Abstract
Despite similarities in their clinical presentation, patients with multiple system atrophy (MSA) have residual sympathetic tone and intact post-ganglionic noradrenergic fibers, whereas patients with pure autonomic failure (PAF) and Parkinson’s disease (PD) have efferent post-ganglionic autonomic denervation. These differences are apparent biochemically, with near normal plasma norepinephrine in MSA but very low levels in PAF, and in neurophysiological testing. These differences are also reflected in the response patients have to drugs that interact with the autonomic nervous system. E.g., the ganglionic blocker trimethaphan reduce residual sympathetic tone and lower blood pressure in MSA but less so in PAF. Conversely, the α2-antagonist yohimbine produces a greater increase in blood pressure in MSA compared to PAF, although significant overlap exists. In normal subjects the norepinephrine reuptake (NET) inhibitor atomoxetine has little effect on blood pressure because the peripheral effects of NET inhibition that result in noradrenergic vasoconstriction, are counteracted by the increase in brain norepinephrine which reduces sympathetic outflow (a clonidine-like effect). In patients with autonomic failure and intact peripheral noradrenergic fibers only the peripheral vasoconstriction is apparent. This translates to a significant pressor effect of atomoxetine in MSA, but not in PAF patients. Thus, pharmacological probes can be used to understand the pathophysiology of the different forms of autonomic failure, assist in the diagnosis, and aid in the management of orthostatic hypotension.
Recent developments in our understanding of the underlying pathophysiology of the discrete clinical forms of autonomic failure, reviewed elsewhere in this issue, have revealed that patients with multiple system atrophy (MSA) are characterized by impairment of central autonomic pathways crucial for autonomic cardiovascular control. In contrast, peripheral postganglionic noradrenergic fibers are at least in part preserved. Indeed, plasma norepinephrine concentrations in the supine position are near normal in these patients [8]. Moreover, positron emission tomography studies revealed intact cardiac uptake of labeled catechols [7]. These observations are consistent with the presence of intact noradrenergic nerve terminals and neuronal catecholamine reuptake mechanisms. Yet, in MSA patients, peripheral sympathetic fibers are disconnected from central nervous input. Therefore, their residual sympathetic tone cannot be modulated by baroreflex or central nervous input, but in can be targeted pharmacologically for the treatment of orthostatic hypotension and supine hypertension. In contrast, patients with pure autonomic failure (PAF) and with Parkinson disease (PD) exhibit neurodegeneration of peripheral postganglionic noradrenergic fibers, as evidenced by profound reductions in plasma norepinephrine concentrations and cardiac uptake of labeled catechols.
These pathophysiological differences are reflected in disease-specific responses to medications targeting the autonomic nervous system. We have taken advantage of these pharmacological probes to understand the pathophysiology of autonomic failure and assist in the management of orthostatic hypotension. Some of this work has been reviewed elsewhere [2, 23, 26].
Baroreflex Failure and Denervation Hypersensitivity
Autonomic failure patients show profoundly exaggerated responses to vasoactive compounds. The degree of the hypersensitivity is variable among patients and appears to depend on the severity of the autonomic impairment. In general patients with severe autonomic failure are about 10-fold hypersensitive to vasoactive medications compared with normal subjects. E.g., alpha-adrenoreceptor agonists produce exaggerated pressor responses in autonomic failure patients. This has been interpreted as due to denervation hypersensitivity, secondary to adrenoreceptors up-regulation in the face of decreased catecholamine exposure. Indeed, increased adrenoreceptor density on circulating blood cells has been reported in earlier studies [1, 4, 11].
Given that peripheral norepinephrine availability is reduced more in PAF than in MSA, denervation hypersensitivity should be greater in PAF patients. Surprisingly, the hypersensitivity to vasoactive medications is also profoundly increased in MSA patients despite their near normal plasma norepinephrine concentrations. Moreover, regardless of the etiology, autonomic failure is associated with excessive responsiveness to other vasoactive agents that do not engage adrenoreceptors. Therefore, the exquisite sensitivity to vasoactive medications in autonomic failure patients cannot solely be explained by adrenergic denervation hypersensitivity. Instead, the phenomenon may be largely explained by lack of baroreflex blood pressure buffering capacity [15]. In both MSA and PAF patients, the baroreflex feedback loop is almost completely interrupted, either in the central nervous system (MSA) or at the level of efferent fibers (PAF). From a diagnostic point of view, hypersensitivity to vasoactive medications cannot be used to differentiate between PAF and MSA because both conditions are associated with similar impairments in baroreflex blood pressure buffering. However, the site of the autonomic lesion produces differential responses to interventions targeting peripheral noradrenergic nerves.
Autonomic Pharmacological Probes
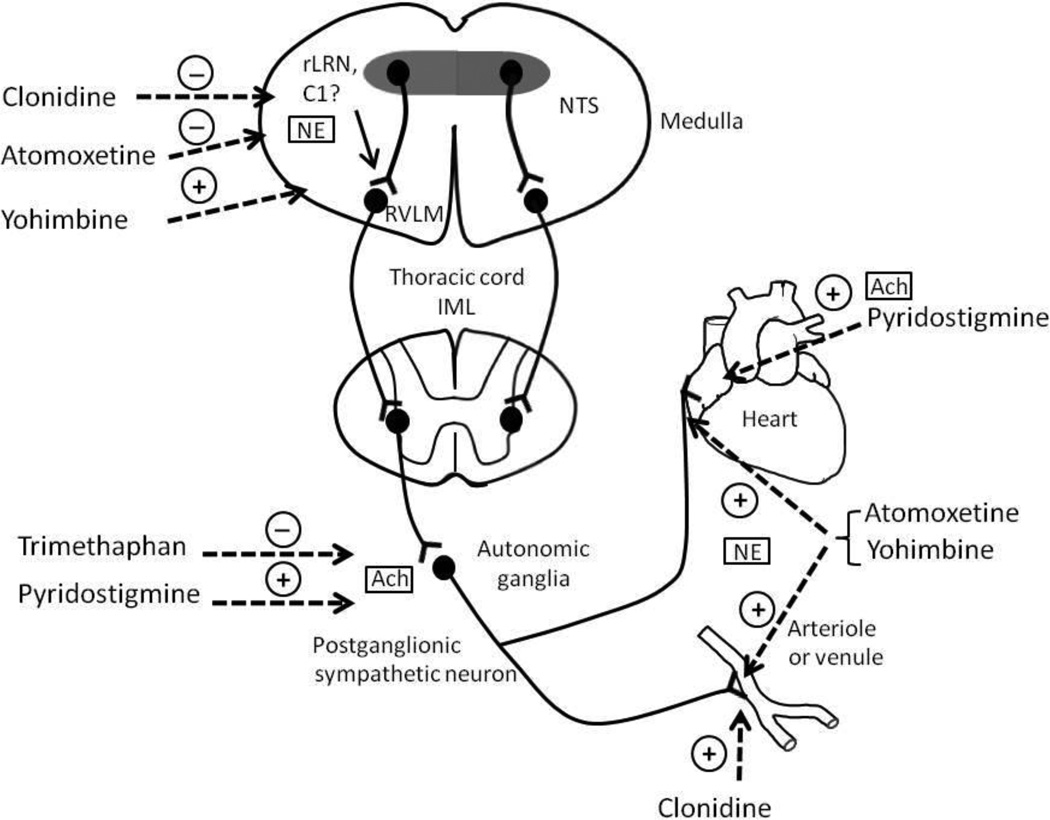
Autonomic drugs could be used as pharmacological probes based on their site of action (Figure 1). Clonidine activates α2-adrenoreceptors in the brain stem inhibiting efferent sympathetic outflow from the rostroventrolateral medulla. Clonidine’s precise site of action is not clear. The nucleus tractus solitarius and C1 area of the rostral part of the nucleus reticularis lateralis have been implicated. Norepinephrine transporter inhibitors including atomoxetine and reboxetine elicit clonidine-like responses in the brainstem by enhancing norepinephrine availability [10, 30]. In contrast, the α2 adrenoreceptor antagonist yohimbine has the opposite effect of clonidine, thus, increasing efferent sympathetic traffic.
Figure 1.
Site of action of the different pharmacological probes that result in either activation (+) or inhibition (−) of sympathetic activity.
Acetylcholine (Ach); C1 area of the NTS (C1); rostral part of the nucleus tractus reticularis lateralis (rLRN); norepinephrine (NE).
All these drugs also act on peripheral noradrenergic nerve fibers. Yohimbine blocks the presynaptic α2-adrenergic receptors that normally restrain norepinephrine release. Norepinephrine transporter inhibitors potentiate norepinephrine in peripheral tissues. Clonidine reduces peripheral norepinephrine release through presynaptic actions and activates postsynaptic vascular α2 adrenergic receptors producing vasoconstriction. The nicotinic receptor blocker trimethaphan interrupts cholinergic neurotransmission at the level of autonomic ganglia, resulting in near complete sympathetic and parasympathetic inhibition. Conversely, pyridostigmine augments autonomic ganglionic neurotransmission through acetyl-cholinesterase inhibition, thus potentiating endogenous acetylcholine. Pyridostigmine also potentiates actions of acetylcholine released from cholinergic postganglionic fibers, which in the heart can produce bradycardia. These pharmacological probes are reviewed below, emphasizing their use in understanding the underlying pathophysiology of primary forms of autonomic failure. Because of their exaggerated responsiveness, autonomic failure patients can also serve as a model to magnify subtle manipulations of norepinephrine turnover on blood pressure. For example, we applied this approach to show that catechol-O-methyltransferase inhibition increases blood pressure in MSA [12].
Trimethaphan
Trimethaphan can be infused intravenously to induce a dose-dependent autonomic ganglionic blockade. The drug induces complete but transient withdrawal of both sympathetic and parasympathetic efferent limbs of the autonomic nervous system [14], which is virtually complete at about 4 to 5 mg/min [5]. Unfortunately, trimethaphan has been taken off the market and is no longer commercially available.
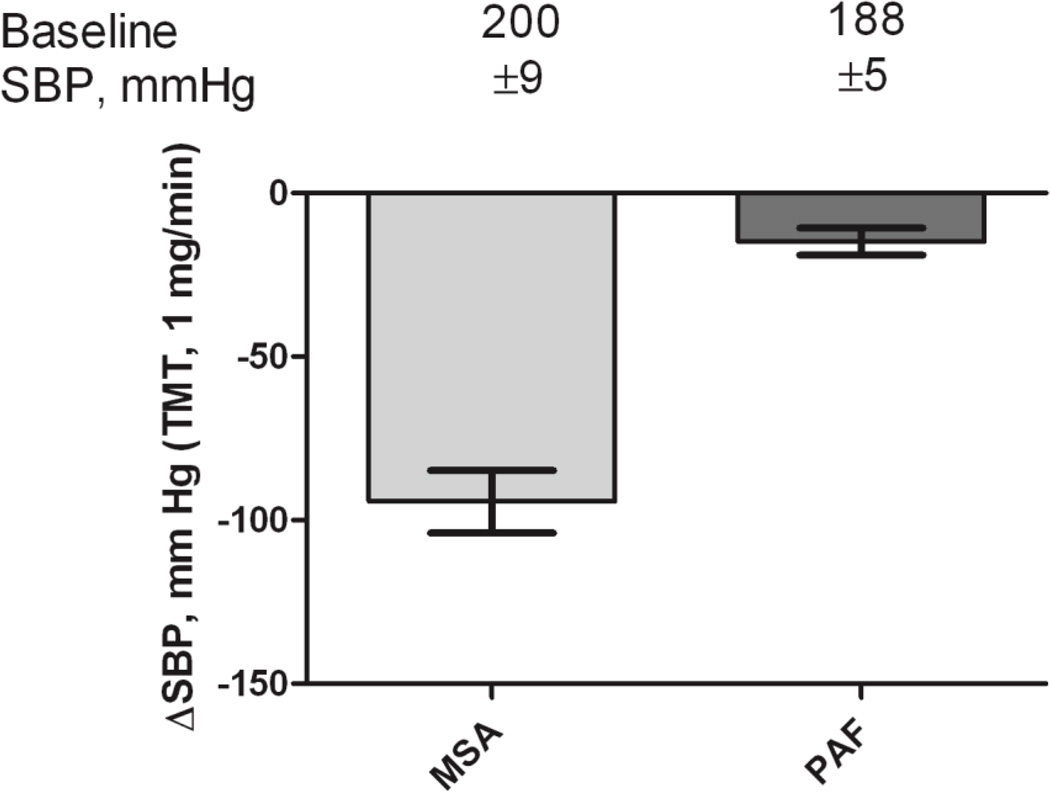
In patients with peripheral forms of autonomic failure (PAF and PD), in whom postganglionic sympathetic and parasympathetic fibers degenerate, interrupting neurotransmission in autonomic ganglia with trimethaphan, therefore, would be inconsequential. Conversely, given that residual sympathetic tone and postganglionic fibers are preserved in MSA, trimethaphan should retain its effectiveness. We have taken advantage of these theoretical considerations [24] and found that in patients with MSA, blood pressure was uniformly and dramatically reduced, (Figure 2), whereas low dose ganglionic blockade had little to no effect in patients with PAF.
Figure 2.
Decrease in systolic blood pressure (SBP) after elimination of residual sympathetic tone with the ganglionic blocker trimethaphan (1 mg/min). Patients with MSA normalized their blood pressure with trimethaphan (SBP during infusion was 96±9 mm Hg) compared with PAF (SBP during infusion 164±6 mm Hg).
Pyridostigmine
Pyridostigmine inhibits acetyl cholinesterase activity, thus increasing cholinergic transmission across the synaptic cleft. Because pre-ganglionic autonomic neurons are cholinergic, pyridostigmine is thought to amplify sympathetic ganglionic neurotransmission. In healthy subjects, traffic to autonomic ganglia is low while supine but activated with standing. In patients with milder form of autonomic failure and incomplete interruption of the baroreflex loop, the medication offers the theoretical advantage of preferentially increasing sympathetic neurotransmission during hypotensive episodes elicited by standing and in proportion to their needs [29]. Furthermore, the risk of worsening supine hypertension, which occurs with most other pressor agents, may be reduced.
Considering the mode of action of pyridostigmine, patients with residual postganglionic noradrenergic fibers (i.e., MSA) should respond more to enhanced autonomic ganglionic neurotransmission compared with patients with peripheral autonomic dysfunction (i.e., PAF or PD). In practice there is a large overlap in the pyridostigmine response between patients with peripheral and central autonomic lesions, possibly because the number of patients in each group may have been too low to detect a difference. In any case, patients with relatively preserved baroreflex function had a greater response [29], supporting the idea that the pyridostigmine response is related to the degree of residual sympathetic function. This agrees with the observation that the drug is not effective in patients with severe autonomic failure [27].
Clonidine
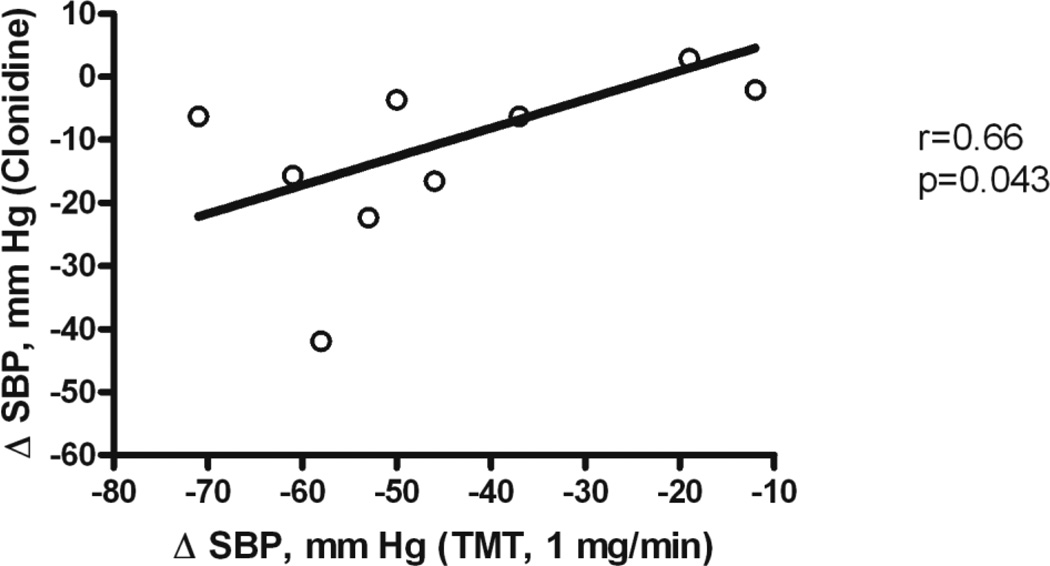
Because supine hypertension in MSA is driven by residual sympathetic tone, we hypothesized that clonidine would be particularly efficacious in reducing nocturnal blood pressure and the resulting pressure natriuresis. Indeed, clonidine, given at 8 PM, decreased nighttime blood pressure [25]. We observed the maximal depressor response of about 30 mm Hg 6 to 8 hours following drug administration. Remarkably, antihypertensive response to clonidine did not differ between MSA and PAF patients. Possibly, we included PAF patients with some degree of residual sympathetic tone. We find the response to trimethaphan a better tool to discriminate the contribution of residual sympathetic tone to supine hypertension between PAF and MSA because sympathetic inhibition is more complete and is not obscured by the direct vascular actions lf clonidine. Clonidine’s hypotensive response was modestly correlated with the magnitude of residual sympathetic tone, as determined by the magnitude of reduction in blood pressure induced by trimethaphan (Figure 3). Thus supine hypertension and nocturnal natriuresis in autonomic failure, secondary to residual sympathetic tone, can be ameliorated with clonidine. However, in patients with severe PAF without residual sympathetic tone, clonidine, particularly when given in higher doses, can elicit paradoxical increases in blood pressure [20]. The response is explained by unopposed vascular α2-adrenoreceptor activation, in the setting of impaired baroreflex blood pressure buffering. While this response is rarely seen at oral clonidine doses of 0.1 mg used to treat nocturnal supine hypertension, this potentially dangerous response ought to be considered in the individual risk-benefit assessment in these patients.
Figure 3.
Decrease in SBP with clonidine significantly correlates with the decrease in SBP during trimethaphan infusion (1 mg/min) in 11 patients with autonomic failure. These results are consistent with residual sympathetic tone contributing to supine hypertension in autonomic failure.
Yohimbine
The selective α2 adrenoreceptor antagonist yohimbine is the pharmacological counterpart to the partial α2-agonist clonidine, and therefore has opposite cardiovascular actions. Yohimbine acts centrally to stimulate sympathetic outflow. In the periphery, the drug enhances norepinephrine release from adrenergic terminals. The overall cardiovascular effects of yohimbine result from both central and peripheral actions [9]. Owing to its pharmacological actions, which facilitate sympathetic activation, yohimbine potentiates pressor and heart rate responses to cold pressor and isometric handgrip testing and Valsalva maneuvers in healthy subjects [6].
Yohimbine produces a significant pressor response in autonomic failure patients and attenuates orthostatic hypotension at doses that would have negligible effects in normal subjects [13, 18]. In contrast, yohimbine does not raise blood pressure in patients with congenital inability to synthesize norepinephrine because of dopamine β-hydroxylase deficiency [21]. These observations imply that the actions of yohimbine are primarily mediated by its interaction with the sympathetic nervous system and that even patients with severe forms of autonomic failure have some degree of residual sympathetic tone that can be harnessed by this drug. In fact, the magnitude of the pressor response to yohimbine in autonomic failure is comparable to that of the direct α adrenergic agonist and sympathomimetic phenylpropanolamine [13]. Yohimbine is no longer commercially available, but can be obtained from compounding pharmacies.
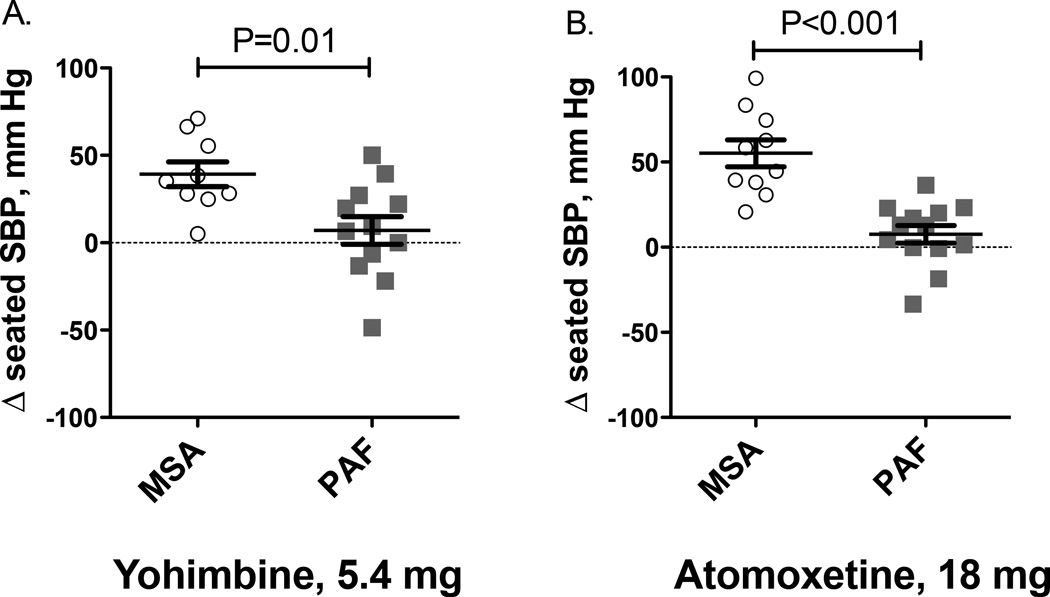
Patients with MSA tend to respond more to yohimbine compared with PAF, but there is significant overlap between groups (Figure 4A). The likely explanation is residual sympathetic tone in MSA because the pressor response to yohimbine was strongly correlated with the decrease in blood pressure produced by trimethaphan [24]. Moreover, the yohimbine-induced pressor response was greater in MSA patients with supine hypertension, known to be mediated by residual sympathetic tone, compared with MSA patients without supine hypertension [24].
Figure 4.
Treatment effect of yohimbine (Panel A) and atomoxetine (Panel B) in patients with multiple system atrophy (MSA) and pure autonomic failure (PAF).
Atomoxetine
The selective norepinephrine transporter (NET) blocker atomoxetine is commonly used in the treatment of attention deficit hyperactivity disorder in children and adults. Neuronal norepinephrine reuptake inhibition would be expected to increase neurotransmitter concentrations in the neuroeffector junction. The mechanism would tend to increase blood pressure in the periphery. However, the peripheral increase in norepinephrine availability appears to be counteracted by central nervous clonidine-like sympatholytic action mediated through central α2-adrenoreceptors [10, 30]. The effect of norepinephrine reuptake inhibition can be dissected in human subjects by assessing the pressor response to atomoxetine in patients with distinct autonomic lesions involving central nervous or peripheral autonomic structures. We hypothesized that norepinephrine reuptake inhibition blockade would induce a pressor effect in patients with intact peripheral sympathetic fibers (MSA with central autonomic impairment), but not in patients with PAF and peripheral autonomic denervation. Indeed, atomoxetine acutely increased seated and standing systolic blood pressure in MSA patients by about 50 mm Hg compared with placebo (Figure 4B). In contrast, in PAF patients, atomoxetine did not elicit a pressor response; seated and standing systolic blood pressure increased by less than 5 mm Hg with atomoxetine compared with placebo [28].
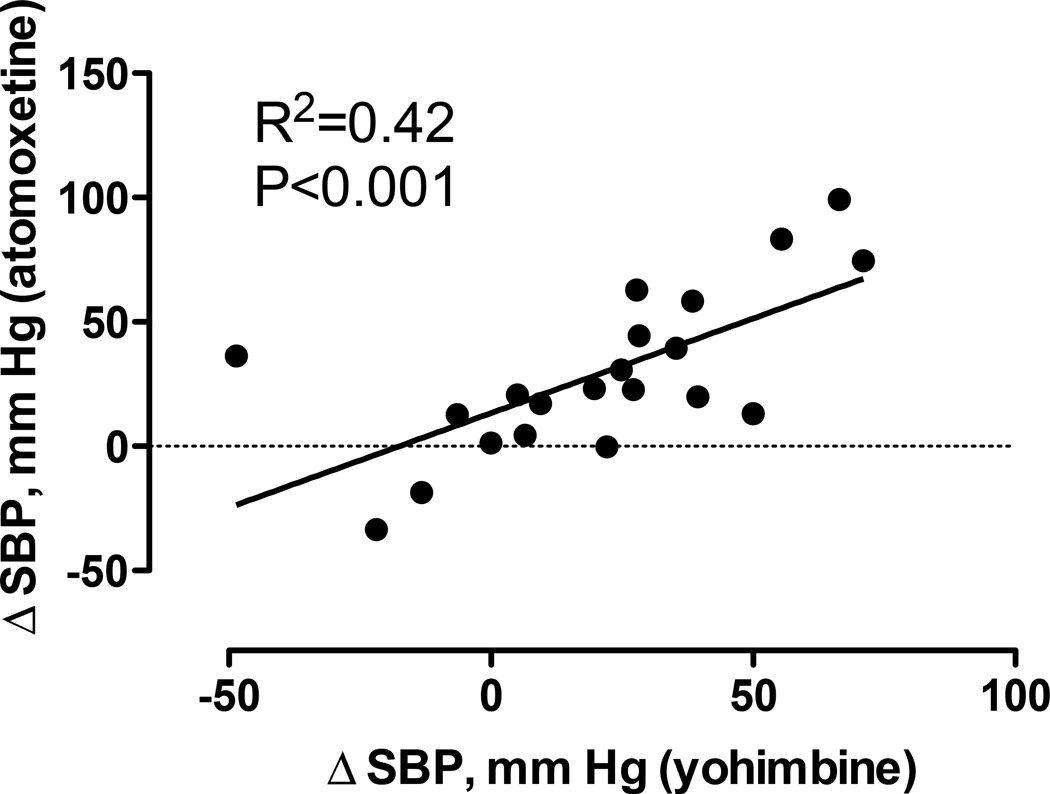
We should note that these initial studies were conducted in patients with late stage disease, allowing for the clinical differentiation between MSA and PAF. Atomoxetine may produce a greater increase in blood pressure in PAF patients with less severe autonomic dysfunction and some degree of residual sympathetic tone [19]. In fact, there was a direct correlation between the pressor response of atomoxetine to the one elicited by yohimbine, Figure 5.
Figure 5.
Panel A. Systolic blood pressure measurements (y-axis) with 18 mg of atomoxetine and placebo in patients with central autonomic failure or MSA. SBP increased significantly with atomoxetine 18 mg (P=<0.001); the asterisk (*) indicates P=<0.001.
Panel B. Systolic blood pressure measurements with atomoxetine and placebo in patients with peripheral autonomic failure (PAF, PD+). No significant changes in SBP were observed (P=0.08).
Can Pharmacology Guide Us to the Site of Residual Sympathetic Tone in MSA?
The observation that clonidine reduces blood pressure in MSA patients, whereas atomoxetine increases it, provides insight in the underlying pathophysiology. Clonidine is known to act as an agonist of α2-adrenergic receptors in the central nervous system to reduce sympathetic tone and this central effect is the likely mechanism of action for the decrease in blood pressure produced by this agent in MSA patients. In fact, clonidine-induced blood pressure reduction in autonomic failure patients is associated with a decrease in sympathetic tone as determined by reduction in plasma norepinephrine [33]. Furthermore, the depressor response is proportional to the patient’s residual sympathetic tone determined by their response to trimethaphan [25]. For atomoxetine to have a clonidine-like effect and reduce sympathetic tone, it requires tonic presynaptic release of norepinephrine in relevant central nervous system centers and intact neural connections to peripheral sympathetic nerves. Both are impaired in MSA patients and, therefore, atomoxetine’s peripheral pressor actions, which depend on residual sympathetic outflow with tonic norepinephrine release, are unopposed.
Three important clinical implications can be derived from the response to atomoxetine in autonomic failure patients. First, these results suggest that intact central autonomic function is essential for preventing hypertension in normal subjects; i.e, central clonidine-like actions are important in preventing hypertension in individuals without autonomic impairment. Second, the pressor effect of atomoxetine can be exploited therapeutically to improve MSA patients with orthostatic hypotension, in cases not responsive to other pharmacological agents. Orthostatic blood pressure in such patients increased by nearly 50 mm Hg, a goal achieved by few other medications, including the α2-adrenergic receptor antagonist yohimbine or the sympathomimetic phenylpropanolamine [13]. Patients should use this drug at least 60 minutes before upright activities and avoid the supine position for 3 to 4 hours because their blood pressure will remain elevated throughout this period. Atomoxetine may not be as useful in patients with peripheral autonomic disorders, but such patients may respond to direct α-adrenergic receptor agonists. Remarkably, norepinephrine reuptake inhibition also improves orthostatic tolerance in healthy subjects [22]. Third, the response to norepinephrine reuptake inhibition can provide insight about the level of the lesion in autonomic disorders.
Differentiation between MSA and PAF/PD is a common diagnostic challenge, particularly in patients with early stage disease, and pharmacological tests may provide additional information complementing routine clinical assessment and imaging. Because there is no curative treatment for these disorders, an accurate diagnosis is important mainly because of its prognostic value; MSA patients have a dismal prognosis with average life expectancy of 9 years from disease onset [32], whereas peripheral autonomic failure syndromes carry a better prognosis. Correct differentiation between MSA, PAF, and PD may become more important for clinical trials testing disease-modifying medications, which require the enrollment of patients early in the course of their disease.
Table 1 summarizes the clinical and biochemical finding that differentiate these conditions. Assessment of neuronal alpha-synuclein deposition in skin biopsies is another promising approach that may aid in the differential diagnosis of autonomic disorders [31]. In this context, the pharmacological tools discussed above may aid in narrowing down the location of the lesion within the autonomic nervous system. It is important to note, however, that none of the tests summarized in Table 1, including the response to atomoxetine or trimethaphan described here, has been rigorously validated in patients with early stage disease posing particular problems when the differential diagnosis would be most helpful. We strongly believe that further research should be conducted to develop diagnostic algorithms for the correct diagnosis of early stage degenerative autonomic disorders. Such diagnostic tools will be essential for conducting clinical trials on disease modifying therapies and for the translation into routine clinical care.
Table 1.
Clinical, Biochemical and Pharmacological Differences in Autonomic Disorders
| MSA | PAF/PD | Ref | |
|---|---|---|---|
| Evidence of degeneration of efferent postganglionic noradrenergic fibers in PAF/PD | |||
| Plasma norepinephrine | ←→ | ↓↓ | [8] |
| Cardiac Uptake MIBG or Flurodopa | ←→ | ↓↓ | [3, 7] |
| LFSBP | ←→ | ↓↓ | [5] |
| Evidence of impaired CNS pathways in MSA | |||
| Hypotension-induced vasopressin release | ↓↓ | ←→ | [16] |
| Clonidine-induced growth hormone release | ↓↓ | ←→ | [17] |
| Pharmacologic evidence of residual sympathetic tone | |||
| BP response to trimethaphan | ↓↓ | ←→ | [24] |
| BP response to atomoxetine | ↑↑ | ↑ | [28] |
References
- 1.Bannister R, Boylston AW, Davies IB, Mathias CJ, Sever PS, Sudera D. Beta-receptor numbers and thermodynamics in denervation supersensitivity. J.Physiol. 1981;319:369–377. doi: 10.1113/jphysiol.1981.sp013914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biaggioni I. Clinical pharmacology of autonomic failure. In: Low PAB E, editor. Clinical Autonomic Disorders. Lippincott Williams & Wilkins; 2008. pp. 307–316. [Google Scholar]
- 3.Braune S, Reinhardt M, Schnitzer R, Riedel A, Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology. 1999;53:1020–1025. doi: 10.1212/wnl.53.5.1020. [DOI] [PubMed] [Google Scholar]
- 4.Davies B, Sudera D, Sagnella G, Marchesi-Saviotti E, Mathias C, Bannister R, Sever P. Increased numbers of alpha receptors in sympathetic denervation supersensitivity in man. J Clin Invest. 1982;69:779–784. doi: 10.1172/JCI110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson RM, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension. Assessment by blood pressure variability and ganglionic blockade. Journal of Hypertension. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MR, Hollister AS, Robertson D. Influence of yohimbine on blood pressure, autonomic reflexes and plasma catecholamines in humans. Hypertension. 1983;5:772–778. doi: 10.1161/01.hyp.5.5.772. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DS, Holmes C, Cannon RO, Eisenhofer G, Kopin IJ. Sympathetic cardioneuropathy in dysautonomias. N Engl J Med. 1997;336:696–702. doi: 10.1056/NEJM199703063361004. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DS, Polinsky RJ, Garty M, Robertson D, Brown RT, Biaggioni I, Stull R, Kopin IJ. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Annals of Neurology. 1989;26:558–563. doi: 10.1002/ana.410260410. [DOI] [PubMed] [Google Scholar]
- 9.Grossman E, Rea RF, Hoffman A, Goldstein DS. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. American Journal of Physiology. 1991;260:R142–R147. doi: 10.1152/ajpregu.1991.260.1.R142. [DOI] [PubMed] [Google Scholar]
- 10.Heusser K, Tank J, Diedrich A, Engeli S, Klaua S, Kruger N, Strauss A, Stoffels G, Luft FC, Jordan J. Influence of sibutramine treatment on sympathetic vasomotor tone in obese subjects. Clin Pharmacol Ther. 2006;79:500–508. doi: 10.1016/j.clpt.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Hui KKP, Conolly ME. Increased numbers of beta receptors in orthostatic hypotension due to autonomic dysfunction. N Engl J Med. 1981;304:1473–1476. doi: 10.1056/NEJM198106113042407. [DOI] [PubMed] [Google Scholar]
- 12.Jordan J, Lipp A, Tank J, Schroder C, Stoffels M, Franke G, Diedrich A, Arnold G, Goldstein DS, Sharma AM, Luft FC. Catechol-o-methyltransferase and blood pressure in humans. Circulation. 2002;106:460–465. doi: 10.1161/01.cir.0000022844.50161.3b. [DOI] [PubMed] [Google Scholar]
- 13.Jordan J, Shannon JR, Biaggioni I, Norman R, Black BK, Robertson D. Contrasting actions of pressor agents in severe autonomic failure. AJM. 1998;105:116–124. doi: 10.1016/s0002-9343(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 14.Jordan J, Shannon JR, Black BK, Lance RH, Squillante MD, Costa F, Robertson D. N(N)-nicotinic blockade as an acute human model of autonomic failure. Hypertension. 1998;31:1178–1184. doi: 10.1161/01.hyp.31.5.1178. [DOI] [PubMed] [Google Scholar]
- 15.Jordan J, Tank J, Shannon JR, Diedrich A, Lipp A, Schroder C, Arnold G, Sharma AM, Biaggioni I, Robertson D, Luft FC. Baroreflex buffering and susceptibility to vasoactive drugs. Circulation. 2002;105:1459–1464. doi: 10.1161/01.cir.0000012126.56352.fd. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann H, Oribe E, Miller M, Knott P, Wiltshire-Clement M, Yahr MD. Hypotension-induced vasopressin release distinguishes between pure autonomic failure and multiple system atrophy with autonomic failure. Neurology. 1992;42:590–593. doi: 10.1212/wnl.42.3.590. [DOI] [PubMed] [Google Scholar]
- 17.Kimber JR, Watson L, Mathias CJ. Distinction of idiopathic Parkinson's disease from multiple-system atrophy by stimulation of growth-hormone release with clonidine. Lancet. 1997;349:1877–1881. doi: 10.1016/s0140-6736(96)10168-9. [DOI] [PubMed] [Google Scholar]
- 18.Onrot J, Goldberg MR, Biaggioni I, Wiley R, Hollister AS, Robertson D. Oral yohimbine in human autonomic failure. Neurology. 1987;37:215–220. doi: 10.1212/wnl.37.2.215. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L, Raj SR, Robertson D, Biaggioni I, Shibao CA. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2014;64:1235–1240. doi: 10.1161/HYPERTENSIONAHA.114.04225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson D, Goldberg MR, Hollister AS, Wade D, Robertson RM. Clonidine raises blood pressure in idiopathic orthostatic hypotension. AJM. 1983;74:193–199. doi: 10.1016/0002-9343(83)90607-1. [DOI] [PubMed] [Google Scholar]
- 21.Robertson D, Goldberg MR, Onrot J, Hollister AS, Thompson JC, Wiley R, Robertson RM. Isolated failure of autonomic noradrenergic neurotransmission: evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder C, Birkenfeld AL, Mayer AF, Tank J, Diedrich A, Luft FC, Jordan J. Norepinephrine transporter inhibition prevents tilt-induced pre-syncope. J Am Coll Cardiol. 2006;48:516–522. doi: 10.1016/j.jacc.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder C, Jordan J, Kaufmann H. Management of neurogenic orthostatic hypotension in patients with autonomic failure. Drugs. 2013;73:1267–1279. doi: 10.1007/s40265-013-0097-0. [DOI] [PubMed] [Google Scholar]
- 24.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 25.Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension. 2006;47:522–526. doi: 10.1161/01.HYP.0000199982.71858.11. [DOI] [PubMed] [Google Scholar]
- 26.Shibao C, Okamoto L, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol Ther. 2012;134:279–286. doi: 10.1016/j.pharmthera.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibao C, Okamoto LE, Gamboa A, Yu C, Diedrich A, Raj SR, Robertson D, Biaggioni I. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56:847–851. doi: 10.1161/HYPERTENSIONAHA.110.154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, Robertson D, Biaggioni I. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. doi: 10.1161/HYPERTENSIONAHA.107.089961. [DOI] [PubMed] [Google Scholar]
- 29.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J.Neurol.Neurosurg.Psychiatry. 2003;74:1294–1298. doi: 10.1136/jnnp.74.9.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003;107:2949–2954. doi: 10.1161/01.CIR.0000072786.99163.FE. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Gibbons CH, Lafo J, Freeman R. alpha-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81:1604–1610. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- 33.Young TM, Asahina M, Watson L, Mathias CJ. Hemodynamic effects of clonidine in two contrasting models of autonomic failure: multiple system atrophy and pure autonomic failure. Movement disorders : official journal of the Movement Disorder Society. 2006;21:609–615. doi: 10.1002/mds.20755. [DOI] [PubMed] [Google Scholar]