Abstract
Acute and chronic inflammation responses characterize the vascular remodelling processes in atherosclerosis, restenosis, pulmonary arterial hypertension, and angiogenesis. The functional and phenotypic changes in diverse vascular cell types are mediated by complex signalling cascades that initiate and control genetic reprogramming. The signalling molecule's signal transducer and activator of transcription 3 (STAT3) plays a key role in the initiation and continuation of these pathophysiological changes. This review highlights the pivotal involvement of STAT3 in pathological vascular remodelling processes and discusses potential translational therapies, which target STAT3 signalling, to prevent and treat cardiovascular diseases. Moreover, current clinical trials using highly effective and selective inhibitors of STAT3 signalling for distinct diseases, such as myelofibrosis and rheumatoid arthritis, are discussed with regard to their vascular (side-) effects and their potential to pave the way for a direct use of these molecules for the prevention or treatment of vascular diseases.
Keywords: Signal transducer and activator of transcription 3, Atherosclerosis, Restenosis, Angiogenesis, Translational medicine
1. Introduction
Complex vascular remodelling processes are the substrate and hallmark of vascular diseases like atherosclerosis, post-angioplasty restenosis and pulmonary artery hypertension but are also essential to maintain vascular integrity or to regenerate vascular structures where needed.
Cellular adaption and functional reprogramming under the distinct conditions is tightly controlled by complex signalling cascades.1–3 In this context, the signal transducer and activator of transcription 3 (STAT3) has been extensively described as a central signalling molecule that controls cellular adaption in response to environmental stimuli or stress in endothelial cells, smooth muscle cells, and circulating/inflammatory cells.
In response to a wide range of growth factors and cytokines including interferons, IL-5, IL-6, and IL-10, epidermal growth factor, and the hormone leptin STAT3 is phosphorylated by receptor-associated tyrosine kinases [e.g. Janus kinase (JAK) 2], forms heterodimers, and translocates to the nucleus, where it acts as a transcription factor. Additionally, the transcriptional activity of STAT3 is dependent on its delicate balance with other STATs, such as STAT1 or STAT5. Moreover, multiple regulating factors affect the STAT3 signalling response by alteration of the STAT3 phosphorylation status, regulation of STAT3 gene expression, or interference of STAT3/DNA interactions. Beyond this, tyrosine kinase-independent phosphorylation as well as non-transcriptional mechanisms of action have been shown for STAT3 (Figure 1).4–6 Despite these numerous co-regulating factors, the IL-6/JAK2/STAT3 axis is certainly the most thoroughly investigated and probably also the most important signalling sequence to modulate cellular functions. This is supported by the promising results of recent clinical trials, which demonstrated that inhibitors of the IL-6/JAK2/STAT3 axis are excellent tools for the treatment of a large number of inflammatory myeloproliferative and malignant diseases (Table 1). Since these inhibitors and other STAT3-targeting strategies are about to find their way to clinical application, we believe that it is important to highlight the possible (side-)effects that such therapeutics will have on the integrity of the vascular system.
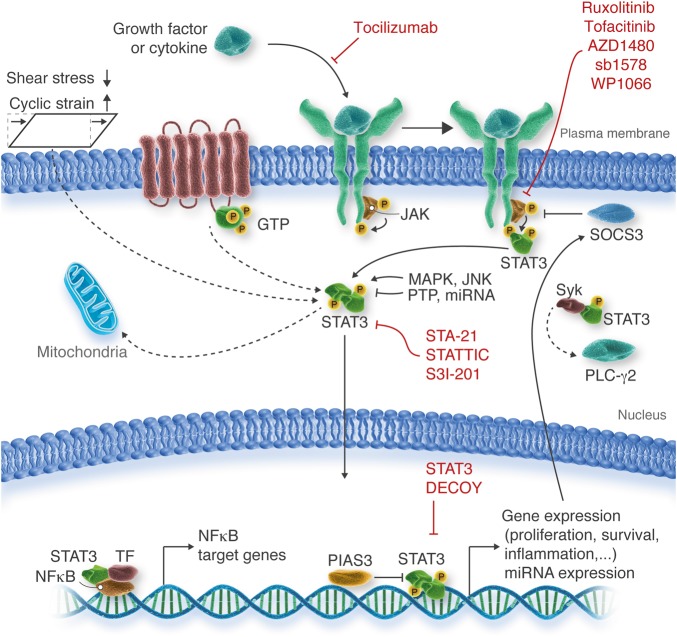
Figure 1.
Physiological regulation and pharmacological inhibition of the STAT3 signalling pathway. STAT3 is a key player in cell signalling and transcription in response to a wide range of stimuli and in various ways. The most relevant STAT3 signalling pathways in vascular diseases are indicated with continuous lines; less well-investigated STAT3 signalling pathways are indicated with broken lines. GTP, guanosine triphosphate; JAK, janus kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; miRNA, microRNA; NFκB, nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells; P, phosphate residue; PIAS3, protein inhibitor of activated STAT; PLC-γ2, phospholipase C, γ2 isoform; PTP, protein tyrosine phosphatase; SOCS3, suppressor of cytokine signalling; Syk, spleen tyrosine kinase; TF, transcription factor.
Table 1.
Targeted therapies in clinical trials affecting STAT3 phosphorylation status
| Name | Mechanism | Condition | Clinical Trial Phase | ClinicalTrials.gov Identifier | Preclinical effects on vascular function |
|---|---|---|---|---|---|
| Direct inhibition | |||||
| STA-21 | STAT3 SH2 domain inhibition | Psoriasis | Phase 1/27 | NCT01047943 | Not investigated |
| STAT3 DECOY | Disruption of STAT3-DNA interaction | Head and neck cancer | Phase 08 | NCT00696176 | Inhibition of tumour angiogenesis9 |
| STATTIC | STAT3 SH2 domain inhibition | Preclinical | Inhibition of neointima formation,10 tumour angiogenesis,11 and vascular dysfunction12 | ||
| S3I-201 | STAT3 SH2 domain inhibition | Preclinical | Inhibition of tumour angiogenesis13 and vascular dysfunction12 | ||
| Indirect inhibition by targeting STAT3 upstream signalling | |||||
| Ruxolitinib | JAK1/2 inhibition | Myelofibrosis | FDA approved | Not investigated | |
| Tocilizumab | IL-6 receptor antibody | Rheumatoid arthritis | FDA approved | Inhibition of tumour angiogenesis14 | |
| Tofacitinib | pan-JAK inhibition | Rheumatoid arthritis | FDA approveda | Inhibition of tumour angiogenesis15 | |
| AZD1480 | JAK1/2 inhibition | Solid malignancies, primary myelofibrosis | Phase 1 | NCT00910728, NCT01112397, NCT01219543 | Inhibition of tumour angiogenesis16 |
| sb1578 | JAK2 inhibition | Rheumatoid arthritis | Phase 1 | NCT01235871 | Not investigated |
| WP1066 | JAK2 inhibition | Brain cancer | Phase 1 | NCT01904123 | Inhibition of neointima formation17 and tumour angiogenesis,18 increased atherosclerotic plaque stability,19 |
aTofacitinib is currently approved for the treatment of rheumatoid arthritis in the USA and Russia. It was not approved by the European regulatory agencies because of concerns over efficacy and safety.
It is surprising, that the growing amount of knowledge on STAT3’s function in vascular diseases has not been reviewed so far. Therefore, we provide an overview on the recent knowledge gained on the impact and function of STAT3 during the most common pathologic vascular remodelling processes and diseases. We also summarize the current promising therapeutic clinical strategies that interfere with STAT3 signalling in the setting of non-cardiac, malignant diseases and discuss the potential effects of these treatment modalities on vascular function and pathological vascular remodelling.
2. Angiogenesis, endothelial function, and regeneration
Angiogenesis and endothelial regeneration are in the focus of both cardiovascular and cancer research. Specifically, these processes are interesting with respect to tumour angiogenesis, endothelial dysfunction, and vasa vasorum neovascularization in atherosclerosis and to re-endothelialization in restenosis and PAH. The principal functional remodelling events, namely EC proliferation, migration, and survival, as well as the selective degradation of the basement membrane and the extracellular matrix, require STAT3 activation.20–22 STAT3 has been described to affect the expression of a broad range of angiogenic and angiostatic mediators including basic fibroblast growth factor, matrix metalloproteinase (MMP-)2 and MMP-9, and the probably best identified and most important inducer of angiogenesis, vascular endothelial growth factor (VEGF).21,23,24 In addition to the binding site for STAT3, the VEGF gene promoter contains binding sites for several potential transcription factors.25 Even the binding of several of these transcription factors depends on STAT3 coaction. STAT3 is therefore suggested to be a proangiogenic key regulator of the VEGF gene, either alone or in a complex with coactivators.26–29
The role of STAT3 in tumour angiogenesis appears to be very diverse due to the vast array of different tumour cell types. Overall, the activation of STAT3 always exhibits a proangiogenic phenotype in tumour angiogenesis. The putative antiangiogenic effects of STAT3 inhibitors might be responsible, at least in part, for their clinical success in oncology.
Meanwhile, the role of angiogenesis in cardiology is ambiguous. Assuming STAT3 acts in a generally proangiogenic manner, the inhibition of STAT3 most likely worsens endothelial dysfunction and re-endothelialization while on the other hand reducing vasa vasorum neovascularization and, thereby, plaque progression.30 Surprisingly, we found that the re-endothelialization process was not impaired following femoral artery dilation in the presence of a STAT3 inhibitor.17 The underlying mechanism may involve miRNA-92a, which is a member of the STAT3-controlled miRNA cluster 17/92.31 miRNA-92a is found to be up-regulated in murine atherosclerotic and neointimal lesions and controls endothelial proliferation, migration, and the expression of a range of chemokines and adhesion molecules under pathological conditions. The inhibition of miRNA-92a accelerates the re-endothelialization process, decreases the neointimal lesion size as well as the atherosclerotic plaque burden, and promotes a more stable lesion phenotype.32,33
Withal, angiogenesis after myocardial infarction was also found to be mediated by STAT3 activity.34 While mice completely deficient in STAT3 are not viable, cardiomyocyte-specific deletion of STAT3 was shown to be associated with a reduction in myocardial capillary density.35,36 Interestingly, this knockout did not alter VEGF expression levels in cardiomyocytes but caused increased expression levels of antiangiogenic genes, i.e. connective tissue growth factor, thrombospondin-1, and tissue inhibitor of metalloproteinase-1.35 Furthermore, the cardiomyocyte STAT3 is necessary to stimulate the production and release of endogenous erythropoietin which, in turn, is needed to maintain the endothelial differentiation potential of cardiac stem cells.37,38 Thus, STAT3 emerged as a factor that regulates the cardiac microenvironment by regulating the release of factors that impact endothelial regeneration.
Hence, the STAT3-dependent regulation of endothelial growth, differentiation and function appears to be complex and diverse. Further investigation is needed to clarify the temporal and spatial function of STAT3 in EC in vascular regeneration and remodelling.
3. Role of STAT3 in vascular diseases
The closely related regulation of proliferation, survival, migration, and differentiation of various cell types becomes unbalanced in a wide range of vascular diseases.2,3 STAT3 is a critical regulator of genes controlling these functional conditions. Therefore, it is logical that many publications address the function of STAT3 in vascular diseases (Figure 2).
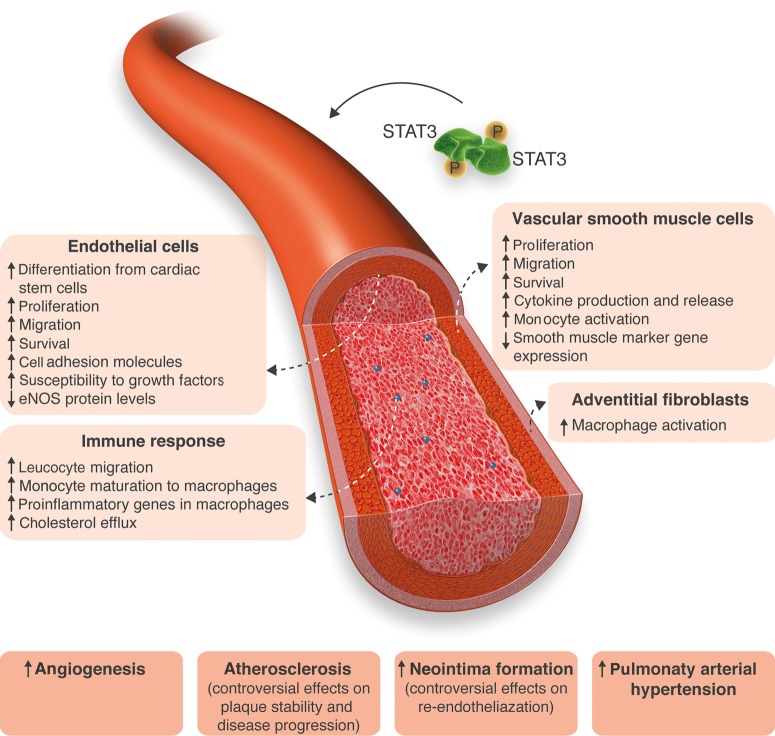
Figure 2.
STAT3 in vascular diseases. STAT3 is shown to be activated in response to mitogenic stimuli in different cell types in vitro, in in vivo models of numerous vascular diseases, and in patients suffering from cardiovascular diseases. STAT3 activation causes functional changes in most cell types, leading to a more undifferentiated and activated phenotype and thereby contributing to vascular lesion formation.
3.1. Atherosclerosis
Endothelial dysfunction, the recruitment of VSMCs from the medial—and of VSMC progenitors from the adventitial—to the intimal layer, and inflammation are cornerstones of the development of atherosclerotic lesions.2,39 STAT3 plays a key role in these processes: STAT3 phosphorylation markedly increased in atherosclerotic lesions of ApoE knockout mice on a cholesterol-rich diet, which underscores a critical role for activated STAT3 proteins in the pathogenesis of atherosclerosis in vivo.40 This assumption is underlined by following observational clinical studies: in 2000, Ridker et al.41 identified plasma concentration of the STAT3-activating cytokine IL-6 as a risk predictor for myocardial infarction. More recently, an Austrian single-centre study evaluated the frequency and severity of coronary artery disease (CAD) in patients with myeloproliferative disorders. The investigators found the frequency of the JAK2 V617F gain-of-function mutation to be more than twice as high in patients with CAD compared with patients without coronary disease.42 This mutation in the JAK2 gene is known to be associated with myeloproliferative diseases. However, its occurrence has also been reported in the healthy population.43 Further studies will have to clarify whether this mutation is also an independent risk factor for CAD in an otherwise healthy population.
Endothelial dysfunction is characterized, inter alia, by the retention of biochemically modified—primarily oxidized—cholesterol-containing low-density lipoprotein (LDL) particles. These particles induce the expression of adhesion molecules that capture leucocytes on the cell surface and initiate an inflammatory process.44 These adhesion molecules, namely intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin and potentially others, are up-regulated in response to STAT3 phosphorylation in endothelial cells (EC) and promote the recruitment of inflammatory cells to the vessel wall.45,46
VSMC recruitment and function in atherosclerosis include a complex interplay of several functional effects, especially proliferation, migration, and extracellular matrix degradation and synthesis. These effects are largely mediated by cytokines. For example, oncostatin M, a monocyte and T-lymphocyte-specific cytokine present in human carotid artery plaques, contributes to proatherogenic VSMC function in a STAT3-dependent manner.19,47 As in other cell types, such as macrophages, STAT3 regulates the transcription of a considerable number of cytokines also within VSMCs, making VSMCs important promoters of inflammation.48,49 The cross-talk between monocytes and VSMCs enhances IL-6 expression and STAT3 activation, leading to the up-regulation of several cytokines in monocytes. This effect in turn results in enhanced reactive oxygen species production in VSMCs; both of these effects contribute substantially to atherosclerotic lesion formation.50,51
Additional data suggest that STAT3 controls the inflammatory cascade more extensively. Specifically, a network analysis of inflammatory genes differentially expressed in CAD revealed a list of 184 transcription factors potentially involved in orchestrating the inflammatory response in atherosclerosis. STAT3 was identified as one of the three transcription factors controlling the regulation of the inflammatory key genes identified in this analysis.52 Macrophages internalizing oxidized LDL yield large foam cells, which in turn further fuel the inflammatory process. IL-6 induced activation of the JAK2/STAT3 pathway in adventitial fibroblasts and macrophages promotes atherosclerotic lesion formation in Ang II-infused LDLR−/− mice.53 Moreover, STAT3 signalling has been shown to be involved in IL-13-dependent foam cell formation in addition to STAT1 and STAT6 signalling.54
Both STAT3 and its regulators show altered expression profiles in atherosclerotic lesions. Suppressor of cytokine signalling (SOCS) 1 and 3, inhibitors of STAT3 signalling, were detected to be highly expressed in the shoulder region of human coronary artery plaques and in murine atherosclerotic lesions, most likely as an endogenous reaction to limit the excessive STAT3 activation in these regions. Despite an enhanced expression of these inhibitors, STAT3 activation is enhanced in these plaque regions. SOCS3 inhibition accelerated atherosclerotic lesion size, leucocyte content, and chemokine expression, while overexpression of SOCS3 prevented STAT3 phosphorylation, VSMC proliferation and inflammatory gene expression.55
However, some studies raise questions on the detrimental role of STAT3 in the pathogenesis of atherosclerosis: adenoviral overexpression of human STAT3 in LDLR−/− mice on a high-fat diet has been shown to impair atherosclerotic plaque burden and to reduce aortic inflammatory cell infiltration.56 Additionally, lipid loading in human macrophages was found to increase IL-6 secretion and thereby STAT3 phosphorylation. This effect in turn promoted cholesterol efflux and attenuated the macrophage pro-inflammatory phenotype.57 This finding is underscored by the fact that phosphorylation of STAT3 is associated with markers of stability in human carotid atherosclerotic plaques.58 Notably, none of these studies investigated the effects of specific STAT3 inhibition on atherosclerotic lesion development.
Lastly, the two alternatively spliced isoforms of STAT3, STAT3α, and the truncated isoform STAT3β, have contrasting effects on the development of atherosclerosis. STAT3β lacks the carboxy-terminal transactivation domain but retains dimerization and DNA-binding functions, behaving in a dominant-negative fashion. STAT3α is assumed to exert most pro-oncogenic functions, while STAT3β is found to promote the expression of certain anti-inflammatory genes.59 Mice deficient in both STAT3β and apolipoprotein E exhibited enhanced atherosclerotic plaque formation, most likely due to the unopposed action of STAT3α.60
Thus, the detailed mechanistic role of STAT3 isotypes in the pathogenesis of atherosclerosis is complex and conceivably isotype-dependent. But given that STAT3 signalling in general influences ECs, VSMCs, and leucocyte function in a rather proatherogenic manner and that interfering with the STAT3 pathway in vivo prevents atherosclerotic lesion formation, strategies which inhibit STAT3 signalling seem to have rather protective than adverse effects on the progression of atherosclerosis.
3.2. Neointima formation
Restenosis paradoxically occurs after procedures performed to treat stenotic atherosclerotic lesions, e.g. coronary angioplasty and stent implantation. We and others observed a significant increase in protein expression and phosphorylation of STAT3 in the developing neointimal lesion in a mouse model of wire-induced injury three weeks after dilatation.17,61,62 Closely examining the signalling background of even a portion of the subsequent activated target genes exposes the wide diversity of functional VSMC regulation observed in the appropriate assays. Cyclin D1, for example, plays a decisive role in the regulation of cell-cycle progression, leading to VSMC proliferation and migration and thickening of the neointimal lesion.17,62 Survivin, another strongly regulated gene, is known as a central regulator of VSMC viability in neointima formation after injury.63 Building on this, we showed the expression of survivin to be essentially STAT3-dependent in this context.17 Even the orchestration of the inflammatory response is found to be under STAT3 control. In this context, we demonstrated that STAT3 inhibition also prevents the up-regulation of the chemokine (C-C motif) ligand 5, also known as RANTES following vascular injury in vivo. Cytokines such as monocyte chemoattractant protein-1 and RANTES not only cause leucocyte infiltration but also mitigate VSMC migration.64,65
A further very important functional change after vascular injury includes the phenotypic modulation of VSMCs. This change from a contractile to a rather less differentiated, synthetic phenotype is a prerequisite for cell proliferation and migration.66 Although the exact role of STAT3 in this condition is not well investigated, a recent study using the STAT3 inhibitor apigenin found increased differentiation-marker gene expression after treatment with apigenin in vitro, suggesting that STAT3 is also involved in the differentiation process of VSMC.67 Howbeit, VSMC marker gene expression crucially depends on the balance between STAT1 and STAT3 and might even be impaired by STAT3 activation under some circumstances.68
All of these findings indicate that the inhibition of STAT3 signalling by means of specific inhibitors attenuates the proliferative and migratory response of VSMC following vascular injury and thus can prevent restenosis in vivo.10,17,67 Importantly, the re-endothelialization process, and thus vascular regeneration and healing, were not impaired after local STAT3 inhibition in our hands. Thus, specific pharmacological STAT3 inhibitors hold promise to serve as novel therapeutic tools for the prevention of vascular re-occlusion following angioplasty.
3.3. Pulmonary arterial hypertension
The pathogenesis of PAH is characterized by increased proliferation and suppressed apoptosis of both pulmonary EC and VSMC, which leads to vascular thickening and stiffening and turns a ‘high-flow-low-resistance’ system into a ‘low-flow-high-resistance’ system.69 STAT3 controls the underlying functional changes of the involved cell types even in this vascular disease.
A very early but crucial clinical finding in PAH research was the reduced expression of the endothelial nitric oxide synthase (eNOS) in the lungs of patients with pulmonary hypertension.70 More than a decade later, STAT3 was found to play a key role in the regulation of eNOS expression: binding of p-STAT3 to the eNOS promoter impairs its activity, thereby decreasing eNOS protein levels and NO production.71
Only 2 years earlier, a role for STAT3 in PAH was first suggested by Masri et al., who found increased p-STAT3 levels in the pulmonary arteries from patients with idiopathic PAH compared with those from healthy subjects. Cultured pulmonary artery ECs from PAH patients were more susceptible to growth factors than the ECs from controls and exhibited higher proliferation rates, higher migration rates, and enhanced survival. These effects were markedly reduced in the presence of AG-490, an inhibitor of JAK2/STAT3 signalling.72 Similar effects were described for PAH VSMCs. The expression of STAT3 and its target genes, nuclear factor of activated T cells (NFAT) and provirus integration site for Moloney murine leukaemia virus (Pim1) were shown to be increased in human and experimental PAH. Their inhibition decreased the proliferative response and promoted apoptosis in PAH VSMCs.73
Very recently published work has revealed a considerable inflammatory impact of STAT3 in PAH. Human and bovine adventitial fibroblasts from hypertensive pulmonary arteries activate macrophages through IL-6 and STAT3 signalling, a pathway very similar to the above-mentioned cross-talk of monocytes and VSMCs in atherosclerosis. STAT3 haplodeficiency attenuated this macrophage activation, whereas complete STAT3 deficiency increased macrophage activation due to compensatory STAT1 up-regulation.74
Heritable subtypes of pulmonary arterial hypertension include ones caused by germline mutations in the bone morphogenetic protein receptor 2 (BMPR2) gene, which encodes a transforming growth factor-β (TGF-β) receptor.75 Altered surface expressions of BMPR2 have also been observed in non-genetic forms of pulmonary hypertension and several animal models mimicking the disease, underscoring the important role of BMPR2 in disease development.76–78 Further studies have shed more light on the molecular mechanisms regulating BMPR2 expression. BMPR2 expression was found to be directly regulated by the miRNA cluster 17/92, the expression of which is controlled by STAT3 signalling.31 Specifically, the presence of this STAT3/BMPR2 axis could be confirmed to be active in pulmonary artery VSMCs.79 Another miRNA regulated by STAT3 in PAH is miRNA-204, which is suppressed following STAT3 expression, leading to uninhibited NFAT expression. This effect then contributes to the proliferative and antiapoptotic phenotype of PAH VSMC.80 In summary, it appears reasonable to speculate that the modulation of STAT3 signalling might emerge also as a promising approach to prevent or reverse pulmonary vascular narrowing.
4. Current therapeutic strategies targeting STAT3 signalling
Targeting the STAT3 pathway is an upcoming therapeutic approach to the treatment of a rising number of inflammatory or proliferative diseases, e.g. myelofibrosis, myeloproliferative disorders, rheumatoid arthritis, and colitis ulcerosa, which also has a modulating effect on vascular cell function (Table 1).81–85 Interfering the STAT3 signalling network came to the focus of clinical attention when genetic variations for JAK2 were identified in various proliferative diseases. Likely the most profound finding is a change of valine to phenylalanine at amino acid 617 (V617F) within the JH2 ‘kinaselike’ domain of JAK2. This substitution is catalytically active and can phosphorylate and activate the kinase domain, leading to an over-activation of JAK2 with consecutive STAT3 and STAT5 heterodimer formation in myeloproliferative diseases.86,87 This mutation appears to render haematopoietic cells more sensitive to growth factors and increases the risk of thrombo-embolic events due to high platelet counts in patients with myeloproliferative syndromes.
Two years after identifying the V617F mutation, the first specific JAK2 inhibitors were designed.88,89 Only another 2 years later, one of these inhibitors, ruxolitinib, was approved by the US Food and Drug Administration (FDA) for the treatment of myelofibrosis based on results of the COMFORT trials.90 Today, two more drugs exerting inhibitory effects on STAT3 signalling have been approved by the FDA for the treatment of rheumatoid arthritis: tofacitinib, a pan-JAK inhibitor, and tocilizumab, a monoclonal antibody targeting the IL-6 receptor.
The above-discussed positive effects of STAT3 inhibition on vascular remodelling processes are all the more exciting, given that patients with myelofibrosis or rheumatoid arthritis are at increased risk of cardiovascular disease by virtue of their autoimmune disease and related vascular changes. Therefore, ruxolitinib, tofacitinib, and tocilizumab could prevent the life-threatening complications (i.e. myocardial infarction) that are associated with these diseases, not only by treating the underlying condition but also by direct protection of vascular function. In contrast, the initially less well-understood effects of STAT3 inhibition on endothelial cell function raised concerns regarding a possible aggravation of endothelial and vascular dysfunction which might rather increase the numbers of major adverse cardiac events. Given this discrepancy and the raised concerns, the evaluation of cardiovascular events was included in the design of further studies investigating the effects of the FDA-approved STAT3 inhibitors.
So far, the effect on vascular function and disease has only been evaluated for tocilizumab. First, an indirect hint was provided by a large genetic study in which 25 458 individuals suffering from CAD were compared with 100 740 healthy controls. This study found an association of an IL-6 receptor variant (which represents the same molecular defect as achieved by a pharmacological blockade with tocilizumab) with decreased odds of coronary events.91 Consistent with this study, some smaller trials focused on lipid levels as surrogates for vascular risk in patients treated with tocilizumab: the authors of the MEASURE study found elevated levels of LDL, total cholesterol, and triglycerides in patients treated with tocilizumab. However, at the same time, HDL particles were altered towards an anti-inflammatory composition.92 More importantly, and besides its effects on lipid metabolism, tocilizumab has been shown to improve endothelial function and reduce arterial stiffness, clearly indicating a positive net effect of a strategy that interferes with IL-6 signalling on vascular function and integrity.93
At least two on-going phase 4 trials focus now on the cardiovascular risk or benefit of tocilizumab in patients with rheumatoid arthritis.
One study is designed as a single-centre trial with patients suffering from rheumatoid arthritis. The investigators focus on cardiovascular risk measurements and defined the primary outcome measures as the proportion of changes in Framingham Point Scores at baseline and 52 weeks (NCT01752335).
In another large, randomized, open-label, parallel-group, multi-centre study, the rate of ischaemic cardiovascular events is evaluated in patients treated with tocilizumab in comparison with etanercept, which inhibits tumour necrosis factor signalling and has FDA approval to treat rheumatoid arthritis. The investigators enrolled more than 3000 patients with moderate-to-severe rheumatoid arthritis and history of CAD or presence of CAD risk factors. The primary endpoint is the occurrence of major adverse cardiac events, defined as a composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke within 5 years (NCT01331837).
A Norwegian group from Oslo went a step further and hypothesized that administration of tocilizumab in patients with non-ST elevation myocardial infarction may interrupt the self-perpetuating inflammatory loops of cardiovascular damage resulting in improved plaque stability with potential secondary beneficial effects on myocardial damage. Therefore, they initiated a randomized, double-blind, placebo-controlled phase 2 trial including a total of 120 patients. To evaluate effect of tocilizumab on the acute inflammatory response, they chose measurements of high-sensitivity C-reactive protein as a primary measure endpoint. Secondary endpoints include inter alia vascular effects, i.e. endothelial function as assessed by tonometry and coronary flow reserve at baseline and 6 months, as well as vascular and cardiac regeneration and infarct size as assessed by echocardiography and MRI at 6 months (NCT01491074). The results of these studies are not yet published but are eagerly awaited and will provide further information about the potential risks or benefits of inhibiting the IL-6 pathway and maybe of STAT3-targeting treatments for cardiovascular diseases.
Owing to its anti-inflammatory properties however, there are some side-effects of tocilizumab, especially an increased risk of infections, a fact which could limit the success of this drug, given that the vast majority of patients included in the studies are vulnerable to infections as a result of multimorbidity.94 Thus, the evaluation of more specific drugs acting further downstream in the IL-6 signalling cascade might be worthwhile. Of the group of JAK inhibitors, only two current studies defined cardiovascular events following tofacitinib treatment as a primary safety end point (NCT01519089, NCT02092467). To our knowledge, none of the on-going clinical trials investigating the effects of ruxolitinib that are registered at ClinicalTrials.gov focus explicitly on this topic but defined ‘duration and severity of adverse events’ as secondary outcome measures.
Even more specific effects can likely be achieved by the use of new direct STAT3 inhibitors. Mechanistically, most of them act through blockage of phosphotyrosine residue binding sites called Src-homology 2 (SH2) domains necessary for STAT3 receptor binding and dimerization. At least three of these compounds were reported to have potent and favourable effects. The small molecule inhibitors S3I-201 and STATTIC protect against Ang II-induced oxidative stress, endothelial dysfunction, and hypertension.12 Two inhibitors have already reached clinical phase trials. Of these inhibitors, STA-21 was shown to successfully treat psoriatic lesions in a small, non-randomized dermatologic phase 1/2 trial (NCT01047943).7 The administration of a STAT3 decoy oligonucleotide was evaluated in a clinical phase 0 trial of head and neck tumours (NCT00696176). This decoy obtains a double-stranded DNA with great homology to the promoter region of STAT3 target genes and blocks STAT3 signalling through interception of activated STAT3 molecules. Notably, no dose-limiting toxicities were reported, while decreased target gene expressions were observed.8
Likely, the most promising novel substance is WP1066, a JAK2 inhibitor, which achieved outstanding results in the treatment of malignant as well as vascular diseases. In preclinical studies, WP1066 not only exhibited a significant impact on the prevention of tumour angiogenesis but also successfully prevented neointima formation and contributed to plaque stability in atherosclerosis.17–19 Very recently, a US group collaborating with the National Institutes of Health and the National Cancer Institute initiated a phase 1 trial of WP1066 for the treatment of glioblastoma and central nervous system melanoma (NCT01904123). Moreover, in contrast to other STAT3-inhibiting molecules which are currently in clinical evaluation, in vivo studies demonstrated excellent tissue penetration by WP1066.95,96 Considering that the main target cells in the treatment of vascular remodelling processes are VSMCs and adventitial cells, this property of WP1066 is not to be underestimated.
Apart from targeted therapies, a group of well-known and highly important cardiovascular drugs have an impact on STAT3 signalling. Statins—inhibitors of the enzyme 3-hydroxy-3-methyl-glutaryl (HMG)-coenzyme A (CoA)-reductase—exert part of their pleiotropic (non-cholesterol related) beneficial vascular effects by interfering with the JAK/STAT signalling pathway. Statins were shown to effectively modulate the inflammatory response in atherosclerotic lesions by reducing IL-6 levels, thereby decreasing STAT3 phosphorylation and increasing SOCS3 expression.40,97 The combination of statins with aspirin and/or indomethacin can even completely abolish IL-6 production and prevent STAT3 phosphorylation in VSMC/monocyte cocultures.51 Fluvastatin combined with a selective Ang II type 1 receptor blocker, valsartan, prevented STAT3 phosphorylation and exerted inhibitory effects on neointima formation.98 Considering the biochemical action of these well-established drugs, novel specific STAT3 inhibitors might likewise have clinical success.
Despite the undoubtedly positive effects of STAT3 inhibition on pathological vascular remodelling processes, it is important to consider that STAT3 is a key essential factor to maintain normal cardiac function.99,100 Moderate reduction in STAT3 activity appears to be tolerated quite well and might even be advantageous in some inflammatory conditions, i.e. immune-mediated myocarditis, where STAT3 activation is known to be crucial.101–103 Notwithstanding this, therapies aiming at systemic STAT3 inhibition under conditions that are already associated with reduced STAT3 activity in organs, such as the heart (e.g. doxorubicin pre-treatment, heart failure), may be detrimental because such therapies might further promote heart failure, which is, in fact, a major cause for terminating anti-tumour therapies.37 A personalized medicine approach with regard to organ specific STAT3 conditions might be required to determine whether a therapy targeting STAT3 is beneficial with regard to the entire organism. Thus, the pre-evaluation of patients, including the assessment of cardiac function and the knowledge of potentially cardiotoxic co-therapies, is needed prior to the initialization of a systemic STAT3-targeting medical therapy.
Moreover, for the treatment of single atherosclerotic lesions, a local application might be a favourable option, given that local application using drug-eluting stents or balloons may exert potent effects with negligible systemic side effects.
5. Conclusions
Over the last two decades, STAT3 signalling has been identified as a central pathway for the activation and reprogramming of vascular cells especially under pathological conditions. Moreover, it has been shown that this pathway initiates and fuels pathological vascular remodelling processes. The current success of well-tolerated STAT3-interfering therapies in oncologic, haematologic and chronic inflammatory diseases, as well as the results of different novel and specific STAT3 inhibitors in numerous clinical trials, strongly suggest that this therapeutic strategy might also offer substantial benefits in the treatment of vasculoproliferative diseases such as atherosclerosis, restenosis, and pulmonary arterial hypertension. Thus, the results of the recently initiated first clinical trials evaluating specifically the cardiovascular effects of these novel STAT3 inhibitors are eagerly awaited.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (Cluster of excellence REBIRTH). Funding to pay the Open Access publication charges for this article was provided by the Deutsche Forschungsgemeinschaft (Cluster of excellence REBIRTH).
Acknowledgements
We thank our colleagues for helpful discussions and apologize to all whose work was not properly cited due to the space constraints of a short review.
Conflict of interest: none declared.
References
- 1.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol 2010;30:1890–1896. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 2002;8:1249–1256. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 4.Grote K, Luchtefeld M, Schieffer B. Janus under stress—role of JAK/STAT signaling pathway in vascular diseases. Vascul Pharmacol 2005;43:357–363. [DOI] [PubMed] [Google Scholar]
- 5.Wincewicz A, Sulkowska M, Rutkowski R, Sulkowski S, Musiatowicz B, Hirnle T, Famulski W, Koda M, Sokol G, Szarejko P. STAT1 and STAT3 as intracellular regulators of vascular remodeling. Eur J Intern Med 2007;18:267–271. [DOI] [PubMed] [Google Scholar]
- 6.Haghikia A, Hoch M, Stapel B, Hilfiker-Kleiner D. STAT3 regulation of and by micrornas in development and disease. Jak-Stat 2012;1:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, Iiyama T, Asao N, DiGiovanni J, Sano S. STAT3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a STAT3 inhibitor. J Invest Dermatol 2011;131:108–117. [DOI] [PubMed] [Google Scholar]
- 8.Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S, Freilino ML, Shi H, Li C, Ly D, Rapireddy S, Etter JP, Li PK, Wang L, Chiosea S, Seethala RR, Gooding WE, Chen X, Kaminski N, Pandit K, Johnson DE, Grandis JR. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov 2012;2:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein JD, Sano D, Sen M, Myers JN, Grandis JR, Kim S. Stat3 oligonucleotide inhibits tumor angiogenesis in preclinical models of squamous cell carcinoma. PLoS One 2014;9:e81819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwaiberger AV, Heiss EH, Cabaravdic M, Oberan T, Zaujec J, Schachner D, Uhrin P, Atanasov AG, Breuss JM, Binder BR, Dirsch VM. Indirubin-3′-monoxime blocks vascular smooth muscle cell proliferation by inhibition of signal transducer and activator of transcription 3 signaling and reduces neointima formation in vivo. Arterioscler Thromb Vasc Biol 2010;30:2475–2481. [DOI] [PubMed] [Google Scholar]
- 11.Carbajo-Pescador S, Ordonez R, Benet M, Jover R, Garcia-Palomo A, Mauriz JL, Gonzalez-Gallego J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer 2013;109:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AW, Kinzenbaw DA, Modrick ML, Faraci FM. Small-molecule inhibitors of signal transducer and activator of transcription 3 protect against angiotensin II-induced vascular dysfunction and hypertension. Hypertension 2013;61:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurbuz V, Konac E, Varol N, Yilmaz A, Gurocak S, Menevse S, Sozen S. Effects of AG490 and S3I-201 on regulation of the JAK/STAT3 signaling pathway in relation to angiogenesis in trail-resistant prostate cancer cells. Oncol Lett 2014;7:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, Oike Y, Endo M, Ibusuki M, Hiraki A, Nakayama H, Yoshitake Y, Shinohara M, Ando Y. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res 2009;15:5426–5434. [DOI] [PubMed] [Google Scholar]
- 15.Zgheib A, Pelletier-Bonnier E, Levros LC, Jr, Annabi B. Selective JAK/STAT3 signalling regulates transcription of colony stimulating factor-2 and -3 in concanavalin-A-activated mesenchymal stromal cells. Cytokine 2013;63:187–193. [DOI] [PubMed] [Google Scholar]
- 16.Xin H, Herrmann A, Reckamp K, Zhang W, Pal S, Hedvat M, Zhang C, Liang W, Scuto A, Weng S, Morosini D, Cao ZA, Zinda M, Figlin R, Huszar D, Jove R, Yu H. Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res 2011;71:6601–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel JM, Dutzmann J, Bielenberg W, Widmer-Teske R, Gunduz D, Hamm CW, Sedding DG. Inhibition of STAT3 signaling prevents vascular smooth muscle cell proliferation and neointima formation. Basic Res Cardiol 2012;107:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, Hayakawa M, Sumitomo M, Asano T. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer 2010;102:1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demyanets S, Kaun C, Rychli K, Pfaffenberger S, Kastl SP, Hohensinner PJ, Rega G, Katsaros KM, Afonyushkin T, Bochkov VN, Paireder M, Huk I, Maurer G, Huber K, Wojta J. Oncostatin M-enhanced vascular endothelial growth factor expression in human vascular smooth muscle cells involves PI3K-, P38 MAPK-, Erk1/2- and STAT1/STAT3-dependent pathways and is attenuated by interferon-gamma. Basic Res Cardiol 2011;106:217–231. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev 2008;28:185–200. [DOI] [PubMed] [Google Scholar]
- 22.Hilfiker-Kleiner D, Limbourg A, Drexler H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc Med 2005;15:152–157. [DOI] [PubMed] [Google Scholar]
- 23.Funamoto M, Fujio Y, Kunisada K, Negoro S, Tone E, Osugi T, Hirota H, Izumi M, Yoshizaki K, Walsh K, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem 2000;275:10561–10566. [DOI] [PubMed] [Google Scholar]
- 24.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002;21:2000–2008. [DOI] [PubMed] [Google Scholar]
- 25.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991;266:11947–11954. [PubMed] [Google Scholar]
- 26.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003;22:1517–1527. [DOI] [PubMed] [Google Scholar]
- 27.Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and SP1. Int J Cancer 2005;115:202–213. [DOI] [PubMed] [Google Scholar]
- 28.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene 2005;24:3110–3120. [DOI] [PubMed] [Google Scholar]
- 29.Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J 2005;19:1296–1298. [DOI] [PubMed] [Google Scholar]
- 30.Langheinrich AC, Michniewicz A, Sedding DG, Walker G, Beighley PE, Rau WS, Bohle RM, Ritman EL. Correlation of vasa vasorum neovascularization and plaque progression in aortas of apolipoprotein E(−/−)/low-density lipoprotein(−/−) double knockout mice. Arterioscler Thromb Vasc Biol 2006;26:347–352. [DOI] [PubMed] [Google Scholar]
- 31.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type ii through a novel STAT3-microrna cluster 17/92 pathway. Circ Res 2009;104:1184–1191. [DOI] [PubMed] [Google Scholar]
- 32.Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, Bonauer A, Boon RA, Fischer A, Bauersachs J, van Rooij E, Dimmeler S, Sedding DG. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res 2014;103:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microrna-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res 2014;114:434–443. [DOI] [PubMed] [Google Scholar]
- 34.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the JAK-STAT pathway in cardiomyocytes. Nat Med 2005;11:305–311. [DOI] [PubMed] [Google Scholar]
- 35.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 2004;95:187–195. [DOI] [PubMed] [Google Scholar]
- 36.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600. [DOI] [PubMed] [Google Scholar]
- 37.Hoch M, Fischer P, Stapel B, Missol-Kolka E, Sekkali B, Scherr M, Favret F, Braun T, Eder M, Schuster-Gossler K, Gossler A, Hilfiker A, Balligand JL, Drexler H, Hilfiker-Kleiner D. Erythropoietin preserves the endothelial differentiation capacity of cardiac progenitor cells and reduces heart failure during anticancer therapies. Cell Stem Cell 2011;9:131–143. [DOI] [PubMed] [Google Scholar]
- 38.Westenbrink BD, Ruifrok WP, Voors AA, Tilton RG, van Veldhuisen DJ, Schoemaker RG, van Gilst WH, de Boer RA. Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure. Cardiovasc Res 2010;87:30–39. [DOI] [PubMed] [Google Scholar]
- 39.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA 2008;105:9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Li D, Yan W, Li W. Pravastatin prevents aortic atherosclerosis via modulation of signal transduction and activation of transcription 3 (STAT3) to attenuate interleukin-6 (IL-6) action in ApoE knockout mice. Int J Mol Sci 2008;9:2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–1772. [DOI] [PubMed] [Google Scholar]
- 42.Muendlein A, Gasser K, Kinz E, Stark N, Leiherer A, Rein P, Saely CH, Grallert H, Peters A, Drexel H, Lang AH. Evaluation of the prevalence and prospective clinical impact of the JAK2 V617F mutation in coronary patients. Am J Hematol 2014;89:295–301. [DOI] [PubMed] [Google Scholar]
- 43.Sidon P, El Housni H, Dessars B, Heimann P. The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia 2006;20:1622. [DOI] [PubMed] [Google Scholar]
- 44.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 45.Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011;218:44–52. [DOI] [PubMed] [Google Scholar]
- 46.Yang XP, Irani K, Mattagajasingh S, Dipaula A, Khanday F, Ozaki M, Fox-Talbot K, Baldwin WM, 3rd, Becker LC. Signal transducer and activator of transcription 3alpha and specificity protein 1 interact to upregulate intercellular adhesion molecule-1 in ischemic-reperfused myocardium and vascular endothelium. Arterioscler Thromb Vasc Biol 2005;25:1395–1400. [DOI] [PubMed] [Google Scholar]
- 47.Albasanz-Puig A, Murray J, Preusch M, Coan D, Namekata M, Patel Y, Dong ZM, Rosenfeld ME, Wijelath ES. Oncostatin M is expressed in atherosclerotic lesions: a role for Oncostatin M in the pathogenesis of atherosclerosis. Atherosclerosis 2011;216:292–298. [DOI] [PubMed] [Google Scholar]
- 48.Sahar S, Dwarakanath RS, Reddy MA, Lanting L, Todorov I, Natarajan R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: a novel cross-talk in the pathogenesis of atherosclerosis. Circ Res 2005;96:1064–1071. [DOI] [PubMed] [Google Scholar]
- 49.Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res 2002;91:427–433. [DOI] [PubMed] [Google Scholar]
- 50.Gan AM, Pirvulescu MM, Stan D, Simion V, Calin M, Manduteanu I, Butoi E. Monocytes and smooth muscle cells cross-talk activates stat3 and induces resistin and reactive oxygen species production [corrected]. J Cell Biochem 2013;114:2273–2283. [DOI] [PubMed] [Google Scholar]
- 51.Loppnow H, Zhang L, Buerke M, Lautenschlager M, Chen L, Frister A, Schlitt A, Luther T, Song N, Hofmann B, Rose-John S, Silber RE, Muller-Werdan U, Werdan K. Statins potently reduce the cytokine-mediated IL-6 release in SMC/MNC cocultures. J Cell Mol Med 2011;15:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair J, Ghatge M, Kakkar VV, Shanker J. Network analysis of inflammatory genes and their transcriptional regulators in coronary artery disease. PLoS One 2014;9:e94328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Recinos A, 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR. Angiotensin ii induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis 2007;194:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yakubenko VP, Hsi LC, Cathcart MK, Bhattacharjee A. From macrophage interleukin-13 receptor to foam cell formation: mechanisms for αMβ2 integrin interference. J Biol Chem 2013;288:2778–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortiz-Munoz G, Martin-Ventura JL, Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O, Munoz-Garcia B, Fernandez-Vizarra P, Ortega L, Egido J, Gomez-Guerrero C. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:525–531. [DOI] [PubMed] [Google Scholar]
- 56.Khan JA, Cao M, Kang BY, Liu Y, Mehta JL, Hermonat PL. AAV/hSTAT3-gene delivery lowers aortic inflammatory cell infiltration in LDLR KO mice on high cholesterol. Atherosclerosis 2010;213:59–66. [DOI] [PubMed] [Google Scholar]
- 57.Frisdal E, Lesnik P, Olivier M, Robillard P, Chapman MJ, Huby T, Guerin M, Le Goff W. Interleukin-6 protects human macrophages from cellular cholesterol accumulation and attenuates the proinflammatory response. J Biol Chem 2011;286:30926–30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med 2009;206:2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marino F, Orecchia V, Regis G, Musteanu M, Tassone B, Jon C, Forni M, Calautti E, Chiarle R, Eferl R, Poli V. STAT3β controls inflammatory responses and early tumor onset in skin and colon experimental cancer models. Am J Cancer Res 2014;4:484–494. [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J, Baldwin WM, 3rd, Lee CY, Desiderio S. STAT3β mitigates development of atherosclerosis in apolipoprotein E-deficient mice. J Mol Med 2013;91:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seki Y, Kai H, Shibata R, Nagata T, Yasukawa H, Yoshimura A, Imaizumi T. Role of the JAK/STAT pathway in rat carotid artery remodeling after vascular injury. Circ Res 2000;87:12–18. [DOI] [PubMed] [Google Scholar]
- 62.Wang D, Liu Z, Li Q, Karpurapu M, Kundumani-Sridharan V, Cao H, Dronadula N, Rizvi F, Bajpai AK, Zhang C, Muller-Newen G, Harris KW, Rao GN. An essential role for gp130 in neointima formation following arterial injury. Circ Res 2007;100:807–816. [DOI] [PubMed] [Google Scholar]
- 63.Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med 2002;8:987–994. [DOI] [PubMed] [Google Scholar]
- 64.Kovacic JC, Gupta R, Lee AC, Ma M, Fang F, Tolbert CN, Walts AD, Beltran LE, San H, Chen G, St Hilaire C, Boehm M. Stat3-dependent acute rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J Clin Invest 2010;120:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh NK, Wang D, Kundumani-Sridharan V, Van Quyen D, Niu J, Rao GN. 15-Lipoxygenase-1-enhanced SRC-Janus kinase 2-signal transducer and activator of transcription 3 stimulation and monocyte chemoattractant protein-1 expression require redox-sensitive activation of epidermal growth factor receptor in vascular wall remodeling. J Biol Chem 2011;286:22478–22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest 2000;106:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan H, Gao L, Zhu L, Yan L, Fu M, Chen C, Dong X, Wang L, Huang K, Jiang H. Apigenin attenuates neointima formation via suppression of vascular smooth muscle cell phenotypic transformation. J Cell Biochem 2012;113:1198–1207. [DOI] [PubMed] [Google Scholar]
- 68.Kirchmer MN, Franco A, Albasanz-Puig A, Murray J, Yagi M, Gao L, Dong ZM, Wijelath ES. Modulation of vascular smooth muscle cell phenotype by STAT-1 and STAT-3. Atherosclerosis 2014;234:169–175. [DOI] [PubMed] [Google Scholar]
- 69.Paulin R, Meloche J, Bonnet S. STAT3 signaling in pulmonary arterial hypertension. Jak-Stat 2012;1:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995;333:214–221. [DOI] [PubMed] [Google Scholar]
- 71.Sud N, Black SM. Endothelin-1 impairs nitric oxide signaling in endothelial cells through a protein kinase Cdelta-dependent activation of STAT3 and decreased endothelial nitric oxide synthase expression. DNA Cell Biol 2009;28:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L548–L554. [DOI] [PubMed] [Google Scholar]
- 73.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Cote J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 2011;123:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 2014;193:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 76.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002;105:1672–1678. [DOI] [PubMed] [Google Scholar]
- 77.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, Schermuly RT, Eickelberg O. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2007;27:1072–1078. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;290:L450–L458. [DOI] [PubMed] [Google Scholar]
- 79.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, Lauzon-Joset JF, Breuils-Bonnet S, Tremblay E, Biardel S, Racine C, Courture C, Bonnet P, Majka SM, Deshaies Y, Picard F, Provencher S, Bonnet S. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc 2013;2:e005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011;208:535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366:787–798. [DOI] [PubMed] [Google Scholar]
- 82.Madan B, Goh KC, Hart S, William AD, Jayaraman R, Ethirajulu K, Dymock BW, Wood JM. SB1578, a novel inhibitor of JAK2, FLT3, and c-Fms for the treatment of rheumatoid arthritis. J Immunol 2012;189:4123–4134. [DOI] [PubMed] [Google Scholar]
- 83.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W, Study AI. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 84.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010;363:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–2386. [DOI] [PubMed] [Google Scholar]
- 86.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR, Cancer Genome P. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–1061. [DOI] [PubMed] [Google Scholar]
- 87.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779–1790. [DOI] [PubMed] [Google Scholar]
- 88.Duan Z, Bradner J, Greenberg E, Mazitschek R, Foster R, Mahoney J, Seiden MV. 8-Benzyl-4-oxo-8-azabicyclo[3.2.1]oct-2-ene-6,7-dicarboxylic acid (SD-1008), a novel Janus kinase 2 inhibitor, increases chemotherapy sensitivity in human ovarian cancer cells. Mol Pharmacol 2007;72:1137–1145. [DOI] [PubMed] [Google Scholar]
- 89.Lin Q, Meloni D, Pan Y, Xia M, Rodgers J, Shepard S, Li M, Galya L, Metcalf B, Yue TY, Liu P, Zhou J. Enantioselective synthesis of Janus kinase inhibitor INCB018424 via an organocatalytic aza-Michael reaction. Org Lett 2009;11:1999–2002. [DOI] [PubMed] [Google Scholar]
- 90.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH, Jr, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: measure, a randomised, placebo-controlled study. Ann Rheum Dis 2015;74:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 2011;219:734–736. [DOI] [PubMed] [Google Scholar]
- 94.Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol 2010;20:222–232. [DOI] [PubMed] [Google Scholar]
- 95.Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, Fuller GN, Fokt I, Grimm EA, Schmittling RJ, Archer GE, Jr, Sampson JH, Priebe W, Heimberger AB. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res 2008;14:5759–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stechishin OD, Luchman HA, Ruan Y, Blough MD, Nguyen SA, Kelly JJ, Cairncross JG, Weiss S. On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells. Neuro Oncol 2013;15:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, Mach F. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol 2005;25:1231–1236. [DOI] [PubMed] [Google Scholar]
- 98.Horiuchi M, Cui TX, Li Z, Li JM, Nakagami H, Iwai M. Fluvastatin enhances the inhibitory effects of a selective angiotensin II type 1 receptor blocker, valsartan, on vascular neointimal formation. Circulation 2003;107:106–112. [DOI] [PubMed] [Google Scholar]
- 99.Haghikia A, Ricke-Hoch M, Stapel B, Gorst I, Hilfiker-Kleiner D. STAT3, a key regulator of cell-to-cell communication in the heart. Cardiovasc Res 2014;102:281–289. [DOI] [PubMed] [Google Scholar]
- 100.Hilfiker-Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 2014;11:364–370. [DOI] [PubMed] [Google Scholar]
- 101.Camporeale A, Marino F, Papageorgiou A, Carai P, Fornero S, Fletcher S, Page BD, Gunning P, Forni M, Chiarle R, Morello M, Jensen O, Levi R, Heymans S, Poli V. STAT3 activity is necessary and sufficient for the development of immune-mediated myocarditis in mice and promotes progression to dilated cardiomyopathy. EMBO Mol Med 2013;5:572–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, Muller W, Scherr M, Theilmeier G, Ernst M, Hilfiker A, Drexler H. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 2010;122:145–155. [DOI] [PubMed] [Google Scholar]
- 103.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation 2003;107:320–325. [DOI] [PubMed] [Google Scholar]