Abstract
Matrix metalloproteinase-9 (MMP-9) is an extracellular protease that is induced hours after injury to peripheral nerve. This study shows that MMP-9 gene deletion and neutralization with MMP-9 antibody reduce macrophage content in injured wild-type nerves. In mice with delayed Wallerian degeneration (WldS), MMP-9 and tumor necrosis factor alpha (TNFα) decline in association with the reduced macrophage recruitment to injured nerve that characterizes this strain of mice. We further determined that TNFα acts as an MMP-9 inducer by establishing increased MMP-9 levels after TNFα injection in rat sciatic nerve in vivo and primary Schwann cells in vitro. We found reduced MMP-9 expression in crushed TNFα knockout nerves that was rescued with exogenous TNFα. Finally, local application of MMP-9 on TNFα−/− nerves increased macrophage recruitment to the lesion. These data suggest that TNFα lies upstream of MMP-9 in the pathway of macrophage recruitment to injured peripheral nerve.
Keywords: TNF null, Real-time RT-PCR, Taqman, macrophage migration, MMP, Schwann cell
Introduction
Identifying the early genes that modulate peripheral nerve Wallerian degeneration is important to developing targeted therapeutic intervention. MMP-9, or gelatinase B, belongs to the matrix metalloproteinase (MMP) family of Ca2+-activated, Zn2+-dependent extracellular proteases (Woessner, 1994) and degrades type IV collagen of basal laminae throughout tissues, including Schwann cell basal lamina and collagen barriers of endothelial and perineurial cells in the peripheral nervous system. MMP-9 is barely detectable in uninjured peripheral nerve and induced by such injuries as chronic constriction (Shubayev and Myers, 2000), crush (La Fleur et al., 1996; Kherif et al., 1998; Ferguson and Muir, 2000; Platt et al., 2003; Demestre et al., 2004), transection (Siebert et al., 2001; Hughes et al., 2002), and toxins (Talhouk et al., 2000). MMP-9 activity is linked to experimental models of neuropathic pain (Leppert et al., 1999; Shubayev and Myers, 2000, 2002; Talhouk et al., 2000; Misko et al., 2002) and is elevated in patients with symptomatic neuropathy (Leppert et al., 1999; Mawrin et al., 2003; Renaud et al., 2003; Gurer et al., 2004). It is produced mainly by Schwann cells and endoneurial macrophages (La Fleur et al., 1996; Shubayev and Myers, 2002) and promotes blood nerve barrier degradation, demyelination, and macrophage infiltration in injured nerve (Kieseier et al., 1999; Siebert et al., 2001).
The factors that cause upregulation of MMP-9 in the peripheral nervous system have not been identified. Tumor necrosis factor alpha (TNFα) is a pleotropic cytokine of an early gene family that has been repeatedly implicated in the pathogenesis of Wallerian degeneration (Stoll et al., 2002). TNFα deletion studies have identified macrophage recruitment as the main TNFα function in damaged nerve (Liefner et al., 2000; Siebert and Bruck, 2003). Although the consequences of TNFα upregulation such as demyelination and macrophage migration (Wagner and Myers, 1996b) are known to be synergistic with MMP-9, how and whether MMP-9 and TNFα interact in peripheral nerve are unknown. There are several different molecular levels at which they can be linked. For example, TNFα induces MMP-9 expression in many systems (Saren et al., 1996; Nagase, 1997; Singer et al., 1999; Genersch et al., 2000; Kauppinen and Swanson, 2005), while MMP-9 has the ability to activate TNFα release from its transmembrane precursor (Gearing et al., 1994) and to inactivate TNFα-mediated signaling by sequestering TNF receptors TNFRI (p55) and TNFRII (p75) (Williams et al., 1996). In the CNS, TNFα has been shown to induce MMP-9 expression (Rosenberg et al., 1995), and correlative studies in sciatic nerve indicate that endoneurial MMP-9 expression follows TNFα expression temporally and spatially (La Fleur et al., 1996; Shubayev and Myers, 2000, 2002), suggesting that if linked, TNFα is likely to act as an MMP-9 inducer.
The purpose of this study was to determine whether TNFα induces injury-specific MMP-9 in peripheral nerve and whether these factors are functionally linked with macrophage recruitment—using wild-type, MMP-9 knockout, TNFα knockout, and slow (WldS) models of Wallerian degeneration.
Results
MMP-9 controls macrophage recruitment into injured nerve
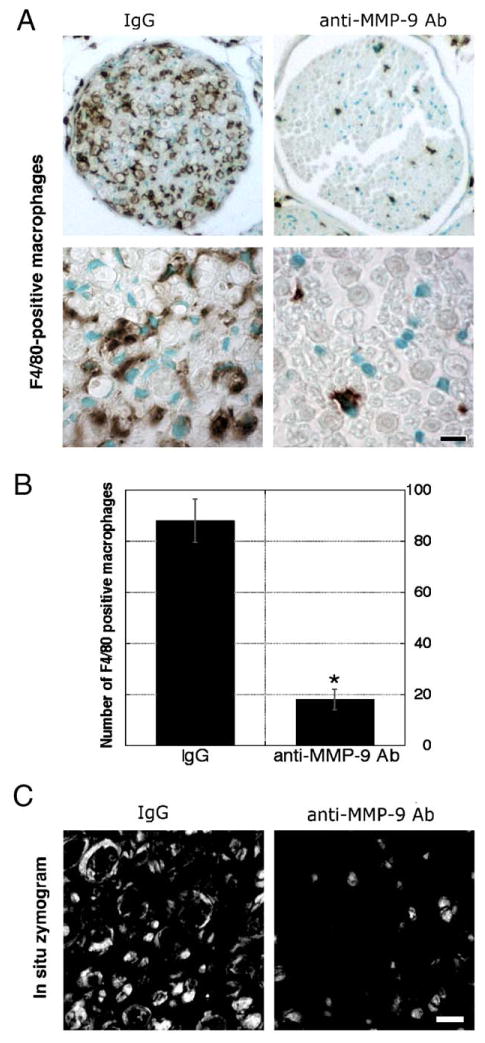
One week after injury, crushed nerves have high macrophage content (Bendszus and Stoll, 2003). Neuropathological assessment of plastic-embedded MMP-9 knockout mouse nerves (Fig. 1) showed a striking reduction in axonal degeneration and macrophage content at 1 week after crush injury, as compared by control mouse nerves. Administration of MMP-9 neutralizing antibody for a week by intraperitoneal injections once daily, starting immediately after mouse sciatic nerve crush, was assessed by immunohistochemistry for a macrophage-specific F4/80 antigen (Figs. 2A, B) showing high infiltration of macrophages in vehicle (rabbit IgG)-treated tissue, while MMP-9 inhibition resulted in reduced macrophage content, consistent with an earlier report (Siebert et al., 2001). In situ zymography confirmed the efficacy of MMP-9 inhibition in the corresponding nerves (Fig. 2C), demonstrating residual intraaxonal gelatinolytic activity in anti-MMP-9-treated tissue due to a second gelatinolytic protease, an MMP-2 (Shubayev and Myers, 2002).
Fig. 1.
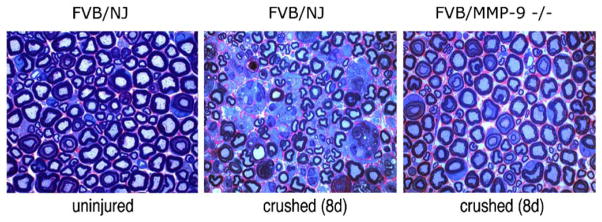
Neuropathology of injured MMP-9 knockout nerves. Plastic-embedded sciatic nerve sections (0.75 μm) of MMP-9−/− and control FVB/NJ mice. Note that macrophage content is increased in FVB/NJ nerves after crush and is reduced after MMP-9 deletion. Sections are stained with methylene blue Azure II. Objective magnification, ×100. Micrographs are representative of 8 mice/group.
Fig. 2.
Anti-MMP-9 therapy reduces macrophages content. (A) F4/80 immunoreactivity in crushed mouse (wild-type, C57BL) sciatic nerves (10 μm) after daily i.p. treatment with MMP-9-neutralizing antibody (50 mg/kg/day) or vehicle (rabbit IgGs) for a week. (B) Densitometric image analysis of F4/80-positive macrophage number shows an 8-fold decrease after MMP-9 inhibition (graph, *P < 0.05). (C) In situ zymography shows gelatinolytic activity in the vehicle-treated nerve that was inhibited after MMP-9 neutralization. Objective magnification, ×10 and ×100 (scale bars = 5 μm). Micrographs are representative of 4 mice/group.
MMP-9 and TNFα expression is diminished in WldS nerves
To correlate the levels of MMP-9 and TNFα expression with macrophage content in degenerating nerve, we used the model of WldS degeneration (Coleman and Ribchester, 2004). In normal mice at 6 h after sciatic nerve injury, MMP-9 and TNFα expression are elevated, and, at 5 days after injury, TNFα is released from its precursor (Shubayev and Myers, 2000). We used these time-points to assess MMP-9 and TNFα mRNA and protein levels in WldS mice.
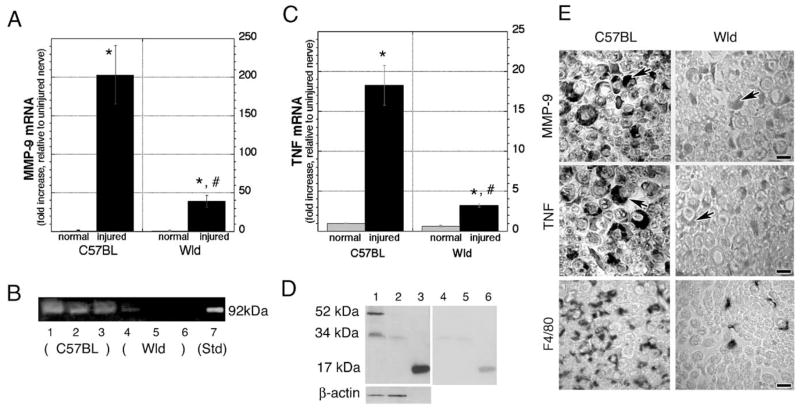
Real-time RT-PCR for MMP-9 (Fig. 3A) showed a 203 ± 37-fold increase in MMP-9 in injured wild-type C57BL nerves relative to uninjured control. In contrast, MMP-9 mRNA was elevated only 39 ± 8-fold in WldS nerves after injury. This corresponds to a 5-fold or 80% decline in MMP-9 mRNA in injured WldS relative to C57BL nerves. Uninjured C57BL and WldS nerves had low but detectable MMP-9 levels that were not significantly different between the two phenotypes. Matching gelatin zymography (Fig. 3B) displayed a reactive 92 kDa gelatinolytic MMP-9 band (against a dark background of undegraded gelatin) in C57BL nerves that was barely detectable in WldS nerves, corresponding to an 87% decline in MMP-9 (P < 0.01). Uninjured wild-type and WldS nerves showed no detectable MMP-9 activity (not shown), as expected (Shubayev and Myers, 2000).
Fig. 3.
MMP-9 and TNFα expression is reduced in crushed WldS nerves. (A) Real-time Taqman RT-PCR for MMP-9, using GAPDH as a normalizer. Data are expressed as the fold increase in crushed (6 h time-point) relative to uninjured nerves (*P < 0.05). Note a significant decline in MMP-9 mRNA in WldS relative to control C57BL nerves (#P < 0.05). One-way ANOVA followed by Tukey’s post-hoc test (n = 20/group). (B) Gelatin zymography showing MMP-9 activity in crushed C57BL nerves (lanes 1–3, representing 3 different samples) that was reduced in WldS nerves (lanes 4–6). MMP-9 standard (lane 7) indicated a clear 92 kDa band against the dark background of undegraded gelatin (n = 6/group). (C) Real-time Taqman RT-PCR for TNFα, using GAPDH as a normalizer. Data are expressed as the fold increase in crushed (6 h time-point) relative to uninjured nerves (*P < 0.05). Note a six-fold decline in TNFα mRNA in injured (#P < 0.05) and a 63% decline in uninjured WldS relative to control C57BL nerves. One-way ANOVA followed by Tukey’s post-hoc test (n = 20/group). (D) Western blot for TNFα in nondenatured crushed wild-type nerves showed 52 and 34 kDa isoforms (lane 1) that were low in WldS nerves (lane 2). Recombinant rat TNFα, a 17 kDa monomer (lane 3), was used for positive control and for preabsorption experiments (lanes 4–6). Gel loading was controlled by β-actin (n = 6/group). (E) Immunohistochemistry for MMP-9, TNFα, and F4/80 in wild-type and WldS nerves at 3 days after crush. Note the reduced Schwann cell reactivity (arrows) for both MMP-9 and TNFα and reduced macrophage (F4/80) content in WldS versus C57BL nerves. Objective magnification, ×100 (scale bars = 5 μm). Micrographs are representative of 4 mice/group.
Real-time RT-PCR for TNFα (Fig. 3C) showed an 18.3 ± 2.5-fold increase in TNFα mRNA in C57BL nerves after injury (P < 0.05), while only a 3.2 ± 0.2-fold increase in WldS nerves, corresponding to a 6-fold or 93% decline in injured WldS relative to control mice (P < 0.05). Before injury, TNFα mRNA was 63% lower in WldS nerves. Western blots for TNFα in matched nondenaturing nerves (Fig. 3D) showed predominant 52 and 34 kDa species at 5 days after nerve crush, representing a trimer and a dimer, respectively, with the former being the most prevalent and potent isoform (Smith and Baglioni, 1987; Wingfield et al., 1987). Both isoforms were declined in WldS nerves. Gel loading was controlled with β-actin at 42 kDa.
Characteristic immunoreactivity in activated Schwann cells was observed for MMP-9 and TNFα in control nerves at 3 days post-crush (Fig. 3E) and was reduced in WldS nerves. Macrophage content in the respective nerve sections identified by macrophage-specific F 4/80 antigen showed low macrophage content in WldS nerves, correlating with the low MMP-9 and TNFα expression.
These data show that MMP-9 and TNFα coordinately decline during WldS degeneration, consistent with the hypotheses that these factors interact during Wallerian degeneration and may have a coordinate role in macrophage recruitment.
TNFα is an MMP-9 inducer in peripheral nerve
TNFα is a known inducer of MMP-9 in many systems (Nagase, 1997), including the CNS (Rosenberg et al., 1995). To analyze the in vivo and in vitro effects of exogenous TNFα treatment on MMP-9 expression, we first microinjected TNFα into the fascicle of crushed rat sciatic nerve and, 48 h after injection, measured MMP-9 expression by real-time RT-PCR (Fig. 4A). Injury produced a 142 ± 24-fold increase in MMP-9 mRNA in the vehicle (BSA) group, while TNFα injection caused a 325 ± 65-fold increase corresponding to a significant (P = 0.046) 2-fold increase in MMP-9 relative to vehicle.
Fig. 4.
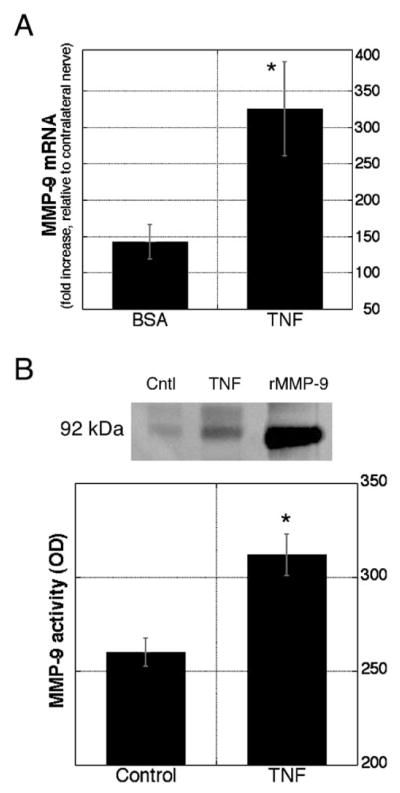
TNFα induces MMP-9 expression in peripheral nerve. (A) TNFα injection into sciatic nerve induced MMP-9 mRNA. Real-time RT-PCR using Taqman probes and primers for rat MMP-9 and GAPDH (normalizer). Data are expressed as the fold increase to contralateral nerve and compared in TNFα-treated with vehicle-treated groups (n = 10/group, *P < 0.05). (B) TNFα treatment of cultured primary Schwann cells induced MMP-9 activity. Gelatin zymography (inverted image) of conditioned media before (lane 1) and after (lane 2) treatment with 50 ng/ml of TNFα. Recombinant MMP-9 was used as positive control (lane 3). MMP-9 levels were assessed by densitometry of 3 samples per each treatment in triplicate experiments (graph, *P < 0.05).
Given that Schwann cells are the first cells to induce MMP-9 in injured sciatic nerve (Shubayev and Myers, 2002), we analyzed the effect of TNFα treatment on MMP-9 levels and proteolytic activity in cultured primary Schwann cells isolated from rat sciatic nerves (Fig. 4B), showing that basal MMP-9 levels in untreated cells were significantly induced with 50 ng/ml of TNFα.
Consistent with the ability of TNFα to induce MMP-9, injured nerves of TNFα knockout mice showed reduced MMP-9 expression at 1 day after crush, relative to control mice (Fig. 5A). Gel loading was controlled with β-actin. To confirm that MMP-9 decline in TNFα−/− nerves was a direct result of TNFα deletion, TNFα−/− nerves were treated locally with exogenous TNFα, which caused an increase in MMP-9 mRNA (Fig. 5B).
Fig. 5.
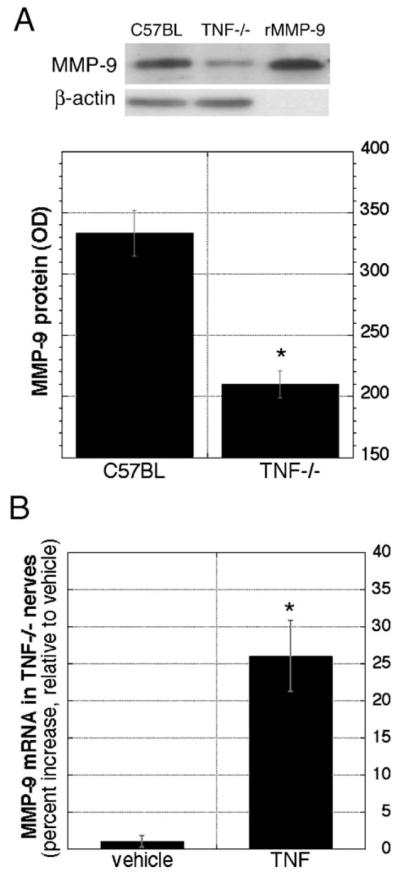
Reduced MMP-9 levels in TNFα knockout nerves are restored by TNFα treatment. (A) Western blot for MMP-9 (92 kDa) in crushed wild-type nerves (lane 1) was of reduced intensity in TNFα knockout (TNF−/−) nerves (lane 2). Recombinant MMP-9 (lane 3) was used for positive control, and β-actin for gel loading control. Densitometric analyses for MMP-9 protein were done using n = 6/group (graph, *P < 0.05). (B) Real-time Taqman RT-PCR for MMP-9 in injured TNFα−/− nerves after TNFα treatment (100 ng), using GAPDH as a normalizer. Data are expressed as the percent increase in TNFα-treated versus BSA-treated nerves. Note a significant increase in MMP-9 mRNA after TNFα−/− nerves were rescued with exogenous TNF (n = 12/group; *P < 0.05).
MMP-9 rescues macrophage recruitment in TNFα knockout mice
TNFα−/− nerves are deficient in their ability to recruit macrophages (Liefner et al., 2000). Consistent with our data indicating that TNFα is an MMP-9 inducer and that MMP-9 controls macrophage migration, we showed that local MMP-9 treatment of TNFα−/− nerves enhanced their ability to recruit macrophages (Fig. 6A), suggesting that MMP-9 mediates TNFα-induced macrophage migration.
Fig. 6.
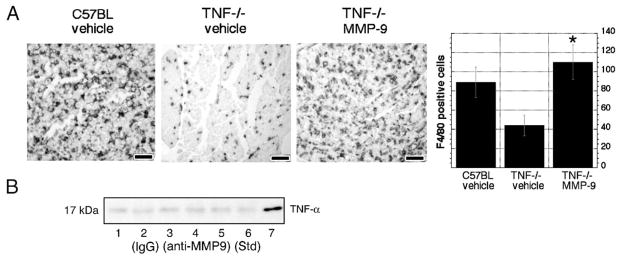
MMP-9 mediates TNFα-induced macrophage recruitment. (A) F4/80 immunoreactivity in control and TNFα−/− nerves 5 days after crush, before and after local MMP-9 (100 ng) treatment. Note high macrophage content in control C57BL nerves that was reduced in TNFα−/− nerves and normalized after MMP-9 treatment. Objective magnification, ×20 (scale bar = 25 μm), micrographs are representative of 4 animals/group. Bar graphs show results of densitometric image analysis (*P < 0.05). (B) Western blot for TNFα in crushed rat sciatic nerves after daily i.p. treatment with anti-MMP-9 antibody (10 μg/day) for a week. Note that the band intensity of a soluble TNFα isoform was not different in nerves of vehicle-treated (lanes 1–3, representing 3 different samples) and MMP-9-inhibited samples (lanes 4–6). A recombinant TNFα protein was used for positive control (lane 7) (n = 3/group).
Since MMP-9 can activate TNFα release from its transmembrane precursor in vitro (Gearing et al., 1994), we tested whether the levels of soluble TNFα changes in injured nerve change after a week of daily treatment with MMP-9 inhibitor (Fig. 6B), when soluble TNFα is present (Shubayev and Myers, 2000). We found no change in soluble TNFα between MMP-9 inhibitor and vehicle groups, suggesting that MMP-9 is not involved in TNFα release and therefore would not promote macrophage migration via TNFα activation, consistent with TNFα deletion studies.
Discussion
TNFα and MMP-9 are important early modulators of nerve injury, and their expression correlates temporally, spatially and functionally (La Fleur et al., 1996; Shubayev and Myers, 2000, 2002). This manuscript is the first to show that TNFα acts as an MMP-9 inducer in peripheral nerve in vivo and in Schwann cells in vitro. It is particularly important to identify regulators of MMP-9 since in peripheral nerve it is induced only with injury. Specifically, MMP-9 protein and activity are undetectable in normal nerve but rapidly increase within 3 h after injury (Shubayev and Myers, 2000). MMP-9 mRNA in normal nerve is low but measurable (La Fleur et al., 1996; Hughes et al., 1998, 2002; Demestre et al., 2004) and increases greater than 200-fold in injured nerve (the changes in the corresponding dorsal root ganglia and spinal cord are only 2- to 3-fold, unpublished observation). These unique characteristics make MMP-9 a sensitive early molecular biomarker of peripheral nerve injury. This study supports earlier observations, that TNFα functions at a lower range of expression, showing 2- to 20-fold induction of TNFα mRNA (La Fleur et al., 1996; Taskinen et al., 2000; Lee et al., 2004). In situ TNFα treatment of cultured Schwann cells and sciatic nerve in vivo caused modest induction in MMP-9, compared to its large increase after sciatic nerve injury. High baseline levels of TNFα in the controls (vehicle-treated tissue and Schwann cells) could have reduced this comparative change. In addition, other cytokines and neurotrophic factors may contribute to MMP-9 induction in degenerating nerve, producing a cumulative TNFα effect in injured nerve.
Schwann cells are the first non-neuronal cells to respond to axonal injury by secreting TNFα (Wagner and Myers, 1996a; Shamash et al., 2002), and these cells showed a TNFα-mediated increase in MMP-9 in vitro. Other cells in the endoneurium may also induce MMP-9 in response to TNFα, such as fibroblasts (Singer et al., 1999) and endothelial cells (Genersch et al., 2000). It remains to be seen if there is a bimodal effect in TNFα regulation of MMP-9 expression or whether its effect is consistent between different Schwann cell phenotypes, especially in light of the roles of MMP-9 and TNFα in myelination.
The present study and earlier reports indicate that macrophage recruitment is the main function of MMP-9 and TNFα (Liefner et al., 2000; Siebert et al., 2001). Our TNFα deletion studies suggest that MMP-9 mediates TNFα-induced macrophage recruitment. In addition, TNFα induces other chemotactic factors, such as monocyte chemoattractant protein-1 (MCP-1) (Subang and Richardson, 2001). It is interesting to note that TNFα is expressed only by pre-phagocytic macrophages (Stoll et al., 1993) and that TNFα deletion reduces macrophage influx without affecting their phagocytic capacity (Liefner et al., 2000). MMP-9 ability to promote macrophage migration after TNFα deletion also suggests that, after being induced by TNFα, MMP-9 promotes macrophage migration independent of TNFα. This is consistent with the hypothesis that MMP-9 is not involved in soluble endoneurial TNFα release in vivo. Other metalloproteases, such as MMP-2 and TNFα converting enzyme (TACE), are likely to control TNFα release (Shubayev and Myers, 2000, 2002).
The WldS mouse strain displays a phenotype of slow Wallerian degeneration due to spontaneous mutation on chromosome 4 (Lyon et al., 1993) that is intrinsic to axons (Lunn et al., 1989; Perry et al., 1990) and is identified as an 85-kb tandem triplication (Coleman et al., 1998) that translates into a neuroprotective chimeric fusion protein of ubiquitin assembly factor E4B (Ube4b) and mononucleotide adenylyltransferase (Nmnat) (Conforti et al., 2000). Using the model of WldS degeneration, we found a positive correlation between TNFα and MMP-9 expression and the macrophage migration capacity of injured nerve. These changes in MMP-9 during WldS degeneration have not been previously reported, but deficiencies in endoneurial levels of Ca2+-dependent proteases have been thought to contribute to WldS degeneration (Glass et al., 1994). Other reports have demonstrated reduced TNFα immunoreactivity in WldS nerves after chronic constriction injury (Sommer and Schafers, 1998) and axotomy (Shamash et al., 2002). In addition to TNFα protein levels, Shamash et al. (2002) showed detectable TNFα mRNA in injured WldS nerves. Herein, we expanded this observation in a quantified approach showing that, although detectable, TNFα mRNA and protein are drastically downregulated in WldS when compared to control mouse nerves. This reduced MMP-9 and TNFα expression may contribute to poor macrophage migration as well as other aspects of WldS degeneration, such as increased tolerance to neuropathic pain (Myers et al., 1996; Sommer and Schafers, 1998). Although endoneurial macrophages produce TNFα and MMP-9 (Shubayev and Myers, 2002), the reduced macrophage content in the WldS phenotype may contribute to reduced MMP-9 and TNFα levels we observed. However, the decline in MMP-9 and TNFα occurs earlier (6 h after nerve crush) than the first hematogenously recruited macrophages appear in nerve (24 h after crush) (Bruck, 1997). Considering that nerve sheath transplants containing wild-type Schwann cells do not cause WldS nerves to revert to the wild-type phenotype (Glass et al., 1993), it is granted that the decline in Schwann cell-derived MMP-9 and TNFα is not causative of slow (WldS) Wallerian degeneration, but rather consequent to the effects of the Ube4b/Nmnat gene on peripheral axons. Increasing evidence suggests that injured WldS nerves undergo a process of axonal atrophy via fundamentally different mechanisms than a rapid Wallerian degeneration of the wild-type phenotype (Beirowski et al., 2005).
In conclusion, we show that TNFα is a key inducer of nerve-injury-specific MMP-9 and that MMP-9 mediates TNFα-induced macrophage migration. It remains to be determined whether other cytokines, such as IL-1β and IFNγ (Nagase, 1997), and neurotrophic factors, such as NGF (Muir, 1994; Shubayev and Myers, 2004), regulate endoneurial MMP-9 and if degenerative and neuroprotective roles of MMP-9 are regulated via independent signaling pathways.
Experimental methods
Animal surgery
Adult female Sprague–Dawley rats (n = 28; 250 g, Harlan Labs), TNFα knockout (TNF−/−, B6,129S6-Tnftm1Gkl/J, n = 52; 20 g, Jackson Labs), MMP-9 knockout (MMP-9−/−, n = 10, FVB.Cg-Mmp9tm1Tvu/, Jackson Labs), mutant C57BL/WldS/Ola (WldS, n = 52; 20 g, Harlan Labs), and control C57BL/6J (n = 88; 20 g, Harlan or Jackson, respectively) or FVB/NJ (n = 10, 20g, Jackson Labs) female mice were anesthetized with isofluorane (IsoSol; Vedco, St. Joseph, MO) or with intraperitoneal injection of a rodent anesthesia cocktail containing 60 mg/kg ketamine (Phoenix Scientific, St. Joseph, MO), 1.2 mg/kg acepromazine (Fermenta Animal Health, Kansas City, MO), and 6.4 mg/kg xylazine (Boehringer Pharmaceuticals, St. Joseph, MO). The sciatic nerve was exposed unilaterally at the mid-thigh level and crushed using fine, smooth surface forceps twice for 2 s each to produce a sciatic nerve crush. All procedures were performed according to protocols approved by the University of California, San Diego and the VA Healthcare System Committee on Animal Research and conform to the NIH Guidelines for Animal Use.
Primary Schwann cell cultures
Schwann cells were isolated from sciatic nerves of 1-day-old Sprague–Dawley rats as previously described (Campana et al., 1998) and further separated from fibroblasts using an anti-fibronectin antibody and rabbit complement. This resulted in approximately 99% pure Schwann cell cultures as assessed by S100 immunofluorescence. Primary Schwann cells were maintained in DMEM containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100μg/ml streptomycin, 21 μg/ml bovine pituitary extract, and 4μM forskolin and incubated at 37°C under humidified 5.5% CO2. Schwann cells were expanded by passing the cells twice after the cultures were established.
MMP-9, TNFα, and MMP-9 inhibitor therapy
Recombinant murine TNFα (R&D Systems) or bovine serum albumin (BSA, Sigma, 0.1%, vehicle) was (1) injected into rat sciatic nerve at 24 h following crush injury using a 33-gauge needle at 250 ng in 5 μl as shown before (Shubayev and Myers, 2001); (2) applied in situ onto a crushed sciatic nerve of TNF−/− mice using a gelfoam at 100 ng in 10 μl; or (3) applied to cultured primary Schwann cells at 1–50 ng/ml for 24 h. Recombinant human MMP-9 (Chemicon) was applied in situ onto a crushed TNFα−/− mouse sciatic nerve using a gelfoam at 100 ng in 10 μl. Rabbit MMP-9-neutralizing antibody (10 μg/day, Chemicon) or normal rabbit IgG (Vector, vehicle) was suspended in PBS and administered by intraperitoneal injection starting 1 h following sciatic nerve crush once daily for 7 days.
Antibodies
The following antibodies were used for immunohistochemistry and Western blotting: goat anti-TNFα (R&D, 1:100), rabbit anti-MMP-9 (Torrey Pines Labs, 1:500), rat anti-mouse F4/80 (Serotec, 1:100), mouse anti-β-actin (Sigma, 1:10,000). Respective normal serum or IgG was used for negative control. Signal specificity was confirmed by preabsorption of primary antibody with recombinant TNFα and MMP-9 proteins.
Quantitative Taqman real-time RT-PCR
Samples were stored in RNA-later (Ambion) at −20°C. Total RNA was extracted with Trizol (Invitrogen), purified on RNeasy mini columns (Qiagen), and treated with RNase-free DNAse I (Qiagen). The RNA purity was verified by OD260/280 absorption ratio of 1.9–2.0. cDNA was synthesized using SuperScript II first-strand RT-PCR kit (Invitrogen). MMP-9 and TNFα gene expression was measured by quantitative real-time polymerase chain reaction (MX4000, Stratagene, La Jolla, CA) using 25 ng of mouse or 50 ng of rat cDNA and 2× Taqman universal PCR master mix (Applied Biosystems) with a one-step program (95°C for 10 min, 95°C for 30 s, and 60°C for 1 min for 50 cycles). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression was used as a normalizer for each sample. Primers and Taqman probes were designed using Primer Express 2.0 software (Applied Biosystems), obtained from Biosearch Technologies (Novato, CA) (Table 1), and their concentrations were optimized using spleen and injured sciatic nerve cDNA (amplification efficiency of 100.1–100.3%). GAPDH expression was confirmed to be not significantly different in injured and uninjured nerves. Duplicate samples without cDNA (no-template control) showed no contaminating DNA.
Table 1.
Primer and probe sequences for TNFα, MMP-9 and GAPDH for Taqman real-time RT-PCRa
| Gene | Type | Sequences (5′–3′) |
|---|---|---|
| MMP-9 mouse (NM_013599) | Forward | CGACGACGACGAGTTGTG |
| Reverse | CATGGGGCACCATTTGAGTT | |
| Probe | AAGGCGTCGTGATCCCCACTTACT | |
| MMP-9 rat (NM_031055) | Forward | GTAACCCTGGTCACCGGACTT |
| Reverse | ATACGTTCCCGGCTGATCAG | |
| Probe | CGCGTCGTGGAGGGAAGGCTC | |
| TNFα mouse (NM_013693) | Forward | GCCACCACGCTCTTCTGT |
| Reverse | GGAGGCCATTTGGGAACT | |
| Probe | TACTGAACTTCGGGGTGATCGGTC | |
| GAPDH mouse (NM_008084) | Forward | CAACGGGAAGCCCATCAC |
| Reverse | CGGCCTCACCCCATTTG | |
| Probe | CTTCCAGGAGCGAGACCCCACTAACA | |
| GAPDH rat (XO2231) | Forward | GAACATCATCCCTGCATCCA |
| Reverse | CCAGTGAGCTTCCCGTTCA | |
| Probe | CTTGCCCACAGCCTTGGCAGC |
Taqman probe containing 5′ reporter FAM and 3′ quencher BHQ-1 dyes (in parentheses, GeneBank accession numbers).
Gelatin zymography
Tissue and cell lysates were prepared with non-reducing, protease-inhibitor-free Laemmli sample buffer; cell conditioned media were supplemented with 0.1% SDS, clarified by centrifugation, and normalized to cell number. Samples containing 25–45 μg of protein (BSA Protein Assay, Pierce) were heated at 55°C for 5 min and run on 10% SDS polyacrylamide gel containing 1 mg/ml of gelatin at 160 V for 90 min (Shubayev and Myers, 2000). The gels were washed in 2.5% Triton X-100, developed at 37°C overnight in 50 mM Tris–HCl, 150 mM NaCl, 5 mM CaCl2, 1 μM ZnCl2, and 0.2 mM sodium azide (pH 7.6), and stained with colloidal blue (Invitrogen), indicating gelatinolytic MMP-9 activity as a clear band on a dark background of undegraded gelatin. Zymography standard containing recombinant human MMP-9 (Chemicon) was used for control.
Western blotting
Nerves were lysed in 63 mM Tris–HCl, 10% glycerol, 2% SDS, 10 mM PMSF, 5 mM EDTA, and protease inhibitor cocktail (Sigma) (pH 6.8) as described (Shubayev and Myers, 2000). Samples containing 85 μg of protein (BSA Protein Assay, Pierce) were reduced with 10% β-mercaptoethanol (Fisher) or analyzed in native conditions without the reducing agent using 10 or 15% SDS-PAGE in a Laemmli system. Transfer to nitrocellulose at 50 V for 60 min in transfer buffer (12 mM TRIS Base, 95 mM glycine, 20% methanol, pH 8.3) was followed by nonspecific binding block in 5% nonfat dry milk (Bio-Rad), a primary antibody (above) incubation overnight at 4°C, HRP-tagged goat anti-mouse or anti-rabbit IgG, and detection with enhanced chemiluminescence (Amersham). Molecular weight was determined using HRP-tagged SDS-PAGE standards (Bio-Rad). Recombinant human MMP-9 (Chemicon) and murine TNFα (R&D) were used for positive controls. The blots were stripped and reprobed for preabsorption or gel loading (β-actin) controls.
Histology and immunohistochemistry
Plastic-embedded nerve sections (0.75 μm) were used for neuropathologic evaluation to avoid the structural artifacts caused by paraformaldehyde fixation and paraffin embedding. Mouse tissues were removed and placed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, osmicated, dehydrated, and embedded in araldite resin. Sections were cut with a glass knife on an automated microtome (Leica) and stained with methylene blue Azure II for light microscopy.
Paraffin-embedded, 4% paraformaldehyde-fixed nerve sections (10 μm) were deparaffinized with xylenes, rehydrated in graded ethanol, PBS, and endogenous peroxidase was blocked with 3% H2O2. Antigen retrieval (Dako, Carpinteria, CA) was applied for 5 min at 95°C then for 20 min at room temperature. Nonspecific binding was blocked with 10% normal rabbit (F4/80, TNFα) or goat (MMP-9) serum followed by primary antibody (above) incubation overnight at 4°C and biotinylated rabbit anti-rat (F4/80), rabbit anti-goat (TNFα), or goat anti-rabbit (MMP-9) antibody (Vector) and avidin–biotin complex (ABC Elite, Vector). Sections were developed with 3′3-diaminobenzidine (DAB, brown, Vector) and, in some cases, counter-stained with methyl green (Fisher).
In situ zymography
Cryosections (10 μm) of unfixed nerves were mounted on Superfrost slides (Fisher) and immersed in reaction buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM CaCl2, and 0.2 mM sodium azide, pH 7.6) supplemented with 40 μg/ml fluorescein-labeled gelatin (Molecular Probes) (Krekoski et al., 2002). The sections were developed for 24 h at 37°C, rinsed with PBS, fixed with 4% paraformaldehyde in phosphate buffer, rinsed with water, and mounted. Gelatinolytic activity in the tissue sections generated fluorescein–gelatin peptides that were detected by fluorescent microscopy.
Data analyses
For real-time RT-PCR, 5–7 samples per group were quantified using the comparative Ct method (Livak and Schmittgen, 2001). Relative mRNA levels were normalized to GAPDH and compared in the experimental group (TNFα-treated, crushed nerve) to the calibrator group (vehicle-treated, normal nerve), and a fold or percent change was determined by the MX4000 as described (Pfaffl, 2001). Zymograms and Western blots were digitized using an EC3 Darkroom (UVP Imaging) and quantified by LabWorks 4.5. Immunoreactivity was analyzed by Openlab 4.0 (Improvision) in 4 areas/section, 4 sections/block, and 4 animals/group. Results from at least three independent experiments were used to obtain the data plotted in all figures. Significance was assessed by ANOVA followed by Tukey’s post-hoc test or by Student’s t test, and significance was set at P < 0.05.
Acknowledgments
We thank Heidi Heckman for expert technical assistance and Amber Millen for help in preparation of the manuscript. Supported by the Department of Veteran Affairs and NIH Grant NS18715.
References
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendszus M, Stoll G. Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging. J Neurosci. 2003;23:10892–10896. doi: 10.1523/JNEUROSCI.23-34-10892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Hiraiwa M, O’Brien JS. Prosaptide activates the MAPK pathway by a G-protein-dependent mechanism essential for enhanced sulfatide synthesis by Schwann cells. FASEB J. 1998;12:307–314. doi: 10.1096/fasebj.12.3.307. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Ribchester RR. Programmed axon death, synaptic dysfunction and the ubiquitin proteasome system. Curr Drug Targets CNS Neurol Disord. 2004;3:227–238. doi: 10.2174/1568007043337436. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demestre M, Wells GM, Miller KM, Smith KJ, Hughes RA, Gearing AJ, Gregson NA. Characterisation of matrix metal-loproteinases and the effects of a broad-spectrum inhibitor (BB-1101) in peripheral nerve regeneration. Neuroscience. 2004;124:767–779. doi: 10.1016/j.neuroscience.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci. 2000;16:157–167. doi: 10.1006/mcne.2000.0859. [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Genersch E, Hayess K, Neuenfeld Y, Haller H. Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: involvement of Ras-dependent and -independent pathways. J Cell Sci. 2000;113(Pt 23):4319–4330. doi: 10.1242/jcs.113.23.4319. [DOI] [PubMed] [Google Scholar]
- Glass JD, Brushart TM, George EB, Griffin JW. Prolonged survival of transected nerve fibres in C57BL/Ola mice is an intrinsic characteristic of the axon. J Neurocytol. 1993;22:311–321. doi: 10.1007/BF01195555. [DOI] [PubMed] [Google Scholar]
- Glass JD, Schryer BL, Griffin JW. Calcium-mediated degeneration of the axonal cytoskeleton in the Ola mouse. J Neurochem. 1994;62:2472–2475. doi: 10.1046/j.1471-4159.1994.62062472.x. [DOI] [PubMed] [Google Scholar]
- Gurer G, Erdem S, Kocaefe C, Ozguc M, Tan E. Expression of matrix metalloproteinases in vasculitic neuropathy. Rheumatol Int. 2004;24:255–259. doi: 10.1007/s00296-003-0380-6. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Wells GM, Clements JM, Gearing AJ, Redford EJ, Davies M, Smith KJ, Hughes RA, Brown MC, Miller KM. Matrix metalloproteinase expression during experimental auto-immune neuritis. Brain. 1998;121 (Pt 3):481–494. doi: 10.1093/brain/121.3.481. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Wells GM, Perry VH, Brown MC, Miller KM. Comparison of matrix metalloproteinase expression during Wallerian degeneration in the central and peripheral nervous systems. Neuroscience. 2002;113:273–287. doi: 10.1016/s0306-4522(02)00183-5. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–2296. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- Kherif S, Dehaupas M, Lafuma C, Fardeau M, Alameddine HS. Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol Appl Neurobiol. 1998;24:309–319. doi: 10.1046/j.1365-2990.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- Kieseier BC, Seifert T, Giovannoni G, Hartung HP. Matrix metalloproteinases in inflammatory demyelination: targets for treatment. Neurology. 1999;53:20–25. doi: 10.1212/wnl.53.1.20. [DOI] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Graham JB, Muir D. Metal-loproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts. J Neurosci. 2002;22:10408–10415. doi: 10.1523/JNEUROSCI.22-23-10408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. NeuroReport. 2004;15:2807–2811. [PubMed] [Google Scholar]
- Leppert D, Hughes P, Huber S, Erne B, Grygar C, Said G, Miller KM, Steck AJ, Probst A, Fuhr P. Matrix metalloproteinase upregulation in chronic inflammatory demyelinating polyneuropathy and nonsystemic vasculitic neuropathy. Neurology. 1999;53:62–70. doi: 10.1212/wnl.53.1.62. [DOI] [PubMed] [Google Scholar]
- Liefner M, Siebert H, Sachse T, Michel U, Kollias G, Bruck W. The role of TNF-alpha during Wallerian degeneration. J Neuroimmunol. 2000;108:147–152. doi: 10.1016/s0165-5728(00)00262-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci U S A. 1993;90:9717–9720. doi: 10.1073/pnas.90.20.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawrin C, Brunn A, Rocken C, Schroder JM. Peripheral neuropathy in systemic lupus erythematosus: pathomorphological features and distribution pattern of matrix metalloproteinases. Acta Neuropathol (Berl) 2003;105:365–372. doi: 10.1007/s00401-002-0653-2. [DOI] [PubMed] [Google Scholar]
- Misko A, Ferguson T, Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem. 2002;83:885–894. doi: 10.1046/j.1471-4159.2002.01200.x. [DOI] [PubMed] [Google Scholar]
- Muir D. Metalloproteinase-dependent neurite outgrowth within a synthetic extracellular matrix is induced by nerve growth factor. Exp Cell Res. 1994;210:243–252. doi: 10.1006/excr.1994.1036. [DOI] [PubMed] [Google Scholar]
- Myers RR, Heckman HM, Rodriguez M. Reduced hyperalgesia in nerve-injured WLD mice: relationship to nerve fiber phagocytosis, axonal degeneration, and regeneration in normal mice. Exp Neurol. 1996;141:94–101. doi: 10.1006/exnr.1996.0142. [DOI] [PubMed] [Google Scholar]
- Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- Perry VH, Brown MC, Lunn ER, Tree P, Gordon S. Evidence that very slow Wallerian degeneration in C57BL/Ola mice is an intrinsic property of the peripheral nerve. Eur J Neurosci. 1990;2:802–808. doi: 10.1111/j.1460-9568.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt CI, Krekoski CA, Ward RV, Edwards DR, Gavrilovic J. Extracellular matrix and matrix metalloproteinases in sciatic nerve. J Neurosci Res. 2003;74:417–429. doi: 10.1002/jnr.10783. [DOI] [PubMed] [Google Scholar]
- Renaud S, Erne B, Fuhr P, Said G, Lacroix C, Steck AJ, Leppert D. Matrix metalloproteinases-9 and -2 in secondary vasculitic neuropathies. Acta Neuropathol (Berl) 2003;105:37–42. doi: 10.1007/s00401-002-0607-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood–brain barrier: an expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol. 2001;114:48–56. doi: 10.1016/s0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Endoneurial remodeling by TNFalpha- and TNFalpha-releasing proteases. A spatial and temporal co-localization study in painful neuropathy. J Peripher Nerv Syst. 2002;7:28–36. doi: 10.1046/j.1529-8027.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Matrix metalloproteinase-9 promotes nerve growth factor-induced neurite elongation but not new sprout formation in vitro. J Neurosci Res. 2004;77:229–239. doi: 10.1002/jnr.20160. [DOI] [PubMed] [Google Scholar]
- Siebert H, Bruck W. The role of cytokines and adhesion molecules in axon degeneration after peripheral nerve axotomy: a study in different knockout mice. Brain Res. 2003;960:152–156. doi: 10.1016/s0006-8993(02)03806-4. [DOI] [PubMed] [Google Scholar]
- Siebert H, Dippel N, Mader M, Weber F, Bruck W. Matrix metalloproteinase expression and inhibition after sciatic nerve axotomy. J Neuropathol Exp Neurol. 2001;60:85–93. doi: 10.1093/jnen/60.1.85. [DOI] [PubMed] [Google Scholar]
- Singer CF, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y. Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur J Biochem. 1999;259:40–45. doi: 10.1046/j.1432-1327.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- Smith RA, Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987;262:6951–6954. [PubMed] [Google Scholar]
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993;45:175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur J Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- Talhouk RS, Hajjar L, Abou-Gergi R, Simaa’n CJ, Mouneimne G, Saade NE, Safieh-Garabedian B. Functional interplay between gelatinases and hyperalgesia in endotoxin-induced localized inflammatory pain. Pain. 2000;84:397–405. doi: 10.1016/s0304-3959(99)00238-9. [DOI] [PubMed] [Google Scholar]
- Taskinen HS, Olsson T, Bucht A, Khademi M, Svelander L, Roytta M. Peripheral nerve injury induces endoneurial expression of IFN-gamma, IL-10 and TNF-alpha mRNA. J Neuroimmunol. 2000;102:17–25. doi: 10.1016/s0165-5728(99)00154-x. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996a;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. NeuroReport. 1996b;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gibbons DL, Gearing A, Maini RN, Feldmann M, Brennan FM. Paradoxical effects of a synthetic metalloproteinase inhibitor that blocks both p55 and p75 TNF receptor shedding and TNF alpha processing in RA synovial membrane cell cultures. J Clin Invest. 1996;97:2833–2841. doi: 10.1172/JCI118739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield P, Pain RH, Craig S. Tumour necrosis factor is a compact trimer. FEBS Lett. 1987;211:179–184. doi: 10.1016/0014-5793(87)81432-1. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The family of matrix metalloproteinases. Ann N Y Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]