Abstract
Study of P2-purinoceptor subtypes has been difficult due to the lack of potent and selective ligands. With the goal of developing high affinity P2-purinoceptor-selective agonists, we have synthesized a series of analogues of adenine nucleotides modified on the purine ring as chain-extended 2-thioethers or as N6-methyl-substituted compounds. Chemical functionality incorporated in the thioether moiety included cyanoalkyl, nitroaromatic, amino, thiol, cycloalkyl, n-alkyl, and olefinic groups. Apparent affinity of the compounds for P2Y-purinoceptors was established by measurement of P2Y-purinoceptor-promoted phospholipase C activity in turkey erythrocyte membranes and relaxation of carbachol-contracted smooth muscle in three different preparations (guinea pig taenia coil, rabbit aorta, and rabbit mesenteric artery). Activity at P2X-purinoceptors was established by measurement of contraction of rabbit saphenous artery and of the guinea pig vas deferens and urinary bladder. All 11 of the 2-thioethers of ATP stimulated the production of inositol phosphates with K0.5 values of 1.5–770 nM, with an (aminophenyl)ethyl derivative being most potent. Two adenosine diphosphate analogues were equipotent to the corresponding ATP analogues. Adenosine monophosphate analogues were full agonists, although generally 4 orders of magnitude less potent. ATP 2-thioethers displayed pD2 values in the range of 6–8 in smooth muscle assay systems for activity at P2Y-receptors. There was a significant correlation for the 2-thioether compounds between the pK0.5 values for inositol phosphate production and the pD2 values for relaxation mediated via the P2Y-purinoceptors in the guinea pig taenia coli, but not for the vascular P2Y-receptors or for the P2X-receptors. At P2X-receptors, no activity was observed in the rabbit saphenous artery, but variable degrees of activity were observed in the guinea pig vas deferens and bladder depending on distal substituents of the thioether moiety. N6-Methyl-ATP was inactive at P2X-receptors, and approximately equipotent to ATP at taenia coli P2Y-receptors. This suggested that hybrid N6-methyl and 2-thioether ATP derivatives might be potent and selective for certain P2Y-receptors, as was shown for one such derivative, N6-methyl-2-(5-hexenylthio)-ATP.
Introduction
Extracellular ATP has a role as a fast cotransmitter that is released in conjunction with norepinephrine and other transmitters at the neuroeffector junctions of many vascular and visceral smooth muscles.1–3 Recently, it was reported that ATP can act as a fast transmitter at synapses between neurons in the coeliac ganglion4,5 and in the central nervous system6 via opening of ligand-gated ion channels.
ATP activates purinergic receptors of the P2-type (P1 designates adenosine receptors with A1, A2, etc., subtypes, reviewed in ref 7). Several major categories of P2-receptors have been defined8 based on differential potencies of various ATP derivatives. P2X-receptors are activated by α,β-methylene-ATP and apparently consist of ligand-gated cation channels.9,10 P2Y-receptors are activated by 2-(methylthio)-ATP and regulate inositol lipid hydrolysis11–15 and possibly other second messenger pathway(s). A less clearly defined subtype of the P2-receptor family, the P2U-receptor,16 also promotes inositol lipid hydrolysis and is activated by ATP and UTP but not by many analogues of ATP, UTP, and ADP. Other cell-specific P2-receptors have been proposed. ADP regulates platelet cell function through P2Z-receptors,2,8,17 and P2Z-receptors on mast cells, fibroblasts, and leukocytes regulate ion permeability.2,8,17,18
Pharmacological, biochemical, and structural characterization of P2-receptors has been relatively limited, and the development of P2-receptor ligands has lagged far behind the development of P1-receptor ligands, in part due to the greater difficulties in synthesis and purification of nucleoside triphosphates. We have designed a series of new analogues with the goal of developing high-affinity, P2Y-receptor-selective, and metabolically stable agonists. Several of these already have been shown to be more potent than ATP in raising Ca2+ levels via P2Y-like receptors in developing chick myotubes.19 These nucleotides are derivatives of 2-(methylthio)-ATP, in which the S-methyl group has been extended to form a functionalized chain, for derivatization by the functionalized congener approach.20 The selectivity of these and related derivatives of ATP have been established in biochemical12,15 and smooth muscle2,21 assay systems, and the results emphasize structural features that markedly enhance P2Y-receptor potency and selectivity.
Results
Synthesis
Due to its high affinity at P2Y-receptors in binding and functional assays,12,22 it seemed that 2MeSATP may serve as a good lead structure for new P2Y-agonists. However, 2MeSATP has been reported to be hydrolyzed by ectonucleotidases19 under the biochemical assay conditions (e.g., 30–35 °C, 20–60 min), which leads to confusion about the nature of the biologically active species. This problem was resolved upon the elaboration and elongation of the side chain of 2-(alkylthio)-ATP derivatives.19 Those “second generation” ligands were highly stable metabolically as indicated by comparison with 2MeSATP and ATP under various conditions of incubation with intact and broken cell preparations.19 The increased stability is probably due to the steric hindrance by the 2-position chain at the ectonucleotidase binding site. Thus, long-chain 2-(alkylthio)-ATP derivatives are lead structures which may provide both potency and stability of the ligands.
Our synthetic aim was as follows: (a) Elaboration of the side chain on C-2 by different distal substituents, e.g. polar groups, hydrogen-bonding functions, and aromatic rings, thus making an additional potential anchor for improving the binding to the receptor. (b) Attaching different functional groups that may serve as precursors to labeled ligands or for attachment of reporter groups. In similar fashion, the concept of functionalized congeners in the design of purines as P1-receptor ligands has been described.20 (c) Combination of 2-thio-substituted ATP with other modifications for enhancement of selectivity and potency. (d) Synthesis of lower homologues, the corresponding AMP and ADP derivatives for comparison with the triphosphates.
Following the above-mentioned guidelines (a) and (b), we synthesized compounds 8–18, bearing an alkyl, olefin, or aromatic side chain, some of which are substituted by polar groups like cyano or nitro (Table I). A two-step synthesis (Figure 1) of those agonists consisted of alkylation of 2-thioadenosine (modified from ref 19) by the appropriate alkyl bromide in a dilute NaOH/MeOH solution at 20–50 °C or with triethylamine in DMF, followed by a one-pot triphosphorylation.23,24 ATP derivatives were obtained in 14–45% yield, and variable amounts of the corresponding AMP derivatives were obtained as well (34%–69% yield). The nucleotides were purified on ion-exchange resin columns (DEAE A-25 Sephadex) using a 0.4–0.6 M NH4HCO3 buffer gradient. The latter was preferred over Et3NH+HCO3−, due to its greater volatility and simpler 1H NMR spectra of the product in its ammonium salt form. In some cases further purification on HPLC was required. All the derivatives tested for biological activity were characterized by HPLC and and 1H and 31P NMR as well as high-resolution FAB spectra, all of which are essential for structure and purity determination.
Table I.
Activity of Nucleotide Analogues in Various Biochemical and Pharmacological Models
| compound | P2Y-purinoceptors | ||||||
|---|---|---|---|---|---|---|---|
| P2X-purinoceptors mediating contractiona (relative to ATP) |
|||||||
| inositol lipid hydrolysis (nM)d |
mediating relaxationa (relative to ATP) |
||||||
| R saph. art.f |
GP vas |
GP bladder |
|||||
| GP taenia | R aortae | R mes. art.e | |||||
| 1, 5′-ATP | 2800 ± 700 | = (6.2)j | = (4.5) | = (6.0) | = | = (3.5)i | =b |
| 2, 5′-ADP | 8000 ± 2000 | =h | + (5.2) | − (5.2) | na | −b | =h |
| 3, 5′-AMP | na | − −h | = (4.8) | − (5.0) | na | na | nah |
| 4, adenosine | na | − − (3.9)k | + (5.7) | = (6.0) | na | na | na |
| 5, 2-Cl-ATP | 72 ± 19 | + + (7.2)b | + (5.8) | = (6.2) | na | = | = |
| 6, 2-MeS-ATP | 8 ± 2 | + + (8.0) | + +b (6.8) | + + (6.5) | na | =b | =b |
| 7, 2-MeS-ADP | 6 ± 3 | + +b | + + | ||||
| 8, 2-(hexylthio)-ATP | 5 ± 1 | + + (7.5) | + + (6.7, >max) | + + (6.7) | na | + | = |
| 9,c 2-(5-hexenylthio)-ATP | 10 ± 4 | + + (7.9) | + + (≈max) | + + (7.0, ≈max) | na | = | + |
| 10, 2-(5-hexenylthio)-ADP | 6.8 ± 3.0 | + + (8.1) | |||||
| 11, 2-(5-hexenylthio)-AMP | 328 ± 43 | = (5.0) | + + (7.0) | + (6.3) | na | na | na |
| 12,c 2-[(phenylethyl)thio]-ATP | 30 ± 17 | + + (7.1) | + + (6.5, <max) | + (6.2, >max) | na | + + | + + |
| 13, 2-[(2-(p-nitrophenyl)ethyl]thio]-ATP | 12 + 4 | + + (8.0) | + + (6.2) | + + (7.0) | 2.6% | + + | − |
| 14, 2-[[(2-(p-nitrophenyl)ethyl]thio]-AMP | 3000 ± 1200 | = (6.5) | + (6.1, <max) | − (5.4) | na | − | − |
| 15, 2-[[2-(p-nitrophenyl)ethyl]thio]-ATP | 1.53 ± 0.21 | ||||||
| 16, 2-(cyclohexylthio)-ATP | 24 ± 4 | + + (8.0) | + (6.3, <max) | + (5.9, <max) | na | + | = |
| 17, 2-[(6-cyanohexyl)thio]-ATP | 10±5 | + + (8.6) | + + (7.0) | + + (6.9) | 9.2% | + + | g |
| 18, 2-[(6-cyanohexyl)thio]-AMP | 37000 ± 13000 | − (4.4) | + (6.2, <max) | 4.5% | na | na | |
| 19, 2-[(7-aminoheptyl)thic]-ATP | 72.8 ± 46.6 | ||||||
| 20, 2-[(7-thioheptyl)thio]-ATP | 773 ± 328 | ||||||
| 21, 2-[(7-thiocyanatoheptyl)thio]-ATP | 25.9 ± 10.0 | ||||||
| 22,c N6-methyl-ATP | 19000 ± 6000 | = (5.8) | − − | na | na | na | naf |
| 23, N6-methyl-2-[(5-hexenyl)thio]-ATP | 26 ± 7 | + (7.2) | + (5.6) | + (6.0) | 1.5% | na | − |
| 24, N6-methyl-2-[(5-hexenyl)thio]-AMP | >100000 | − − | na | na | 6.7% | na | na |
+ + significantly more potent than ATP; + more potent than or equal to ATP; = equal to ATP; − less potent than or equal to ATP; − − significantly less potent than ATP; na not active at the highest concentration tested (usually around 10−5 M).
Compound 22 inhibited the cholinergic twitch. Compounds 9 and 12 caused contractions and increased the chalinergic twitch (only 9 was sustained).
K0.5 (nM) for stimulation of production of inositol phosphates, expressed as the mean ±SEM for three to eight determinations.
Maximum relaxation relative to 2MeSATP (<, ≈, or >) is in parentheses. Numerical value, if given, is pD2 in log molar units.
For the saphenous artery, the responses are given as a percentage relative to the contraction produced by 1 µM α,β-MeATP. The highest concentrations tested were 3–30 µM.
Compound 17 was approximately 100 times more potent than ATP in the bladder, but it produced tonic contractions rather than the phasic contractions of ATP. In the presence of indomethacin (1 µM), it was much less potent than ATP under the same conditions.
Data from ref 22.
Data from ref 33.
6.2 ± 0.08 (n = 38).
Data from ref 38.
Figure 1.
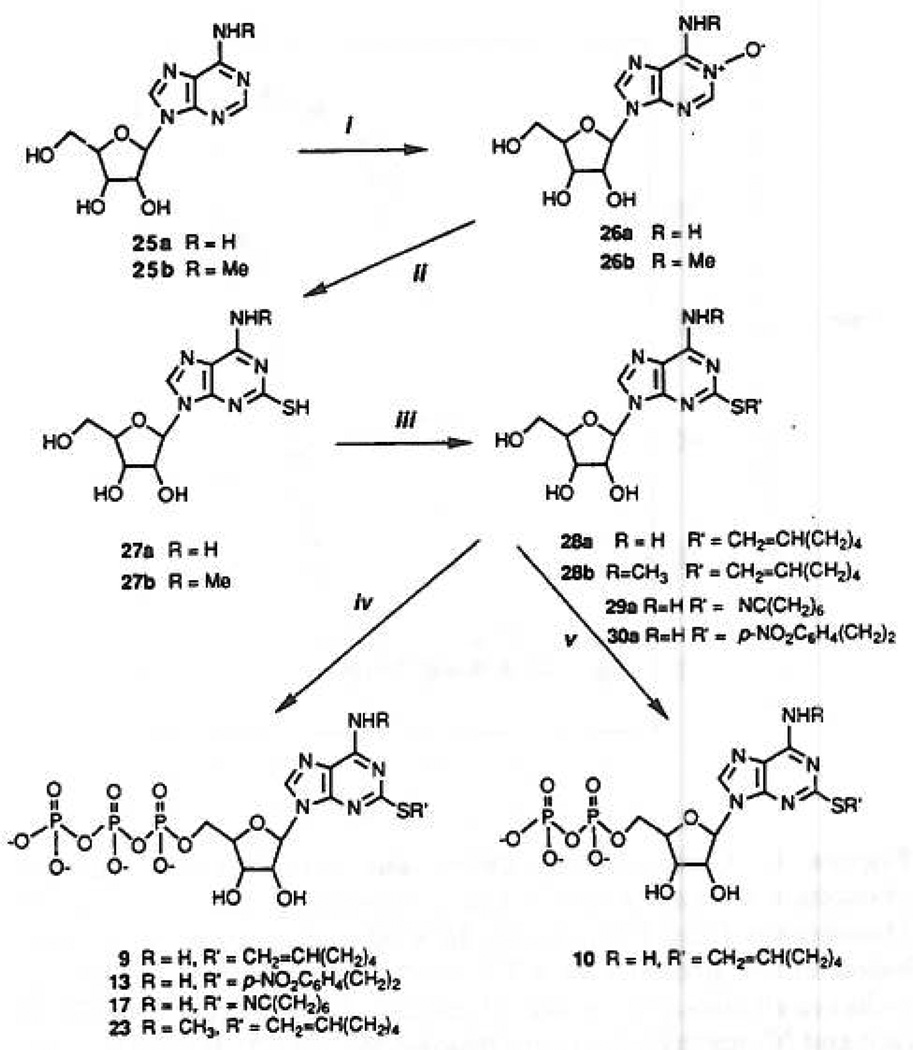
Synthesis of 2-thioether and N6-methyl-ATP analogues. Compound a in this scheme (25–30) refers to R = H, and compound b refers to R = CH3. Compound 27a was synthesized by procedures previously described.13,25 Conditions were: (i) m-chloroperbenzoic acid, 3 days, room temperature; (ii) (1) 5 N NaOH, (2) CS2, MeOH, H2O,120 °C; (iii) RBr, Et3N, DMF; (iv) (1) POCl3, (2) (Bu3NH+)2P2O7H2; (v) (1) POCl3, (2) (Bu3NH+)2-PO4H2. Adenine 5′-triphosphate derivatives synthesized by this method but not shown in figure: 2-(hexylthio)-, 8; 2[(2-phenylethyl)thio]-, 12; and 2-(cyclohexylthio)-, 16. Adenine 5′-monophosphate derivatives also isolated as byproducts of the phosphorylation reactions: 2-(hexenylthio)-, 11; 2-[[2-nitrophenypethyl]thio]-, 14; 2-(6-cyanohexylthio)-, 18; and 2-(6-cyanohexylthio)-N6-methyl-, 24.
The diphosphate derivative, 10, was synthesized by the same method described above, except for using the tributylammonium phosphate salt for condensation with the phosphorodichloridate intermediate instead of the corresponding pyrophosphate salt. This reaction gave rise not only to 2-(5-hexenylthio)-ADP, 10 (~30% yield), but also to the corresponding AMP, 11 (~40% yield), and ATP, 9 (~5% yield), derivatives. The assumed mechanism for the formation of the triphosphate is via a cyclic metatriphosphate intermediate, which eventually undergoes hydrolysis to the open-chain triphosphate.25,26
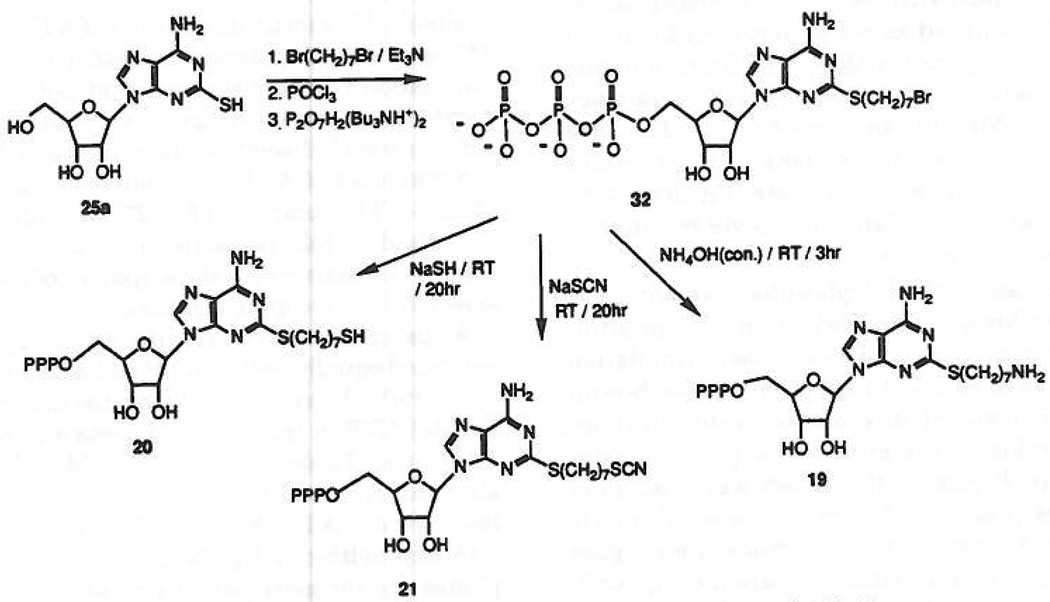
Substitution of the side chain with an amino group was intended for attachment of reporter groups,20 cross-linking to the receptor, immobilization on a solid matrix for affinity chromatography of P2-purinoceptors, or to act as a distal anchor to enhance affinity, perhaps through potential hydrogen bonding with the receptor protein. The synthesis of an arylamino derivative was achieved as follows: 2-[(2-p-aminophenethyl)thio]-ATP, 15, was obtained quantitatively upon PtO2-catalyzed hydrogenolysis of the corresponding nitro compound, 13, at room temperature overnight. An aliphatic amine congener, compound 19, was obtained in three steps from 2-thioadenosine, 25a (Figure 2), which was alkylated by 1,7-dibromoheptane in a dilute NaOH solution. The product, 2-[(7-bromohepty1)-thio] adenosine, 31, was triphosphorylated to provide an alkylating intermediate, 32. This bromoalkyl triphosphate derivative was subsequently exposed to concentrated ammonia for 3 hat room temperature, yielding the desired amino product, 19. Substitution of the bromide in compound 32 with hydrosulfide ion (addition of an excess of NaSH at room temperature for 20 h) gave rise to the thiol derivative, compound 20. Treatment of 32 with aqueous sodium thiocyanate provided compound 21.
Figure 2.
Synthesis of terminally functionalized 2-(alkylthio)-ATP analogues, compounds 19–21.
On the basis of the above mentioned guidelines for the synthesis of a potentially potent and stable agonist, we also sought to enhance selectivity toward P2Y-receptors. The basis for the development of a third generation agonist was the finding that N6-alkyl substitution (e.g. methyl or etheno group bridging N1 and N6, see below and ref 27) caused loss of activity of the corresponding ATP agonist at P2X-receptors at ≤ 10 µM, but not at P2Y-receptors in the ileum or turkey erythrocytes. In light of the possibility that base-modification of ATP might yield P2Y-receptor agonists of very high potency and specificity we have begun a systematic analysis of the activity of 2- and N6-substitutions of the ATP molecule.
Thus, aiming at the synthesis of N6-methyl-ATP analogues, N6-methyladenosine, 25a, was N-oxidized using m-chloroperbenzoic acid in acetic acid (Figure 1), which we found to be more efficient than the hydrogen peroxide procedure.19 Introduction of a 2-thiol group was achieved in a two-step procedure: ring opening of the base-sensitive oxidized pyrimidine to the corresponding oxime by a short reflux in NaOH solution, followed by reaction with CS2, ring closure and N-oxide reduction, done under high temperature and pressure conditions.28 N6-Methyl-2-thioadenosine, 27b, was alkylated by 1-hexen-6-yl bromide in DMF in the presence of Et3N at room temperature to provide 28b, which was phosphorylated to give rise to both triphosphate, 23, and monophosphate, 24, products in 30% and 34% yield, respectively.
P2Y-Purinoceptor-Mediated Activation of Phospholipase C
Turkey erythrocytes have been shown previously to express a P2-purinoceptor that markedly stimulates inositol lipid hydrolysis.11,12 This receptor has been tentatively identified as a P2Y-purinoceptor based on the high potency of 2-(methylthio)-ATP for stimulation of inositol phosphate accumulation and the low potency of agonists, i.e. α,β-MeATP and β,γ-MeATP, that have been previously proposed to be potent P2X-receptor agonists. Similarly, UTP, which activates P2U-receptors, only weakly activated the avian erythrocyte receptor at relatively high concentrations.
Essentially no basal inositol phosphate activity was observed in membranes prepared from [3H]inositol-labeled turkey erythrocytes, and a very small stimulation was observed in the presence of 1 µM GTPγS (not shown). Addition of ATP resulted in a marked concentration-dependent activation of the erythrocyte phospholipase C11,12 as shown in Figure 3; this effect was absolutely dependent on the presence of guanine nucleotides (refs 11, 12 and data not shown). A broad range of analogues of ATP were equally efficacious to the parent compound. Moreover, two commercially available analogues, 2MeSATP, 6, and 2-chloro-ATP, 5, were considerably more potent than ATP.
Figure 3.
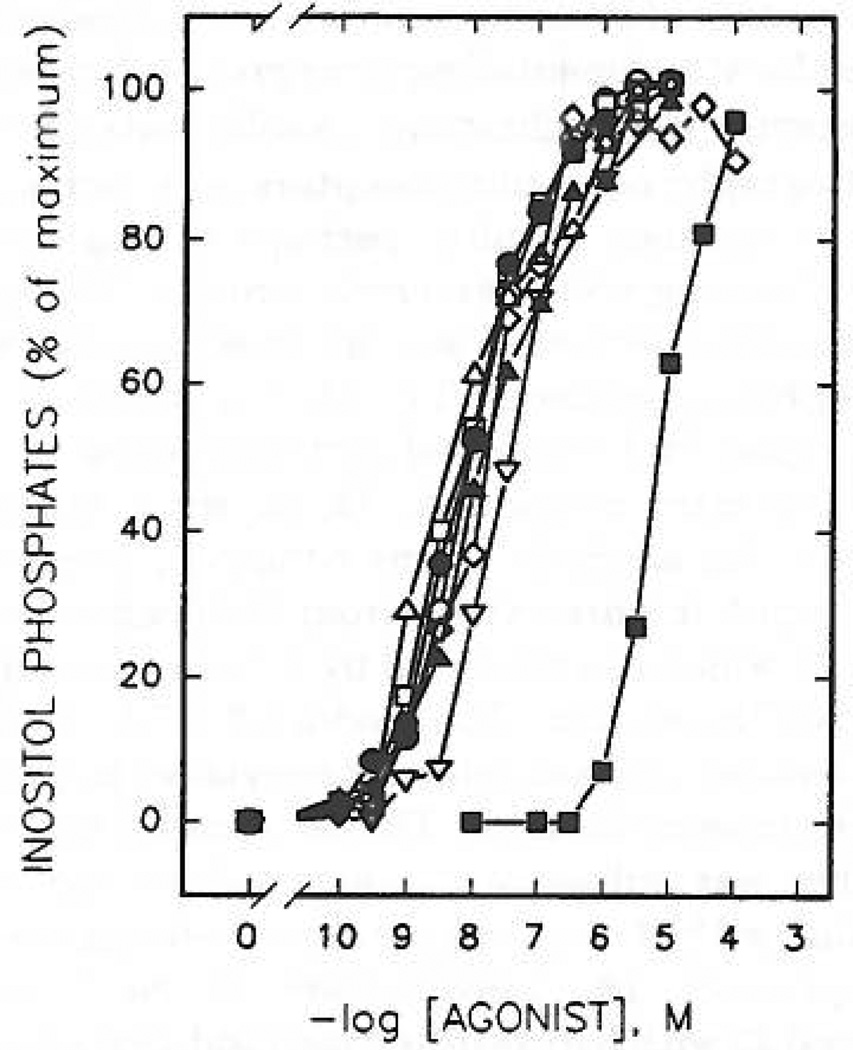
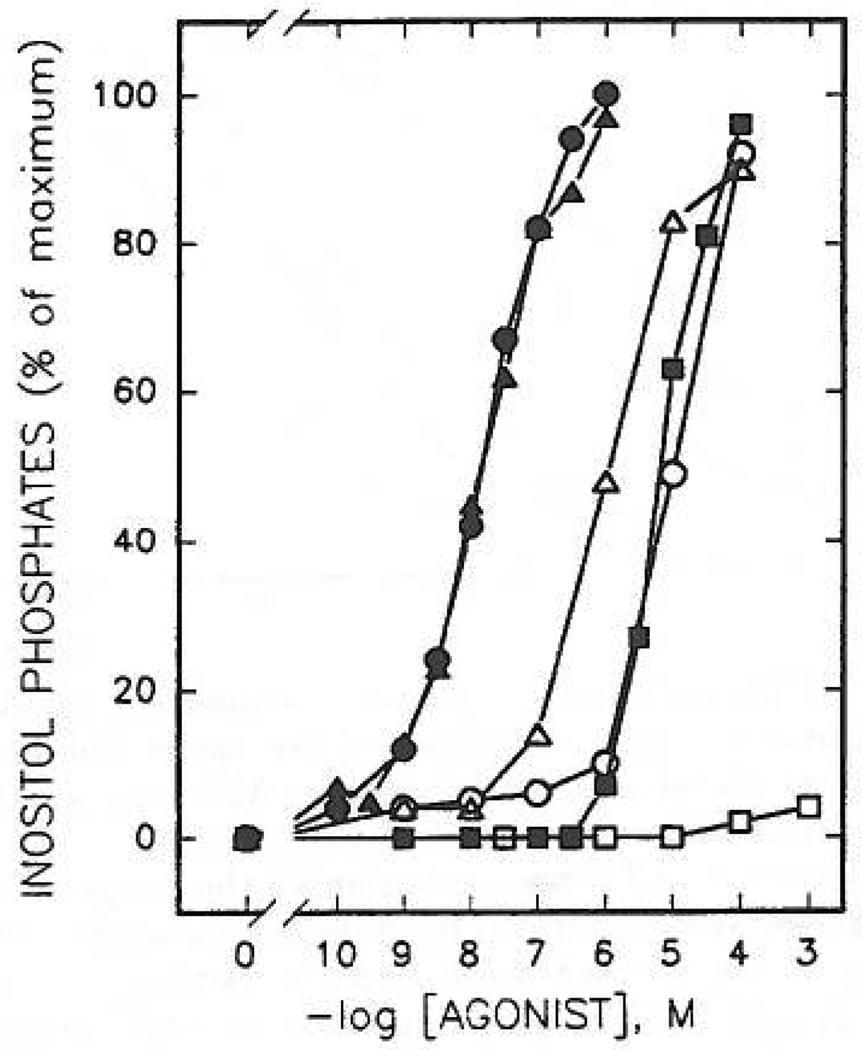
Concentration-dependent stimulation of inositol phosphate formation by 2-thioether derivatives of ATP. Membranes from [3H]inositol-labeled erythrocytes were incubated for 5 min at 30 °C in the presence of the indicated concentrations of ATP, 1 (■); 2-(methylthio)-ATP, 6 (○); 2-(hexylthio)-ATP, 8 (△); 2-(hexenylthio)-ATP, 9 (□); 2-[(phenylethyl)thio]-ATP, 12 (◊); 2-(cyclohexylthio)-ATP, 16 (▽); 2-[(cyanohexyl)thio]-ATP, 17 (●); or 2-[[(p-nitrophenyl)ethyl]thio]-ATP, 13 (▲). Incubation was in the presence of 1µM GTPγS as described in Materials and Methods. The data shown are the average of three to eight experiments carried out in duplicate using different membrane preparations. The average cpm of [3H]inositol phosphates produced in the presence of 1 µM GTPγS alone was 400 cpm (0%). The maximal (100%) level of [3H]inositol phosphates in the presence of GTPγS and adenine nucleotide analogues was at least 5000 cpm with all membrane preparations tested.
2-(Alkylthio)-substituted ATP analogues (compounds 8, 9, 12, 13, 15, 16, 17, 19, 20, and 21) were tested for their capacity to stimulate inositol lipid hydrolysis in turkey erythrocyte membranes. Except for the 7-aminoheptyl and 7-thioheptyl derivatives, 19 and 20, respectively, these thioether derivatives were all at least 2 orders of magnitude more potent (K0.5 values = 5–30 nM) than ATP (Figure 3 and Table I). Thus, most of these compounds were more potent than 2-chloro-ATP, 5 (K0 5 = 72 nM). The thioether derivatives were also equiefficacious to ATP in the turkey erythrocyte membranes.
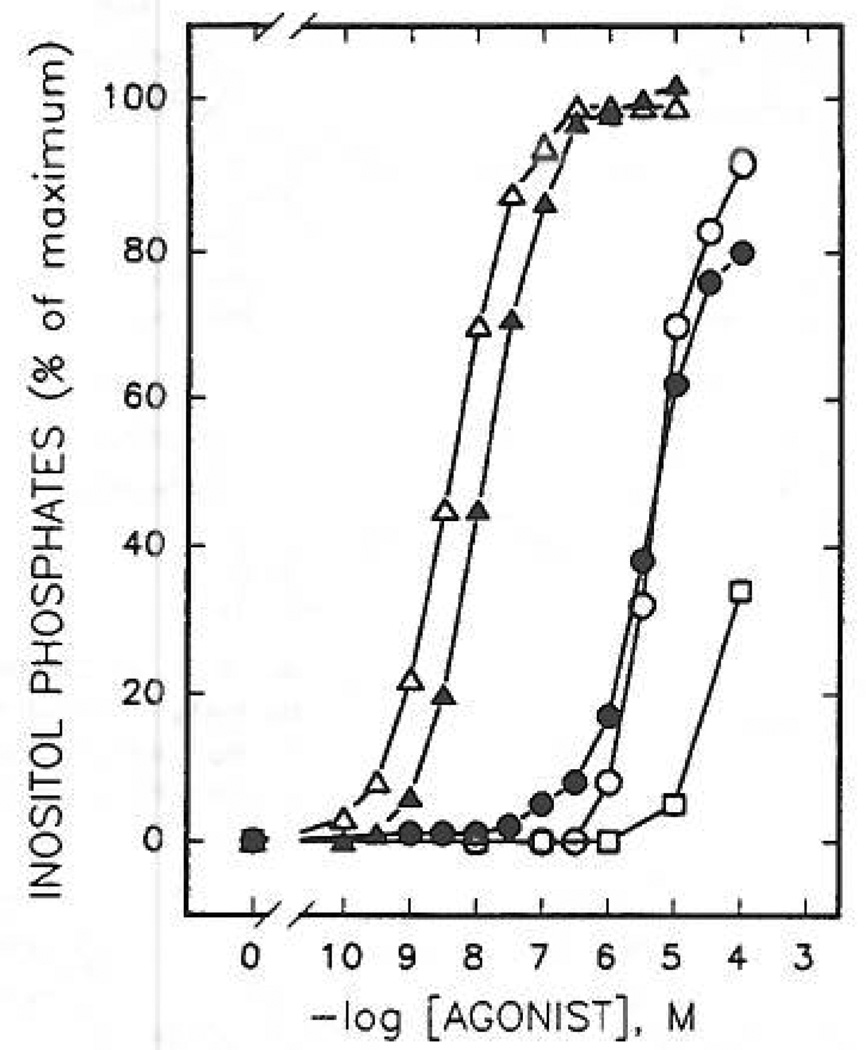
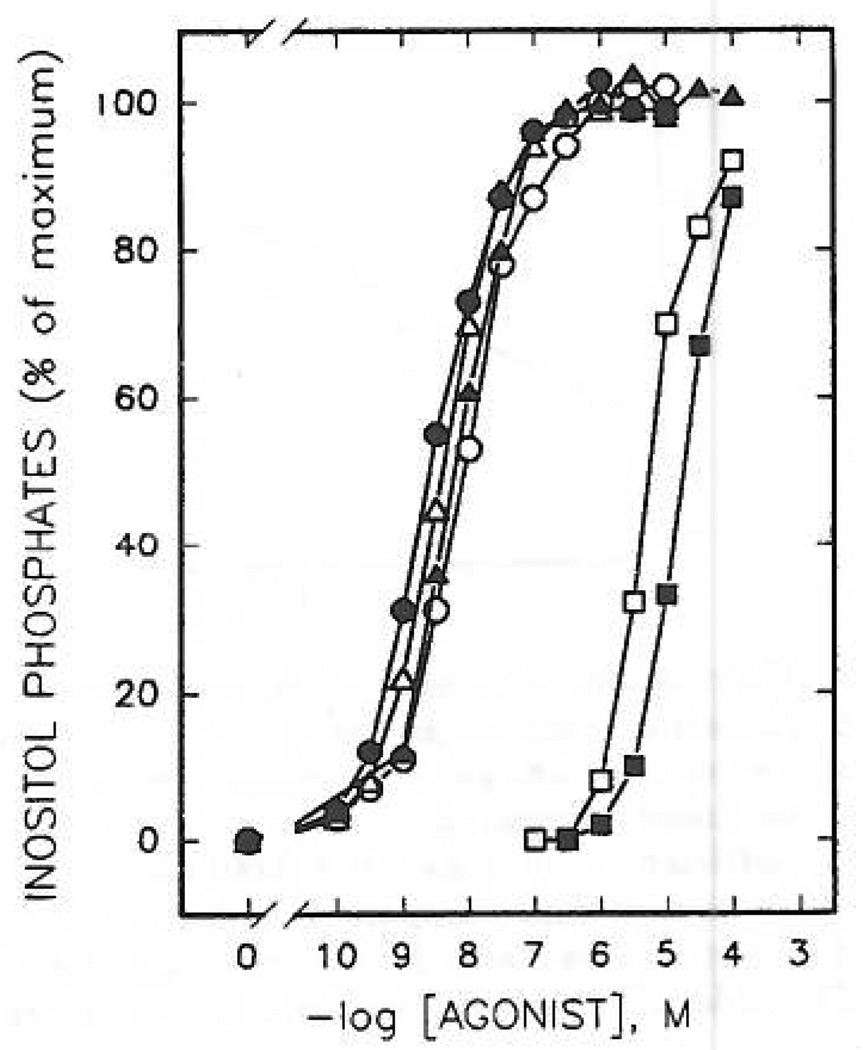
Although a detailed series of compounds is not yet available, N6-substitution, either of ATP itself (compound 22) or of a more potent 2-thioether (compound 23), did not markedly affect the apparent potency at the erythrocyte P2Y-receptor (Figure 4), although P2X-receptor activity was abolished (see below). Upon N6-methylation, the potencies of ATP (compound 1 vs 22) and 2-(hexenylthio)-ATP (compound 9 vs 23) were diminished by only 7-fold and 2.6-fold, respectively. Such hybrid substitutions may be the basis for the development of even more potent, selective P2Y-receptor agonists.
Figure 4.
Concentration-dependent stimulation of inositol phosphate formation by N6- and 2-thioether derivatives of ATP. Membranes from [3H]inositol-labeled erythrocytes were incubated in the presence of ATP, 1 (○); N6-methyl-ATP, 22 (●); 2-(hexenylthio)-ATP, 9 (△); N6-methyl-2-(hexenylthio)-ATP, 23 (▲); and N6-methyl-2-(hexenylthio)-AMP, 24 (□). Incubation was for 5 min at 30 °C in the presence of 1 µM GTPγS. Data shown are from a representative experiment repeated at least three times with similar results. [3H] Inositol phosphate accumulation in the presence of 1µM GTPγS alone was 250 cpm (0%). Maximal levels (100%) of [3H]inositol phosphate accumulation in the presence of GTPγS and a maximal concentration of N6-methyl-2-(hexenylthio)-ATP was 9150 cpm.
As described previously, the erythrocyte receptor was activated equally well by ATP and ADP and very potently by ADPβS. Moreover, as illustrated in Figure 4 and Table I, 2MeSATP, 6 (K0.5 = 8 nM), was equipotent to 2-MeSADP (see Table I), 7 (K0.5 = 6 nM). This relationship also was observed for longer chain analogues, i.e. 2-(5-hexenylthio)-ATP, 9 (K0.5 = 10 nM), was equipotent to 2-(5-hexenylthio)-ADP, 10 (K0.5 = 4 nM). Thus, triphosphates are not more potent activators of the erythrocyte P2Y-receptor than their corresponding ADP analogues.
AMP, 3, had practically no affinity for turkey erythrocyte P2Y-receptors. Curiously, the monophosphate 2-thioether analogues, such as 2-[(6-cyanohexyl)thio]-AMP, 18 (K0.5 = 37 µM), were full agonists (Figure 6), although considerably less potent than the corresponding ATP analogues, 17. A 2-hexenylthio monophosphate derivative, compound 11, however, was only 33-fold less potent at turkey erythrocyte P2Y-receptors than the corresponding triphosphate. This enhancement of potency in adenine monophosphate derivatives containing long-chain 2-thioether groups was incompatable with the N6-methyl modification. Thus, N6-methyl-2-(hexenylthio)adenine monophosphate, compound 24 (Figure 4), was nearly inactive. Several adenosine precursors (2-thioethers), of which the 5′-phosphate derivatives were highly potent, [2-(5-hexenylthio)-and2-[(2-(4-nitrophenyflethyl]-thio] adenosine, 28a and 30a, respectively] were found to be inactive in stimulating production of inositol phosphates at concentrations up to 100 µM.
Figure 6.
Comparative effects of 2-thioether analogues of AMP and ATP on phospholipase C activity in turkey erythrocyte membranes. Membranes from [3H]inositol-labeled erythrocytes were incubated for 5 min at 30 °C in the presence of the indicated concentrations of AMP, 3 (□); ATP, 1 (■); 2-[(cyanohexyl)thio]-AMP, 18 (○); 2-[(cyanohexyl)thio]-ATP, 17 (●); 2-[[(p-nitrophenyl)ethyl]thiol]-AMP, 14 (△); 2-[[(p-nitrophenyl)ethyl]thio]-ATP, 14 (▲). Incubation was in the presence of 1µM GTPγS as described under Materials and Methods. Results shown are from a representative experiment repeated at least three times using different membrane preparations. [3H]inositol phosphate accumulation in the presence of 1µM GTPγS alone was 200 cpm (0%). Maximal levels (100%) of [3H] inositol phosphate accumulation in the presence of GTPγS and a maximal concentration of agonist, e.g. 1µM 2-[(cyanohexyl)thio]-ATP, was 6000 cpm.
A (2-phenylethyl)thio substituent (compound 12) has been substituted on the ring with a 4-nitro (13) or a 4-amino group (15). Compound 15, which is also intended as a substrate for radioiodination, was extremely potent at turkey erythrocyte P2Y-receptors. A comparison of functional group replacement at the distal carbon of a 2-thioheptyl chain (compounds 19, 20, and 21) reveals a wide variation in potency. At this position, an SCN or an NH2 group is favored over an SH group.
P2X- and P2Y-Receptor Smooth Muscle Assays
Pharmacological assays of P2Y-receptors included relaxation of the guinea pig taenia coli,29,30 endothelium-dependent relaxation of the rabbit aorta,21 and endothelium-independent relaxation of the rabbit mesentericartery.31 Pharmacological assays at P2X-receptors29,30,32 included contraction of the saphenous artery of the rabbit and contraction of the vas deferens and bladder of the guinea pig.
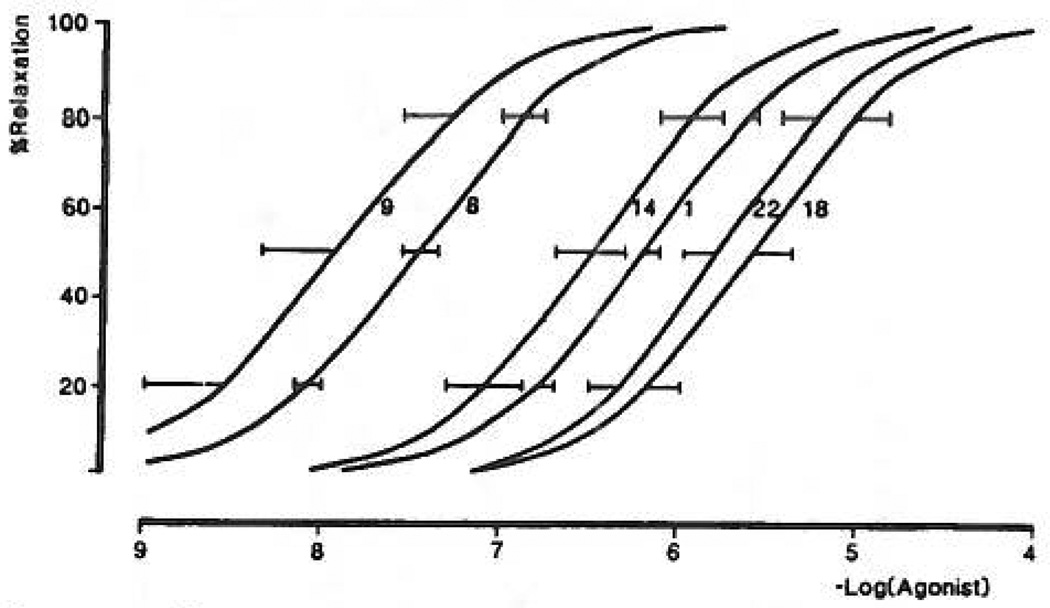
In the pharmacological P2Y-receptor assays, 2-thioether ATP derivatives (compounds 8, 9, 12, 13, 15, 16, 17) were as potent or nearly as potent as 2MeSATP, with EC50 values with pD2 values in the range of 7–8 in the relaxation of guinea pig taenia coli (Table I). As in the turkey erythrocyte response system, 2-[(6-cyanohexyl)thio] AMP, 18, was a full agonist, although much less potent (curve not shown). The 2-hexylthio analogue, 8, was less potent than its corresponding unsaturated derivative, 2-(5-hexenylthio)-ATP, 9 (Figure 7). Curiously, two derivatives, 2-(2-phenylethyl)thio-, 12, and 2-(cyclohexylthio)-ATP, 16, had a greater efficacy than 2MeSATP in the mesenteric artery but less efficacy than 2MeSATP in the rabbit aorta.
Figure 7.
Concentration—response relationships for ATP and its derivatives causing relaxation of the carbachol-contracted guinea pig taenia coli (P2Y-purinoceptor). All curves are the mean of two determinations except for 1 (n = 38), 8 (n = 4), 11 (n = 3), and 14 (n = 5). ATP was tested on all the preparations that the derivatives were tested on. Ordinate axis shows the percentage relaxation of the carbachol-induced contraction; abscissa axis shows −log[agonist]. The pD2 value for 2MeSATP is 8.0 ± 0.15 (ref 33).
In the 2-alkylthio series, the pD2 values were generally greater in the taenia coli than in the rabbit aorta or mesenteric artery. The largest enhancement in potency at the intestinal versus the vascular smooth muscle responses was seen with compound 16, the cyclohexylthio analogue, which was 63-fold more potent in the taenia coli than in the rabbit aorta, and with compound 17, the cyanohexylthio derivative, which was 79-fold more potent in the taenia coli than in the aorta. Other analogues were between 4- and 25- fold selective for the taenia coli receptor. In contrast, the cyclohexylthio derivative of AMP, compound 18, was 63-fold more potent in the mesenteric artery than on the taenia, although in this artery it had only approximately half the efficacy of 2MeSATP.
The 2-thio-substituted ATP analogues also were evaluated in pharmacological assays at P2X-receptors. As with 2-(methylthio)-ATP, 6, no activity was observed with compounds 8, 9, 12, 16, and 22 in the contraction of the rabbit saphenous artery P2X-receptors, and other triphosphates had minimal activity. In this series, only moderate activity was seen at guinea pig vas deferens P2X-receptors, except for compounds 12, 13, and 17, which were very potent. At bladder P2X-receptors, only 2-[(phenylethyl)-thiol-ATP, 12, was very potent.
In smooth muscle assays, the agonist N6-methyl-ATP, 22, was selective for P2Y-receptors in the taenia coli, where it was approximately equipotent to ATP. In contrast, N6-methyl-ATP was inactive at P2X-receptors at ≤10 µM in all three tissues. On the basis of this observation, a hybrid molecule, 23, was synthesized, incorporating the N6-methyl modification (rendering P2Y-selectivity) and the long-chain thioether (rendering high potency at P2Y-receptors). This compound was inactive at P2X-receptors at ≤10 µM but markedly stimulated smooth muscle P2Y-receptors at low concentrations. These data are consistent with the high potency of 23 for stimulation of turkey erythrocyte phospholipase C (Figure 4).
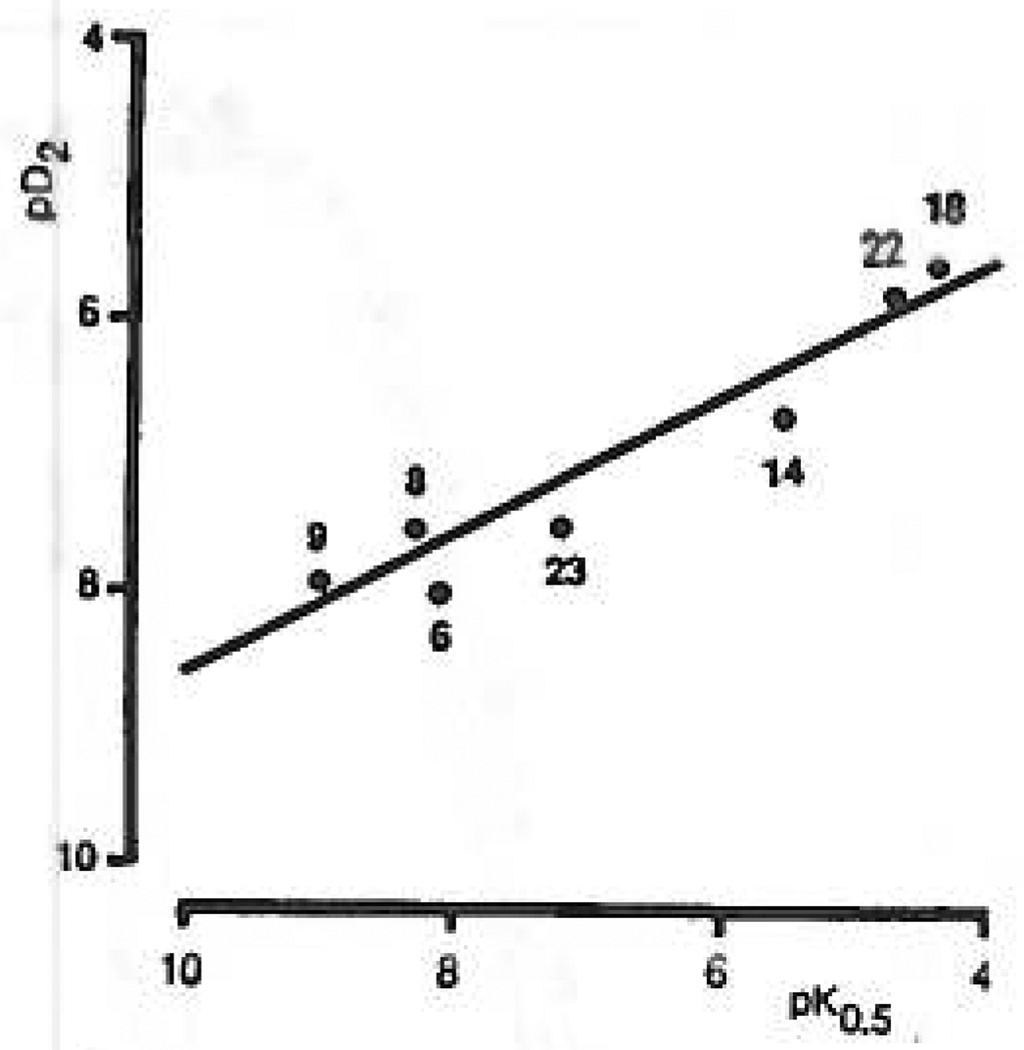
On the basis of our results a series of high-affinity P2Y-purinoceptor agonists have been identified that are much less active or inactive at P2X-purinoceptors. A direct comparison of the relative potencies of these compounds in the phospholipase C assay to potencies at taenia coli test P2Y-receptors is presented in Figure 8. An r value of 0.96 was observed, suggesting that these two receptors are very similar. Although the potencies for these compounds at P2Y-purinoceptors in rabbit aorta and mesenteric artery were highly correlated, these potencies were not well correlated with the values for taenia coli or phospholipase C responses.
Figure 8.
Correlation between turkey erythrocyte P2Y-purinoceptor agonism and guinea pig taenia coli P2Y-purinoceptor agonism, represented by pK0.5 and pD2 values, respectively. The line shows the linear regression of pD2 on pK0.5, which had a correlation coefficient, r, of 0.960 (P < 0.001) for the equation y = 0.5x + 3.59. None of these compounds had significant activity at P2X-purinoceptors (see Table I). The pD2 value for 2-(methylthio)-ATP (2MeSATP) was taken from the literature.24
Discussion
The initial delineation of the P2Y-subclass of P2-purinoceptors principally was based on the selectivity of 2MeSATP for activation of these receptors and their relatively low affinity for α,β-MeATP and β,γ-MeATP. While this subclassification has largely held for P2Y-purinoceptors in a broad range of target tissues and with several physiological and biochemical responses, the limitations accompanying the lack of receptor-selective agonists and antagonists have been considerable. Thus, neither the possibility of the existence of multiple P2Y-purinoceptors nor that of multiple PS-purinoceptor-promoted signaling mechanisms has been adequately addressed. Outside of avian erythrocyte preparations,15 the P2Y-purinoceptor(s) has not been radiolabeled, and only recently was structural information on the receptor published.36
The work described here gives new insight into the chemical features of ATP analogues that favor interaction with P2Y-purinoceptors and describes a series of compounds that should be useful for further characterization of P2Y-purinoceptors. By comparing the structural features for activation of P2Y-purinoceptors with those for activation of smooth muscle P2X-purinoceptors, we have also identified drugs that exhibit high selectivity for interaction at P2Y-versus P2X-purinoceptors. On the basis of the limited structure—activity analyses for activation of P2T- and P2U-purinoceptors, it is unlikely that the high-affinity P2Y-purinoceptor agonists studied here activate P2T- and P2U-receptors. For example, ATP, but not 2MeSATP, activates P2U-purinoceptors,16 and 2MeSADP, but not 2MeSATP, activates P2T-purinoceptors.22,23 We believe that there is a strong possibility that multiple P2Y-purinoceptors exist, e.g. compare the activity of compound 16 at the turkey erythrocyte and rat aorta receptors, although the possibility of species differences in affinity at the same subtype has not been ruled out. Compound 14, on the other hand, is roughly equipotent at all of the P2Y-purinoceptors examined. Thus, various of these new high-affinity analogues may exhibit considerable differences in affinity for potential further subdivisions of the P2Y-purinoceptor subtype. More unambiguously defined model systems or expression of cloned receptors36,37 and selective antagonists are needed to rigorously approach this question. For example, it is not known whether any one of the smooth muscle responses measured in this study involves the action of a single or multiple P2-purinoceptors. The differential activities of many analogues in the P2X-purinoceptor assays is suggestive of multiple subtypes. Thus, compound 12 was inactive in the rabbit saphenous artery and significantly more potent than ATP in both the guinea pig vas deferens and bladder.
The 2-(alkylthio)-ATP analogues, which are highly potent at P2Y-receptors, were previously shown to resist degradation by nucleotidases in brain membrane preparations.19 It is noteworthy that this is the first example of a structural change distal to the triphosphate group itself that renders the analogue stable. This stability is general, since it was apparent in the pharmacological assays in this study, and it will greatly enhance the utility of the 2-thioethers as selective pharmacological tools. Since these compounds are of nanoraolar potency in turkey erythrocytes, they may serve as the basis for the design of molecular probes for ATP receptors. Such probes, potentially including radioligands, fluorescent probes, immobilized ligands for affinity chromatography, affinity labels, and covalently reactive ligands could be obtained using a functionalized congener approach, as has been demonstrated for other classes of purine receptors.20
In summary, we have synthesized a series of novel, long-chain derivatives of 2-(methylthio)-ATP and found them to be of high potency at P2Y-purinoceptors, in some cases surpassing that of the parent compound 2-MeSATP. The most potent compounds in this study in the turkey erythrocytes (K0.5 ≤ nM) were 8, 9, 15, and 17 (triphosphates) and 7 and 10 (diphosphates). There is a high correlation of their biological potencies in two different assays of P2Y-purinoceptor activity, stimulation of phospholipase C in turkey erthyrocytes and relaxation of the taenia coll. This possibly indicates a close similarity between these P2Y-purinoceptors. However the same compounds have different potencies at the P2Y-receptors of rabbit aorta and mesenteric artery, suggesting that P2Y-purinoceptors in the vascular preparation represent a different subtype or that multiple receptors are involved. ATP 2-thioether analogues were active to varying degrees, depending on distal sub stituents of the thioether moiety, at P2X-receptors, in the guinea pig vas deferens and bladder. The conclusions of the structure–activity analysis are (1) N6-methyl modification contributes to selectivity at certain P2Y-receptors. N6-Methyl-ATP derivatives were appreciably active at P2Y-receptors in turkey erythrocytes or the taenia coil, but inactive in the rabbit mesenteric artery. This modification appears to be compatible with the long-chain, potency enhancing 2-thioethers. (2) 2-Thioethers of ADP are equipotent with the ATP analogues in both biochemical and smooth muscle assays. (3) AMP itself is essentially inactive at P2-purinoceptors (very weakly active in the taenia coli), but with the 2-thioether modification, the monophosphates become weak, but full agonists in turkey erythrocyte P2Y-purinoceptors. High potency of one such derivative, compound 18, was observed at the mesenteric artery P2Y-purinoceptors.
Materials and Methods
Chemistry
New compounds were characterized (and resonances assigned) by proton nuclear magnetic resonance using a Varian GEMINI-300 FT-NMR spectrometer. Nucleotides were characterized also by 31P NMR in D2O using H3PO4 as an external reference on a Varian-ASM 100 300-MHz spectrometer. Samples (pD ranged from 5 to 7) were treated with CHELEX-100 (Bio-Rad, Richmond, CA) prior to spectral measurement. Synthetic intermediates and all final products were characterized on a Finnigan MAT mass spectrometer by chemical ionization mass spectrometry (NH3) and high-resolution mass spectrometry. Nucleotides were desorbed from a glycerol matrix under FAB (fast atom bombardment) conditions using 6-kV Xe atoms on a JEOL SX102 spectrometer. N6-Methyladenosine, 2MeSATP, 2MeSADP, and 2-chloro-ATP were obtained from Research Biochemicals, Inc. (Natick, MA). 2-Thioadenosine was the kind gift of Dr. Ray Olsson (Univ. So. Florida, Tampa, FL) or was synthesized as described.19 Purification of nucleotides was achieved on DEAE-A25 Sephadex columns as described below. Where needed final purification was done on a Hewlett-Packard 1090 HPLC system using a semipreparative SynChropak RP-P-100 column (1 × 25 cm, SynChrom, Inc., Lafayette, IN) and a linear gradient of a 0.1 M triethylammonium acetate buffer (TEAA, pH = 7) and acetonitrile (see below) with a flow rate of 3 mL/min ((triethylammonium)4 salt isolated). For analytical purposes, a nucleotide/nucleoside 7U column (250 mm × 4.6 mm, Alltech Associates, Inc., Deerfield IL) was used applying the same gradient as above at a 1 mL/min flow rate. The purity of the nucleotides described below was evaluated on an analytical column in two different solvent systems. One solvent system (I) was 0.1 M TEAA/CH3CN, 80:20 to 40:60, in 20 min. The other (II) was 60 mM ammonium phoshphate and 5 mM tetrabutyl-ammonium phosphate (TBAP) in 90% water/10% methanol (A) and 5 mM TBAP in methanol (B). A concentration gradient from 25% B to 75% B in 8 min was applied. Peaks were detected by UV absorption at 260 nm using a diode array detector. ATP derivatives were generally >91% pure.
N6-Methyladenosine N1-Oxide (26b)
A solution of N6-methyladenosine (2 g, 6.7 mmol) and m-chloroperbenzoic acid (2.3 g, 13.4 mmol) in acetic acid (20 mL) was stirred at room temperature for 2 days. Water (20 mL) was added to the reaction mixture, and a resulting thick precipitate was removed by filtration and discarded. The filtrate was coevaporated repeatedly with water under high vacuum, and the foamy residue was chromatographed on a silica gel column (CHCl3/MeOH, 2:1). The product was obtained as a white solid Imp 145 °C, crystallization from EtOH) in 45% yield (0.9 g). 1H NMR (DMSO): δ 8.62 (s, H-2), 8.55 (s, H-8), 5.88 (d, J = 5.4 Hz, 1H, H-1′), 4.51 (“t”, J = 5.1 Hz, 1H, H-2′), 4.15 (“t”, J = 4.5 Hz, 1H, H-3′), 3.94 (AB q, 1H, H-4′), 3.61 (AB dq, J = 12, 4 Hz, 2H, H-5′), 3.46 (s, 3H, Me) ppm. MS (CI): m/e 298 (MH+). Anal. Calcd for C11H15N5O5: C, 40.12; H, 4.59; N, 21.29. Found: C, 40.44; H, 5.34; N, 20.29.
N6-Methyl-2-thioadenosine (27b)
Compound 26b was converted to 5-amino-1-β-d-ribofurazosylimiclazole 4-(N-methyl)-carboxidoxime by the adaptation of the method of Kikugawa et al.25 and was obtained in a quantitative yield as a yellowish oil. 1H NMR (CD3OD): δ 8.54 (s, 1H, H-2), 7.46 (s, 1H, OH), 5.56 (d, J = 6.2 Hz, 1H, H-1′), 4.47 (t, J = 5.9, 1H, H-2′), 4.23 (dd, J = 5.6, 3.5 Hz, 1H, H-3′), 4.02 (dd, J = 6.0, 3.0 Hz, 1H, H-4′), 3.75 (AB dq, J = 12.0, 3.0 Hz, 2H, H-5′), 2.90 (a, 3H, Me) ppm. 5-Amino-1-β-d-ribofuranosylimidazole 4-(N-methyl)carboxidoxime is thermally unstable and was used immediately or kept at −80 °C.
A heterogeneous solution of 5-amino-1-β-d-ribofuranosylimidazole 4-(N-methyl)carbaxidoxime (0.7 g, 2.5 mmol) in MeOH/H2O/CS2 (19/7/5.5 mL, respectively) was heated in a sealed tube at 120 °C for 5 h. After cooling, the solution was evaporated to dryness and the brownish residue was chromatographed on a silica gel column (CHCl3/MeOH, 2:1, and then MeOH). Final purification was achieved by dissolution of the product in CHCl3/MeOH, 2:1, and then treatment with ether. The product, 27b, was obtained as a light yellowish solid (0.4 g, 51% yield, nip >230 °C, tritutated from ether). 1H NMR (CD3OD): δ 8.14 (s, 1H, H-8), 5.85 (d, J = 5.6 Hz, 1H, H-1′), 4.61 (“t”, J = 5.3 Hz, 1H, H-2′) 4.28 (dd, J = 5.2, 3.6 Hz, 1H, H-3′), 4.12 (“t”, 1H, H-4′), 3.81 (AB dq, J = 12.0, 2.5 Hz, 2H, H-5′), 3.31 (s, 3H, Me) ppm. MS (CI): m/e 314 (MH+). High-resolution FAB (positive ions, glycerol matrix) calcd for C11H16N6O4S (MH+) 314.0923, found 314.0936.
2-(5-Hexenylthio)adenosine Itemileydrate (28a)
2-Thioadenosine (27a, 0.2 g, 0.67 mmol) was dissolved in 0.25 M NaOH (8 mL, 2 mmol). 6-Bromo-1-hexene (0.45 mL, 3.3 mmol) was added, and the solution was stirred at room temperature for 3 h. The reaction mixture was concentrated under reduced pressure (bath temperature 33 °C) and extracted with ether (2 × 2 mL). The aqueous phase was neutralized with 18% HCl and extracted with ethyl acetate (3 × 4 mL). The homogeneous product was obtained after drying and solvent removal as a yellowish solid (0.14 g, 55% yield, mp 94 °C, trituration with ether). 1H NMR (CD3OD): δ 8.16 (s, 1H, H-8), 5.91 (d, J = 5.8 Hz, 1H, H-1′), 5.8 (dm, 1H, olefinic), 4.93 (ddd, J 11. 9.7, 1 Hz, 2H, olefinic), 4.72 (“t”, 1H, J = 5 Hz, H-2′), 4.31 (m, 1H, H-3′), 4.11 (m, 1H, H-4′), 3.83 (m, 2H, H-5′), 3.16 (m, 2H, CH2S), 2.10 (m, 2H, CH2), 1.74 (m, 2H, CH2), 1.56 (m, 2H, CH2) ppm. Anal. Calcd for C16H23N5O4S•0.5H2O: C, 49.22; H, 6.20; N, 17.93. Found: C, 49.46; H, 5.99; N, 17.36.
N6-Methyl-2-(5-hexenylthio)adenosine (28b) was prepared according to the same procedure for 28a in 23% yield (mp >230 °C dec) after column purification (CHCl3/MeOH, 9:1) and trituration with ether. 1H NMR (CD3OD): δ characteristic N6-Me resonance at 3.1 ppm (br s). HRMS: calcd for C17H24N5O4S 395.1611, found 395.1627.
General Nonaqueous Alkylation Procedure
A solution of 2-thioadenosine, 27a, or N6-methyl-2-thioadenosine, 27b (0.3 mmol), and dry Et3N (1.5 equiv) in dry dimethylformamide (2 mL) was stirred at room temperature for 0.5 h. Alkyl bromide (5 equiv) was added and stirring continued for an additional 2.5 h. The reaction solution was cooled in an ice bath, and a small amount of water (ca. 1 mL) was added. The white precipitate was filtered, dried, and chromatographed on a silica gel column (CHCl3/MeOH, 5:1). Final purification was by precipitating the product from CHCl3/MeOH (5:1) solution upon treatment with ether.
2-(6-Cyanohexylthio)adeno s ine Hemi hyd rate (29a)
This compound was prepared according to the above nonaqueous procedure in 72% yield (97 mg). The product was obtained as a yellowish solid (mp 162 °C, crystallized from EtOH/H2O). 1H NMR (CD3OD): δ 8.17 (s, 1H, H-8), 5.92 (d, J = 5.9 Hz, 1H, H-1′), 4.72 (t, J = 5.6 Hz, 1H, H-2′), 4.31 (dd, J = 4.8, 3.5 Hz, 1H, H-3′), 4.11 (AB q, 1H, 11-4′), 3.79 (AB dq, J = 11.4, 2.9 Hz, 2H, H-5′). 2.44 (t, J = 6.9 Hz, 4H, CH2CH2), 1.76 (“t”, 2H, CH2), 1.65 (“t”, 2H, CH2), 1.51 (m, 4H, CH2CH2) ppm. MS (CI): m/e 409 (ME+). Anal. Calcd for C17H24N6O4S• 0.5H2O: C, 48.91; H, 6.04; N, 20.13. Found: C, 48.94; H, 5.86; N, 19.99.
2-[(2-p-Nitrophenethyl)thio)adenosine (30a)
This compound was prepared according to the above nonaqueous procedure in 73% yield (0.23 g). The product was obtained as a yellowish solid (mp 186 °C, crystallized from EtOH/H2O). 1H NMR (CD3OD): δ 8.21 (s, 1H, H-8), 8.16 (d, J = 8.6 Hz, 2H, Ar), 7.57 (d, J = 8.6 Hz, 2H, Ar), 5.98 (d, J = 5.9 Hz, 1H, H-1′), 4.68 (“t”, J = 5.5 Hz, 1H, H-2′), 4.30 (rid, J = 5.1, 3.4 Hz, 1H, H-3′), 4.12 (AB q, 1H, H-4′), 3.79 (AB dq, J = 12.3, 2.9 Hz, 2H, H-5′), 3.43 (m, 2H, CH2Ar), 3.18 (t, 2H, CH2S) ppm. HRMS: calcd for C18H20N6O6 448.1148, found 448.1165.
Nucleoside 5′-Triphosphate (Compounds 8, 9, 12, 13, 16, 17)
The procedure for nucleoside 5′;-triphosphate synthesis was adapted from Kovacs and Ötvös23 and Moffat.24
Preparation of Tri-n-butylammonium Pyrophosphate Solution for Trip hosphate Synthesis
Sodium pyrophosphate decahydrate (6.69 g, 0.015 mol) in water (100 mL) was stirred at room temperature for 10 min until a clear solution was attained. The latter was passed through a column of activated Dowex 50WX-8 200 mesh, H+ form (40 mL of wet resin, 720 mequiv).
The column was washed with deionized water until neutral. The column eluate was collected in a flask (250 mL) containing tri butyl= ine (7.14 mL, 0.03 mol) and EtOH (75 mL) with stirring at 0 °C. The solution became cloudy during elution and became clear when all of the free amine was consumed. Lyophilization yielded a viscous oil. The latter was dissolved in EtOH and evaporated under high vacuum (bath temperature 35–40 °C). The process was repeated three times using dry dimethylformamide (30 mL) as the solvent, resulting in a thick oil which was dissolved in dry dimethylformamide (30 mL) and stored cold over activated molecular sieves.
Preparation of Triethylammoniutn Bicarbonate (TEAB) Buffer
A 1 M solution was prepared by adding dry ice to a 1 M triethylaraine solution in a flask covered tightly by a balloon far ca. 2 h until the pH reached 7.5.
General
All triphosphorylation reactions were carried out in a three-neck flask flame-dried under N2. Nucleosides and Proton Sponge (Aldrich Chemical Co., Milwaukee, WI) were dried overnight in a vacuum oven. Anhydrous solvents were used (trimethyl phosphate, dimethylformamide). Phosphorous oxychloride was distilled and kept under N2.
Typical Procedure
A solution of N6-methyl-2-(5-hexenylthio)adenosine (28b, 0.03 g, 0.076 mmol) and Proton Sponge (0.024 g, 1.5 equiv) in trimethyl phosphate (1 mL) was stirred for 10 min at 0 °C. Phosphorous oxychloride was added dropwise (14 µL, 0.152 mmol), and the clear solution was stirred for 2 hours at 0 °C. A mixture of Bu3N (75 µL) and 0.5 M (Bu3NH+)2 P2O7H2 in dimethylformamide (1 mL) was added at once. After 2 min 0.2 M TEAB solution (7.5 mL) was added, and the clear solution was stirred at room temperature for 45 min. The latter was lyophilized overnight. TLC on a silica gel plate, using propanol/H2O/28% NH4OH (11:2:7) as the eluent, indicated the disappearance of starting material and the formation of a polar product (Rf = 0.3). The spot was typically intensely purple under UV light and dark brown in an I2 chamber. The semisolid obtained after lyophilization was chromatographed at room temperature on a Sephadex DEAE-A25 column, which was swelled in 1 M NaHCO3 in the cold for 3 days (7 × 1.5 cm). The resin was washed with de ionized water (75 mL), using a peristaltic pump, and loaded with the crude reaction residue dissolved in a minimal volume of water. The separation was monitored by UV detection (ISCO, UA-5) at 280 nm. A buffer gradient of 250 mL of water to 250 mL of 0.5 M NH4HCO3 was applied, and 90 5-mL fractions were collected. The relevant fractions were pooled and lyophilized twice to yield a white solid. N6-Methyl-2-(5-hexenylthio)adenosine monophosphate monoammonium salt (24) was obtained in 34% yield (13 mg). 1H NMR (D2O): δ 8.52 (s, 1H, H-8), 6.16 (d, J = 5.4 Hz, 1H, H-1′), 5.80 (m, 1H, vinylic), 3.79 (s, 3H, Me), 2.13 (m, 2H, CH2), 1.79 (m, 2H, CH2), 1.56 (m, 2H, CH2). High-resolution FAB: calcd for C17H25N5SO7P 474.121, found 474.1238 (M2− + 2H+). Retention time: 8.6 min (98% purity) using solvent system II. N6-Methyl-2-(5-hexenylthio)adenosine 5′-triphosphate (23) eluted after the monophosphate and was obtained in 30% yield as the tetraammoniurn salt. 1H NMR (D2O): δ 8.34 (s, 1H, H-8), 6.11 (d, J = 5.7 Hz. 1H, H-1′), 5.91 (m, 1H, vinylic), 5.05 (dd, J = 7.5, 1.6 Hz, vinylic), 4.98 (dd, J = 10.4, 1.6 Hz, 1H, vinylic) (H-2′ is hidden by the water peak), 4.60 (t, J = 4.3 Hz, 1H, H-3′), 4.38 (br.s, 1H, H-4′), 4.24 (m, 2H, H-5′), 3.23 (m, 2H, CH2S), 3.11 (s, 3H, Me), 2.12 (q, J = 7 Hz, 2H, CH2), 1.78 (quintet, J =7 Hz, 2H, CH2), 1.56 (quintet, J = 7 Hz, 211, CH2). 31P NMR: δ −6.0 (br s), −10.9 (d), −21.6 (br s) ppm. High-resolution FAB: calcd for C17H27N5SO13P3 634.0539, found 634.0554 (M4− + 3H+). Retention time: 8.5 min (>98% purity) using solvent system I, 8.8 rain (>98% purity) using solvent system II.
2-(5-Hexenylthio)adenosine 5′-Diphosphate Trisammonium Salt (10)
The reaction was carried out on 0.158 mmol of nucleoside 28a following the typical procedure. In this reaction, however, 0.13 M (Bu3NH+)2PO4H in DMF (6.9 mL, 6 equiv) was used instead of bis(tributylammonium) pyrophosphate solution. TLC taken after workup (silica gel plate; solvent system propanol/28% NH4OH/H2O, 11:8:2) indicated the formation of three products (Rf = 0.33, 0.5, 0.7) in addition to a small amount of starting material. Separation on Sephadex DEAE-A25 column applying 0–0.5 M NH4HCO3 gradient (500 mL of each). Final separation was achieved on a semipreparative column applying a linear gradient of 0.1 M TEAA (pH 8.3)/CH3CN, 80:20 to 40:60 in 20 min (3 mL/min). Mono-, di-, and triphosphate products were obtained in 38% (29.7 mg), 30% (28 mg), and 5% (5.4 mg), respectively. 1H NMR of 10 (D2O): δ 8.40 (s, 1H, H-8), 6.13 (d, J = 5.4 Hz, 1H, H-1′), 4.62 (t, J = 4.7 Hz, 1H, H-3′), 4.37 (m, 1H, H-4′), 4.21 (m, 2H, H-5′) ppm. High resolution FAB: calcd for C16H24O10N5P2S 540.0719, found 540.0728. Retention time: 8.2 min (86% purity) using solvent system I, 7.4 min (86% purity) using solvent system II. NMR of other products: 2-(5-Hexenylthio)adenosine 5′-monophosphate diammonium salt (11). 11H NMR (D2O): δ 8.34 (s, 1H, H-8), 6.13 (d, J = 5.4 Hz, 1H, H-1), 5.91 (dm, 1H, olefinic), 5.01 (dd, J = 11, 9.7 Hz, olefinic), 4.50 (t, J = 4.5 Hz, 1H, H-3′), 4.37 (br s, 1H, H-4′), 4.13(m, 2H, H-5′), 3.21 (m, 2H, CH2S), 2.13 (q, J = 7 Hz, 2H, CH2), 1.77 (m, 2H, CH2), 1.55 (m, 2H, CH2) ppm. High-resolution FAB: calcd for C6H23O7N5PS: 460.1056. Found: 460.1052. Retention time: 9.17 rain (>98% purity) using solvent system I, 7.43 min (>98% purity) using solvent system II. 2-(5-Hexenylthio)adenosine 5′-triphosphate tetraammonium salt (9). 1H NMR (D20): δ 8.41 (s, 1H, H-8), 6.13 (d, J = 5.7 Hz, 1H, H-1′), 4.64 (m, 1H, H-3′), 4.37 (m, 1H, H-4′), 4.24 (dm, 2H, H-5′). High-resolution FAB: calcd for C16H25O13N5P3S 620.0382, found 620.0428. Retention time: 7.7 min (91% purity) using solvent system I, 7.4 min (>98% purity) using solvent system II. Compound 9 was also synthesized by the general triphosphorylation procedure above (19).
2-[2-(p-Nitrophenethyl)thiojadenosine 5′-triphosphate Tetraammonium Salt (13)
This compound was obtained as described above beginning with 0.1 mmol of nucleoside. Purification by ion exchange as above was achieved using a gradient of water and 0.6 M NH4HCO3 (230 mL of each). The triphosphate product 13 was obtained in 37% yield (27.6 mg). 1H NMR (D2O): δ 8.33 (s, 1H, H-8), 7.97 (d, J = 8 Hz, 2H, Ar), 7.45 (d, J = 8 Hz, 2H, Ar), 6.02 (d, J = 5.9 Hz, 1H, H-1′) (H-2′ is hidden by the water peak), 4.60 (t, J = 4.2 Hz, 1H, H-3′), 4.39 (br m, 1H, H-4′), 4.24 (br dm, 2H, H-5′), 3.50 (br m, 2H, CH2), 3.17 (br m, 2H, CH2). 31P NMR (D2O, pD = 6): δ −7.07 (br s), −11.07 (d), −22.68 (br s) ppm. High-resolution FAB: calcd for C18H23N6-O15,P3S 687.0082, found 687.0070 (MH3−). Retention time: 8.0 min (84% purity) using solvent system I, 7.3 min (98% purity) using solvent system II.
The monophosphate analog (14) was obtained in 35% yield (19.2 mg). 1H NMR (D2O): δ 8.42 (s, 1H, H-8), 7.99 (d, J = 8.0 Hz, 2H, Ar), 7.49 (d, J = 8.0 Hz, 2H, Ar), 6.03 (d, J = 5.9 Hz, 1H, H-1′), 4.50 (m, 1H, H-3′), 4.35 (m, 1H, H-4′), 4.00 (t, J = 4 Hz, 2H, H-5′), 3.57 (m, 2H, CH2), 3.22 (m, 2H, CH2). High-resolution FAB: calcd for C18H20N6O9PS 527.0750, found 527.0738. Retention time: 9.6 min (95% purity) using solvent system I, 7.3 min (95% purity) using solvent system II.
2-[(2-p-Aminophenethyl)thio]adenosine 5′-Triphosphate Tetraammonium Salt (15)
Compound 13 (5 mg, 6.7 pmol) dissolved in 0.5 of mL H2O was hydrogenated overnight at room temperature (60 psi) over PtO2 catalyst. After removal of the catalyst by centrifugation, the product was purified by HPLC (retention time 6.5 min on a semipreparative column, using a linear gradient of TEAA/CH3CN 80:20 to 40:60 in 20 min) and obtained in a quantitative yield. 1H NMR (D2O): δ 8.34 (s, 1H, H-8), 7.14 (d, J = 8.3 Hz, 2H, Ar), 6.77 (d, J = 8.3 Hz, 2H, Ar), 6.05 (d, J = 5.7 Hz, 1H, H-1′) (H-2′ is hidden by the water peak), 4.54 (t, J = 4.2 Hz, 1H, H-3′), 4.39 (br m, IH, H-4′, 4.24 (br dm, 2H, H-5′), 3.40 (br m, 2H, CH2), 2.96 (br in, 2H, CH2). High-resolution FAB: calcd for C18H24N6O13P3S 657.0335, found 657.0323 (MH3−).
2-[(6-Cyanohexyl)thio]adenosine 5′-Triphosphate Tetraammonium Salt (17)
The reaction was carried out on 0.11 mmol of nucleoside 29a by the above procedure. A gradient of 0 to 0.5 M aqueous NH4HCO3 (generated from 230 mL of each) was applied during chromatography to obtain the product in 14% yield (10.7 mg). 1H NMR (D2O): δ 8.48 (br s, 1H, H-8), 6.14 (d, J = 5.8 Hz, 1H, H-1′) (H-2′ is hidden by the water peak), 4.58 J = 4.3 Hz, 1H, H-3′, 4.39 (s, 1H, H-4′), 4.25 (br s 2H, H-5′), 3.22 (AB ddd, 2H, CH2S), 2.47 (t, J = 7 Hz, 2H, CH2), 1.77 (t, J = 7 Hz, 2H,CH2) 1.66 (t, J = 7 Hz, 2H, CH2), 1.48 (n, 2H, CH2) ppm. 31P NMR (D2O, pD = 5): δ −5.6 (d), −11.0 (d), −21.5 (t) ppm. High-resolution FAB: calcd for C17H26N6O13P3S 647.0491, found 647.00493 (M4− + 3H+). Retention time: 6.0 min (91% purity) using solvent system I, 6.2 min (98% purity) using solvent system II.
The monophosphate analogue, 18, was obtained in 69% yield (39.6 mg). 1H NMR (D2O): δ 8.46 (s, 1H, H-8), 6.12 (d, J = 6 Hz, 1H, H-1′), 4.51 (m, 1H, H-3′), 4.35 (m, IH, H-4′), 3.99 (m, 2H, H-5′), 3.22 (ddd, J = 12.4, 5.9 Hz, 2H, CH2S), 2.47 (t, J = 7 Hz, 2H, CH2), 1.76 (m, 2H, CH2), 1.66 (m, 2H, CH2), 1.48 (m, 4H, CH2CH2). 31P NMR (D2O, pD = 5): δ–7.4 ppm. High-resolution FAB: calcd for C17H24N6O7PS 487.1165, found 487.1148 (M2− + H+). Retention time: 4.9 min (85% purity) using solvent system I, 6.1 min (87% purity) using solvent system II.
2-[(7-Bromoheptyl)thio]adenosine (31)
2-Thioadenosine (27a, 0.2 g, 0.67 mmol) was dissolved in 0.25 M NaOH (8 mL, 2 mmol). 1,7-Dibromoheptane (0.31 mL, 1.8 mmol) in EtOH (5 mL) was added, and the solution was stirred vigorously at room temperature for 3 h. The solution was concentrated in the rotary evaporator, and the remaining aqueous solution was extracted with ether (2 × 5 mL). The aqueous phase was neutralized with 1 M HCl. MeOH was added as a cosolvent followed by evaporation (2×). The yellowish residue was chromatographed on a silica column using CHCl3/MeOH, 5:1, as the eluent. The oily product was triturated with CHCl3/ether leaving a white solid (0.104 g, 33% yield, mp 137 °C). 1H NMR (CD3OD): δ 8.16 (s, 1H, H-8), 5.92 (d, J = 5.7 Hz, 1H, H-1′), 4.72 (t, 1H, J = 5.6 Hz, H-2′), 4.31. (dd, J = 5.1, 1.5 Hz, 1H, H-3′), 4 11 (dd, 1H, J = 6.3, 3.2 Hz, H-4′), 3.79 (AB dq, J = 12.4, 3 Hz, 2H, H-5′, 3.43 (t, J = 7 Hz, 2H, CH2Br), 3.15 (m, 2H, CH2S), 1.74 (m, 4H, (CH2)2), 1.46 (m, 6H, (CH2)3) ppm. FAB (positive ions, glycerol matrix): 476, 478 (M + 1). Anal. Calcd for C17H26N5O4SBr: C, 42.86; H, 5.50; N, 14.70. Found: C, 42.97; H, 5.52; N, 14.64.
2-[(7-Bromoheptyl)thiojadenosine 5′-Triphosphate Tetraammonium Salt (32)
The reaction was carried out on 0.11 mmol of nucleoside 31 following the typical procedure. A TLC taken after concentrating the crude reaction mixture by lyophilization indicated the formation of product (silica gel, propano1/28% NH4OH/H2O, 11:8:2, Rf = 0.45) in almost quantitative yield. A gradient of 0 to 0.75 M aqueous NH4HCO3 (generated from 500 mL of each) was applied during chromatography to obtain the product in 68% yield (53.2 mg). 1H NMR (D2O): δ 8.37 (br s, 1H, H-8), 6.11 (d, J = 5.7 Hz, 1H, H-1′), 4.75 (br “t”, 1H, H-2′), 4.56 (br “t”, 1H, H-3′), 4.37 (br s, IH, H-4′), 4.22 (br s, 2H, H-5′), 3.44 (t, J = 7 Hz, 2H, CH2Br), 3.18 (m, 2H, CH2S), 1.75 (m, 4H, (CH2)2), 1.36 (m, 6H, (CH2)3) ppm. High-resolution FAB: calcd for C17H28N5O13BrP3S 713.9801, 715.9782, found 713.9787, 715.9807 (MH3−). Retention time: 13.2 min (>98% purity) using solvent system I, 9.3 min using solvent system II.
2[(7-Aminoheptyl)thiojadenosine 5′-Triphosphate Tetrakis(triethylammonium salt) (19)
Compound 32 (8.1 mg, 10 µmol) was dissolved in 28% NH4OH (0.5 mL) and stirred at room temperature for 3 h. The crude mixture was separated on a semipreparative HPLC column (retention time: 7.9 min using a linear gradient of TEAA/CH3CN, 95:5 to 40:60 in 20 min). The product was obtained in 52% yield (5.6 mg) after repeated lyophilizations. 1H NMR (D2O): δ 8.35 (s, 1H, H-8), 6.11 (d, J = 5.4 Hz, 1H, H-1′) (H-2′ is hidden by the water peak), 4.58 (br s, J = 5.4 Hz, 1H, H-3′), 4.40 (br s, 1H, H-4′), 4.24 (br s, 2H, H-5′), 3.21 (q, 26H, CH2S + Et3N), 2.94 (t, J = 7 Hz, 2H, CH2NH2), 1.75 (t, J = 7 Hz, 2H, CH2), 1.62 (m, 2H, CH2), 1.28 (t, J = 7 Hz, 42H, (CH2)3 + Et3N) ppm. High-resolution FAB: calcd for C17H30-N6O13P3S 651.0804, found 651.0778 (MH3−). Retention time: 7.9 min (94% purity) using 0.1 M TEAA/CH3CN, 95:5 to 40:60 in 20 rain, 5.1 min (87% purity) using solvent system II.
2-[(7-Thioheptyl)thioiadenosine 5′-Triphosphate Tetrakis(triethylammonium salt) (20)
Compound 32 (2 mg, 2.5 µmol) was dissolved in a concentrated solution of NaSH (12 mg, in 0.3 mL H2O) and stirred at room temperature for 20 h. The crude mixture was separated on a semipreparative HPLC column, retention time: 13.0 min using a linear gradient of TEAA (pH 8.4)/CH3CN, 80:20 to 40:60 in 20 min. The product was obtained in 60% yield (1.7 mg) after repeated lyophilizations. 1H NMR (D2O): δ 8.35 (s, 1H, H-8), 6.11 (d, J = 5.4 Hz, 1H, H-1′), 4.61 (t, J = 5.4 Hz, 1H, H-3′), 4.37 (br t, 1H, H-4′), 4.24 (br AB q, 2H, H-5′), 3.20 (br s, 28H, CH2S + Et3), 2.62 (t, J = 7 Hz, 2H, CH2-SH), 1.64 and 1.55 (each: m, 2H, CH2), 1.28 (br.s, 42H, (CH2)3 + Et3) ppm. High-resolution FAB: calcd for C17H29N5O13P3S2 668.0416, found 668.0421 (MH3−).
2-[(7-Thiocyanatoheptyl)thio]adenosine 5′-Triphosphate Tris(triethylammonium salt) (21)
Compound 32 (2 mg, 2.5 µmol) was dissolved in a concentrated solution of KSCN (20 mg, ~100 equiv in 0.3 mL of H2O). The solution was stirred at room temperature for 20 h. The crude reaction mixture was separated on a semipreparative HPLC column. The product was obtained in 48% yield (1.2 mg) after three sequential lyophilizations.1H NMR (D2O): δ 8.40 (s, 1H, H-8), 6.13 (d, J = 5.3 Hz, 1H, H-1′), 4.62 (br “t”, 1H, H-3′), 4.39 (br s, 1H, H-4′), 4.24 (m, 2H, H-5′), 3.20 (q, J = 7 Hz, 18H, Et3), 3.02 (m, 2H, CH2S), 1.77 (m, 5H, CH2), 1.43 (m, 7H, CH2), 1.28 (t, J = 7 Hz, 27H, Et3) ppm. High-resolution FAB: calcd for C18H28N6O13P3S2 693.0369, found 693.0358. Retention time: 7.7 min (>98% purity) using solvent system I.
Biological Assays
Stimulation of inositol phosphate formation by ATP analogues was measured in turkey erythrocyte membranes as described.11,12 The K0.5 values were averaged from three to eight independently determined dose–response curves for each compound. Briefly, 1 mL of washed turkey erythrocytes was incubated in inositol-free medium (DMEM; Gibco) with 0.5–1 mCi of 2-[3H] myo-inositol (20 Ci/mmol; American Radiolabelled Chemicals Inc.) for 18–24 h in a humidified atmosphere of 95% air 5% CO2 at 37 °C. Erythrocyte ghosts were prepared by rapid lysis in hypotonic buffer (5 mM sodium phosphate, pH 7.4, 5 mM MgCl2, 1 mM EGTA) as described.12 Phospholipase C activity was measured in 25 µL of [3H]inositol-labeled ghosts (≈175 µg of protein, 200–500 000 cpm/assay) in a medium containing 424 µM CaCl2, 0.91 mM MgSO4, 2 mM EGTA, 115 mM KCl, 5 mM KH2PO4, and 10 mM Hepes, pH 7.0. Assay (100 µL final volume) contained 1µM GTPγS and the indicated concentrations of nucleotide analogues. Ghosts were incubated at 30 °C for 5 min, and total [3H]inositol phosphates were quantitated by anion exchange chromatography as previously described.11,12
Relaxation of the carbachol-contracted guinea pig taenia coli, rabbit aorta, and rabbit mesenteric artery were measured as described.30,31,35 Muscle segments were mounted in organ baths at 37 °C and bathed in oxygenated Krebs solution, and changes in tension in response to increasing concentrations of nucleotide analogues were recorded (at least two determinations). Similarly contraction of the guinea pig isolated urinary bladder detrussor muscle, guinea pig vas deferens, and rabbit saphenous artery was measured as described.30,31,34
Figure 5.
Phospholipase C activity of 2-thioether analogues of ATP and ADP. Membranes from [3H]inositol-labeled erythrocytes were incubated in the presence of the indicated concentrations of ADP, 2 (■); ATP, 1 (□); 2-(methylthio)-ADP, 7 (●); 2-(methylthio)-ATP, 6 (○); 2-(hexenylthio)-ADP, 10 (▲); or 2-(hexenylthio)-ATP, 9 (△). Incubation was for 5 min at 30 °C in the presence of 1 µM GTPγS as described under Materials and Methods. The data shown are from a representative experiment repeated at least three times using different membrane preparations. [3H]Inositol phosphate accumulation in the presence of GTPγS alone was 200–400 cpm (0%). Maximal levels of [3H) inositol phosphates produced by ADP and ATP analogues in the presence of GTPγS (100%) were identical within the same membrane preparation with values ranging from 5000 to 9000 cpm.
Acknowledgment
The Wellcome Trust is thanked for financial support of A.U.Z. Authors thank Dr. Philip J. M. van Galen for helpful discussions and James E. Ruf for technical assistance. This work was supported in part by USPHS Grants GM38213 and GM29536 to T.K.H.
Abbreviations
- ATP
adenosine 5′-triphosphate
- DMSO
dimethyl sulfoxide
- FAB
fast atom bombardment (mass spectroscopy)
- HPLC
high-pressure liquid chromatography
- HRMS
high resolution mass spectroscopy
- MeATP
adenosine 5′-methylenetriphosphate, (α, β) or (β,γ) isomers
- 2MeSATP
2-methylthioadenosine 5′-triphosphate
- TBAP
tetrabutylammonium phosphate
- TEAA
triethylammonium acetate
- TEAB
triethylaromoniurn bicarbonate
- TLC
thin layer chromatography
- EGTA
1,2-bis(2-aminoethoxy)ethane-N,N,N′,N′-tetraacetic acid
- GTPγS
guanosine-5′-O-thiotriphosphate
- Hepes
N-(2-hyd roxyethyl)piperazine-N′-(2-ethanesulfonic acid).
Footnotes
Presented in part at the Amertican Chemical Society 206th National Meeting, Chicago, IL, Aug 25, 1993, Abstract MEDI214.
References
- 1.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 2.Hoyle CHV, Burnstock G. ATP receptors and their physiological roles. In: Stone TW, editor. Adenosine in the Nervous System. London: Academic Press Ltd; 1991. pp. 43–76. [Google Scholar]
- 3.O'Connor SE, Dainty IA, Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol. Sci. 1991;12:137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- 4.Evans RJ, Derkach V, Suprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;57:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 5.Silinsky EM, Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in celiac neurons of the guinea pig. J. Physiol. (London) 1993;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson KA. Adenosine (P1) and ATP (P2)-purinoceptors. In: Hansch C, Sammes PG, Taylor JB, Emmet JC, editors. Comprehensive Medicinal Chemistry. Pergamon: Oxford; 1990. pp. 601–642. Volume Ed. [Google Scholar]
- 8.Gordon JL. Extracelluar ATP: Effects, sources and fate. Biochem. J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- 10.Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol. Sci. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- 11.Harden TK, Hawkins PT, Stephens L, Boyer JL, Downes P. Phosphoinositide hydrolysis by guanosine 5′-(gamma-thio)-triphosphate-activated phospholipase C of turkey erythrocyte membranes. Biochem. J. 1988;252:583–593. doi: 10.1042/bj2520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer JL, Downes CP, Harden TK. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989;264:884–90. [PubMed] [Google Scholar]
- 13.Pirroton S, Boeynaems JM. Transduction mechanisms of P2-purinergic receptors: Role of phospholipase C and calcium. Nucleos. Nucleot. 1991;10:1003–1017. [Google Scholar]
- 14.Haggblad J, Heilbronn E. P2-purinoceptor-stimulated phosphoinositide turnover in chick myotubea. Calcium mobilization and the role of guanyl nucleotide-binding proteins. FEES Lett. 1988;235:133–136. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- 15.Cooper CL, Morris AJ, Harden TK. Guanine nucleotide-sensitive interaction of a radiolabeled agonist with a phospholipase C-linked P2Y-purinergic receptor. J. Biol. Chem. 1989;264:6202–6206. [PubMed] [Google Scholar]
- 16.Dubyak GR. Signal transduction by P2-purinergic receptors for extracellular ATP. Amer. J. Respir. Cell. Mol. Biol. 1991;4:295–300. doi: 10.1165/ajrcmb/4.4.295. [DOI] [PubMed] [Google Scholar]
- 17.Hoyle CHV. Transmission: Purines. In: Burnstock G, Hoyle CHV, editors. Autonomic Neuroeffector Mechanisms. Chur: Harwood Academic Publishers; 1992. pp. 367–407. [Google Scholar]
- 18.Tatham PER, Cusack NJ, Gomperts BD. Characterization of the ATP4−-receptor that mediates permeabilization of rat mast cells. Eur. J. Pharmacol. 1988;147:13–21. doi: 10.1016/0014-2999(88)90628-0. [DOI] [PubMed] [Google Scholar]
- 19.Zimmet J, Järlebark L, van Galen PJM, Jacobson KA, Heilbronn E. Synthesis and biological activity of novel 2-thio derivatives of ATP. Nucleosides Nucleotides. 1993;12:1–20. doi: 10.1080/07328319308016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson KA, Daly JW. Purine functionalized congeners as molecular probes for adenosine receptors. Nucleosides Nucleotides. 1991;10:1029–1038. [Google Scholar]
- 21.Ralevic V, Burnstock G. Roles of P2s-purinoceptors in the cardiovascular system. Circulation. 1991;84:1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Cusack NJ, Hourani S. Design, syntheses and pharmacology of ATP analogues selective for subtypes of P2-purinoceptors. Nucleosides Nucleotides. 1991;10:1019–1028. [Google Scholar]
- 23.Kovacs T, Ötvös L. Simple synthesis of 5-vinyl- and 5-ethynyi-2′-deoxyuridine-5′-triphosphates. TetrahedronLett. 1988;29:4525–4528. [Google Scholar]
- 24.Moffat JG. A general synthesis of nucleoside-5′-triphosphates. Can. J. Chem. 1964;42:599. [Google Scholar]
- 25.Ludwig J. A new route to nucleoside 5′-triphosphates. Acta Biochim. Biophys. Acad. Sci. Hung. 1981;16:131–133. [PubMed] [Google Scholar]
- 26.Nishra NC, Broom AD. A novel synthesis of nucleoside 5′-triphosphates. J Chem. Soc. Chem. Commun. 1991:1276–1277. [Google Scholar]
- 27.Burnstock G, Fischer B, Maillard M, Ziganshin A, Ralevic V, Knight G, Brizzolara A, von Isakovics A, Bayer JL, Harden TK, Jacobson KA. Structure activity relationships for derivatives of adenosine-5′-triphosphate as agonists at P2Y purinoceptors: heterogeneity within P2X- and P2Y-subtypes. Drug Dev. Res. doi: 10.1002/ddr.430310308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikugawa K, Suehiro H, Yanase R, Aoki A. Platelet aggregation inhibitors. IX. Chemical transformation of adenosine into 2-thioadenosine derivatives. Chem. Pharm. Bull. 1977;25:1959–1969. doi: 10.1248/cpb.25.1959. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G, Cusack NJ, Hills JM, MacKenzie I, Meghji P. Studies on the stereoselectivity of the P2-purinoceptor. Br. J. Pharmacol. 1983;79:907–913. doi: 10.1111/j.1476-5381.1983.tb10535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyle CHV, Edwards GA. Activation of PI- and P2Y-purinoceptors by ADP-ribose in the guinea-pig taenia coli, but not of P2X-purinaceptors in the vas deferens. Br. J. Pharmacol. 1992;107:367–374. doi: 10.1111/j.1476-5381.1992.tb12753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnstock G, Warland JJI. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2Y-but not the P2X-purinoceptor. Br. J. Pharmacol. 1987;90:383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welford LA, Cusack NJ, Hourani SM. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur. J. Pharmacol. 1989;141:123–130. doi: 10.1016/0014-2999(87)90418-3. [DOI] [PubMed] [Google Scholar]
- 33.Hoyle CHV, Burnstock G. Purinergic receptors. In: Doods HN, Van Meel JCA, editors. Receptor Data for Biological Experiments: A Guide to Drug Selectivity. New York: Ellis Horwood; 1992. pp. 54–61. [Google Scholar]
- 34.Hoyle CHV, Knight GE, Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br. J. Pharmacol. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubino A, Thomann H, Henlin JM, Schilling W, Criscione L. Endothelium-dependent relaxant effect of neurokinins on rabbit aorta is mediated by the NK1 receptor. Eur. J. Pharmacol. 1992;212:237–240. doi: 10.1016/0014-2999(92)90335-2. [DOI] [PubMed] [Google Scholar]
- 36.Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 37.Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Nat. Acad. Sci. U.S.A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burnstock G, Hills JM, Hoyle CHV. Evidence that the P1-purinoceptor in the guinea pig taenia coli is an A2 subtype. Br. J. Pharmacol. 1984;81:533–541. doi: 10.1111/j.1476-5381.1984.tb10106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]