiramate (200 mg daily) failed to increase abstinence from methamphetamine, although a secondary analysis found a significant reduction in weekly median urinary methamphetamine levels in urine samples in the topiramate group (Elkashef et al., 2012). Another randomized controlled trial found a small but statistically significant effect of topiramate (300 mg daily) in the treatment of cocaine dependence. In that study, topiramate participants maintained cocaine abstinence 16.6% of weeks during the efficacy phase of the study versus 5.8% for the placebo group (p = .02; Johnson et al., 2013).
Perhaps the most unique feature of the present study relative to studies showing modest efficacy of topiramate treating cocaine dependence is that our participants were polysubstance users dually dependent on opiates and cocaine, thus likely representing a more severe addiction pathology including more severe stimulant dependence. In support of this argument is the higher median CSSA score at intake: 28 compared to 20 in Kampman’s (2004) pilot study. It is also possible that co-occurring opioid dependence, and opioid substitution therapy alter the effects of chronic cocaine use on GABA transmission (Kupchik et al., 2014) and on glutamate transmission (Placenza et al., 2008). Buprenorphine appears to increase baseline glutamate levels, blocking the expression of cocaine sensitization while increasing the dopamine response to cocaine (Placenza et al., 2008). The effects of topiramate on these systems may thus differ in dual cocaine and opioid dependence.
Treatment adherence is always a concern in cocaine dependence clinical trials. Conducting the study in a methadone clinic ensured retention and ingestion of at least half the study medication doses through direct observation of dosing. Participants reported to nursing staff ingesting the medications the evening before, although they were inconsistent in returning the empty blister packs, thus preventing a surrogate pill count. Topiramate was well tolerated as participants did not report strong subjective side-effects from the study medications when probed weekly with a side-effect checklist.
An important limitation of the present study is that unlike other clinical trials with voucher incentives, including factorial design combinations of CM and pharmacotherapy (e.g., Poling et al., 2006), the CM groups in this study did not perform better than the non-CM groups. A review of the voucher data showed that the contingency groups received a very high fidelity CM intervention, and voucher amounts were similar to those in previous studies showing that CM increases cocaine abstinence, thus poor fidelity or low reinforcement magnitude are not viable explanations of the lack of a CM effect on cocaine abstinence. One possibility is that delaying the voucher exchange for up to seven days reduced the efficacy of the vouchers (Roll et al., 2000). Another possibility is that requiring payouts in $25 units added further to the delay, especially at the beginning of the voucher program. Despite these design flaws in the CM intervention, descriptive data showed an emerging trend toward the expected effect of the CM intervention on cocaine abstinence. In future trials involving CM, it is of utmost importance to eliminate, or reduce to the fullest extent possible, any delay between confirmation of abstinence and delivery of vouchers.
Topiramate has been found to be effective for the treatment of alcohol dependence. This study included alcohol dependence as a stratification variable, but the groups were unbalanced on this characteristic (perhaps reflecting the relatively low rate of alcohol dependence in the study). More participants with alcohol dependence were randomized to the placebo conditions, suggesting that this imbalance could have favored the topiramate group (not the observed finding). However, the rate of positive BAL was so low overall, precluding any conclusion as to the effect of topiramate on alcohol intake.
There were significantly fewer benzodiazepine positive urine samples in the topiramate groups. Although benzodiazepine dependence was an exclusion criterion, this finding is interesting and suggests that this class of medications could be tested for benzodiazepine abuse in opioid maintenance patients, especially given the risks associated with benzodiazepine use.
The present clinical trial showed that 300 mg/day topiramate was safe but did not significantly increase cocaine abstinence or decrease cocaine craving in cocaine dependent methadone patients.
Supplementary Material
Table 2.
Baseline demographic and drug use characteristics
| Total (N=171) | TOP/CM (N=40) | TOP/Non-CM (N=45) | P/CM (N=39) | P/Non-CM (N=47) | |
|---|---|---|---|---|---|
| Age, M (SD) | 42 (7) | 43 (7) | 41 (7) | 40 (8) | 42 (7) |
| Age 18–40, (%)* | 38 | 33 | 44 | 38 | 36 |
| Age 41–55, (%) | 62 | 68 | 56 | 62 | 64 |
| Race (% AA) | 60 | 58 | 60 | 54 | 68 |
| Gender (% F) | 48 | 43 | 49 | 49 | 51 |
| Education, M (SD) | 11 (1.7) | 11 (2.1) | 11 (1.4) | 12 (1.4) | 11 (1.7) |
| Education <12, (%) | 42 | 40 | 44 | 41 | 40 |
| Education ≥ 12, (%) | 58 | 60 | 56 | 59 | 60 |
| Marital Status (%) | |||||
| Never Married | 62 | 53 | 73 | 69 | 53 |
| Married | 13 | 10 | 9 | 15 | 19 |
| Separated/Divorced/Widowed | 25 | 38 | 18 | 15 | 28 |
| Employment (%) | |||||
| Employed | 26 | 33 | 22 | 28 | 23 |
| Unemployed/Disabled* | 74 | 68 | 78 | 72 | 77 |
| Transfer Methadone Maintenance** (%) | 36 (21) | 13 (33) | 9 (20) | 7 (18) | 7 (15) |
| Clinician CSSA Admission Score, M (SD) | 32 (19) | 34 (19) | 31 (17) | 32 (18) | 33 (20) |
| Clinician CSSA Score ≤20, (%) | 35 | 30 | 36 | 28 | 43 |
| Clinician CSSA Score >20, (%) | 65 | 70 | 64 | 72 | 57 |
| SCID (%) | |||||
| History Alcohol Dependence | 88 (52) | 20 (50) | 21 (47) | 21 (54) | 26 (55) |
| Current Alcohol Dependence*** | 30 (18) | 5 (13) | 5 (11) | 9 (23) | 11 (23) |
| History Sedative Dependence | 31 (18) | 6 (15) | 8 (18) | 7 (18) | 10 (21) |
| History THC Dependence**** | 72 (42) | 13 (33) | 14 (31) | 16 (41) | 29 (62) |
| Current THC Dependence | 18 (25) | 1 (8) | 5 (36) | 4 (25) | 8 (28) |
| Antisocial Personality Disorder | 46 (27) | 10 (25) | 10 (22) | 12 (31) | 14 (30) |
| ASI (past 30 days drug use), M (SD) | |||||
| Alcohol | 4.8 (8.3) | 3.6 (8.1) | 4.5 (7.9) | 6.4 (10.2) | 4.6 (7.1) |
| Heroin | 22.1 (12.0) | 20.3 (13.3) | 20.8 (13.1) | 24.3 (11.2) | 23.2 (10.4) |
| Cocaine | 20.8 (9.0) | 20.7 (8.8) | 21.6 (9.4) | 19.8 (8.9) | 20.9 (9.1) |
| Methadone | 8.2 (12.2) | 10.2 (13.6) | 9.5 (12.4) | 6.9 (12.3) | 6.5 (10.6) |
| Sedatives | 0.3 (0.8) | 0.2 (0.5) | 0.3 (0.8) | 0.2 (0.6) | 0.5 (1.0) |
| THC | 2.7 (7.1) | 0.5 (1.8) | 2.7 (7.2) | 2.7 (7.6) | 4.4 (8.9) |
Where numbers add up to more than 100, it is due to rounding errors.
These participants were on Methadone Maintenance prior to study admission.
A two-factor analysis revealed that participants receiving placebo were significantly more likely to be alcohol dependent that participants receiving TOP.
One participant’s data missing for this diagnosis.
Acknowledgments
The study was supported by grants from the National Institute on Drug Abuse (DA 021808) with additional funds from grant T32 DA07209 and grant K24 DA023186. Were are thankful to Paul Nuzzo for statistical analysis, and to the BPRU research staff, under the leadership of Mary Bailes and Connie Lowery, without whom this project would not have been possible.
Clinical Trial: NCT00685178
Our gratitude for the BPRU team without whom this trial could not be completed: Mary Bailes, Torran Claiborne, Apexa Patel, Bhavika Patel, Gabriel Vera, Tiffany Duren, Paul Nuzzo, Kevin Wein, Michael Gordon, Sharon Henderson, Sherry Thomas, Sylvia Harper, Michele Fulton, Connie Lowery, Diana Beasley, Robin Clay, Lisa Russell, Eric Pittman, Mary Pitts, Shirley Savage, YuZon Wu, Ashley Crowner, Erin Hagner, Alexandra Lowery, Iona Johnson.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
No authors have any financial or other conflict of interest.
This paper has not been published before.
Preliminary results of this study were presented at the College on Drug Dependence in 2010 and 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves OL, Doyle AJ, Clausen T, Gilman C, Bullock R. Evaluation of topiramate neuroprotective effect in severe TBI using microdialysis. Ann N Y Acad Sci. 2003;993:25–34. doi: 10.1111/j.1749-6632.2003.tb07508.x. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Blom TJ, McElroy SL, Keck PE. Preliminary evidence for gender-specific effects of topiramate as a potential aid to smoking cessation. Addiction. 2008;103:687–694. doi: 10.1111/j.1360-0443.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- Bäcktröm P, Hyytiä P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Baltieri DA, Daro FR, Ribeiro PL, de Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103:2035–2044. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858– 871. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berlant JL. Prospective open-label study of add-on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry. 2004;4:24. doi: 10.1186/1471-244X-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H, Rogawski MA. Topiramate reduces excitability in the basolateral amygdala by selectively inhibiting GluK1 (GluR5) kainate receptors on interneurons and positively modulating GABAA receptors on principal neurons. J Pharmacol Exp Ther. 2009;330:558–566. doi: 10.1124/jpet.109.153908. [DOI] [PubMed] [Google Scholar]
- Brodie JD, Case BG, Figueroa E, Dewey SL, Robinson JA, Wanderling JA, Laska EM. Randomized, double-blind, placebo-controlled trial of vigabatrin for the treatment of cocaine dependence in Mexican parolees. Am J Psychiatry. 2009;166:1269–1277. doi: 10.1176/appi.ajp.2009.08121811. [DOI] [PubMed] [Google Scholar]
- Castells X, Kosten TR, Capella D, Vidal X, Colom J, Casas M. Efficacy of opiate maintenance therapy and adjunctive interventions for opioid dependence with comorbid cocaine use disorders: a systematic review and meta-analysis of controlled clinical trials. Am J Drug Alcohol Abuse. 2009;35:339–349. doi: 10.1080/00952990903108215. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Falsetti SA, Saladin ME, Brady KT. Screening for PTSD in a substance abuse sample: psycho- metric properties of a modified version of the PTSD Symptom Scale Self-Report. J Trauma Stress. 1998;11:393–399. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Kahn R, Yu E, Iturriaga E, Li SH, Anderson A, Chiang N, Ait-Daoud N, Weiss D, McSherry F, Serpi T, Rawson R, Hrymoc M, Weis D, McCann M, Pham T, Stock C, Dickinson R, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Li MD, Johnson BA. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297–1306. doi: 10.1111/j.1360-0443.2011.03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gosai K, Kosten TR. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87:1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;17:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61:905–912. doi: 10.1001/archpsyc.61.9.905. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Recent advances in the develpment of treatments for alcohol and cocaine dependence. Focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Wang XQ, Penberthy JK, Javors MA, Seneviratne C, Liu L. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70:1338–1346. doi: 10.1001/jamapsychiatry.2013.2295. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, LaLumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133:94–99. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 2004;172:225–229. doi: 10.1007/s00213-003-1634-4. [DOI] [PubMed] [Google Scholar]
- Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6:829–836. doi: 10.1016/j.jpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, Kalivas PW. Cocaine Dysregulates Opioid Gating of GABA Neurotransmission in the Ventral Pallidum. J Neursci. 2014;34:1057–1066. doi: 10.1523/JNEUROSCI.4336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche SM, Helmers SL. The new antiepileptic drugs. JAMA. 2004;291:605– 614. doi: 10.1001/jama.291.5.605. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. J Subst Abuse Treat. 1999;16:129–135. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Nickel MK, Nickel C, Mitterlehner FO, Tritt K, Lahmann C, Leiberich PK, Rother WK, Loew TH. Topiramate treatment of aggression in female borderline personality disorder patients: a double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65:1515–1519. doi: 10.4088/jcp.v65n1112. [DOI] [PubMed] [Google Scholar]
- Nickel MK, Nickel C, Kaplan P, Lahmann C, Muhlbacher M, Tritt K, Krawczyk J, Leiberich PK, Rother WK, Loew TH. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495–499. doi: 10.1016/j.biopsych.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Nickel C, Lahmann C, Tritt K, Muehlbacher M, Kaplan P, Kettler C, Krawczyk J, Loew TH, Rother WK, Nickel MK. Topiramate in treatment of depressive and anger symptoms in female depressive patients: a randomized, double-blind, placebo-controlled study. J Affect Disord. 2005;87:243–252. doi: 10.1016/j.jad.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placenza FM, Rajabi H, Stewart J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology. 2008;200:347–355. doi: 10.1007/s00213-008-1210-z. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219– 228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Roll JM, Reilly MP, Johanson CE. The influence of exchange delays on cigarette versus money choice: a laboratory analog of voucher-based reinforcement therapy. Exp Clin Psychopharmacol. 2000;8:366–370. doi: 10.1037//1064-1297.8.3.366. [DOI] [PubMed] [Google Scholar]
- Rubio G, Martinez-Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. J Clin Psychopharmacol. 2009;29:584–589. doi: 10.1097/JCP.0b013e3181bfdb79. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of attendance by unemployed methadone patients in a job skills training program. Drug Alcohol Depend. 1996a;41:197–207. doi: 10.1016/0376-8716(96)01252-5. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996b;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, Segal SD, Sheehan M, Roache JD, Bickel WK, Jasinski D, Watson DW, Miller SR, Somoza P, Winhusen T. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA. 2013;70:630– 637. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Mind Garden. Redwood City, VA: 1983. State-Trait Inventory for Adults. [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, Bennett M. Topiramate augmentation in treatment-resistant obsessive-compulsive disorder: a retrospective, open-label case series. Depress Anxiety. 2006;23:1–5. doi: 10.1002/da.20118. [DOI] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Griffiths RR. Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend. 2005;80:369–337. doi: 10.1016/j.drugalcdep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Zeger S, Liang KY. An overview of methods for the analysis of longitudinal data. Stats Med. 1992;11:1825–1839. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.