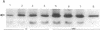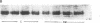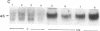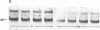Abstract
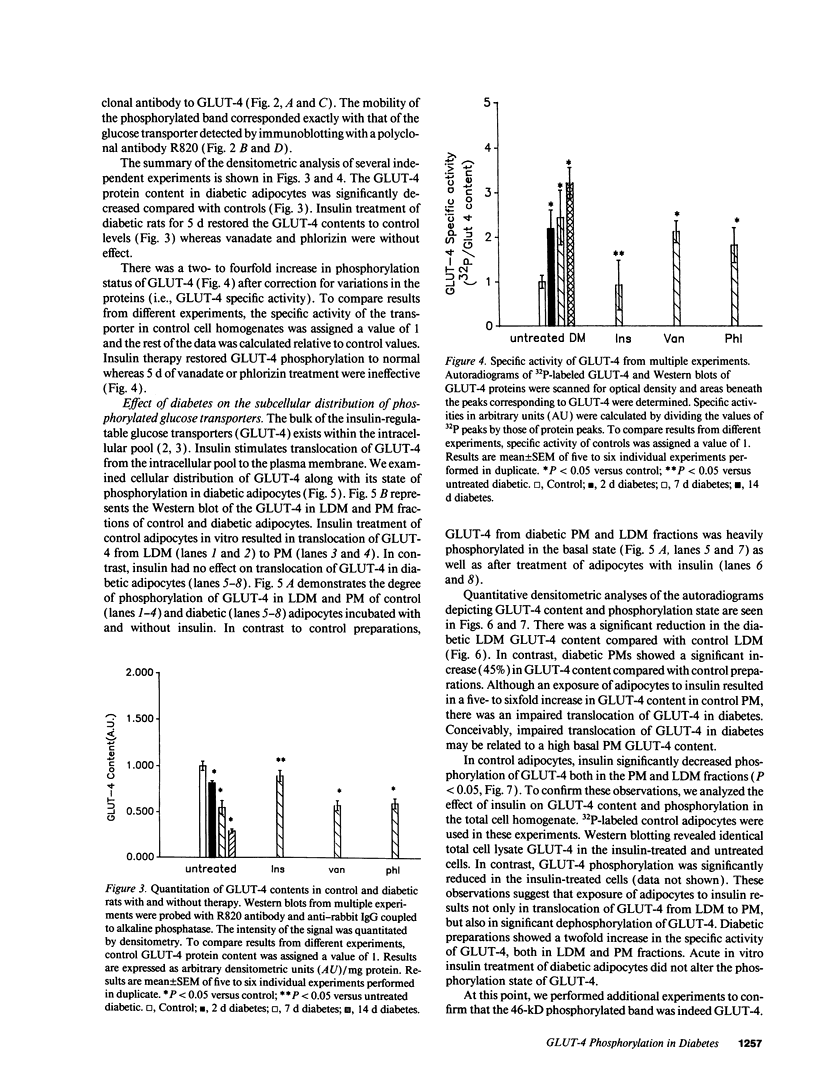
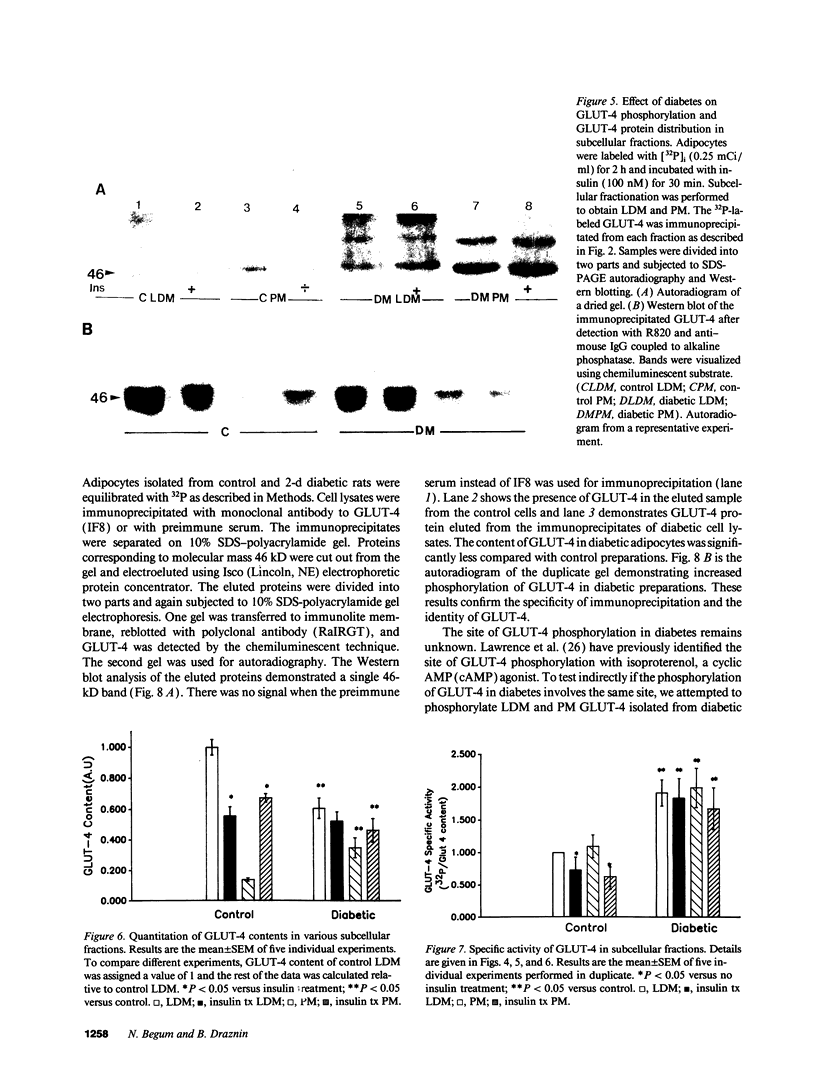
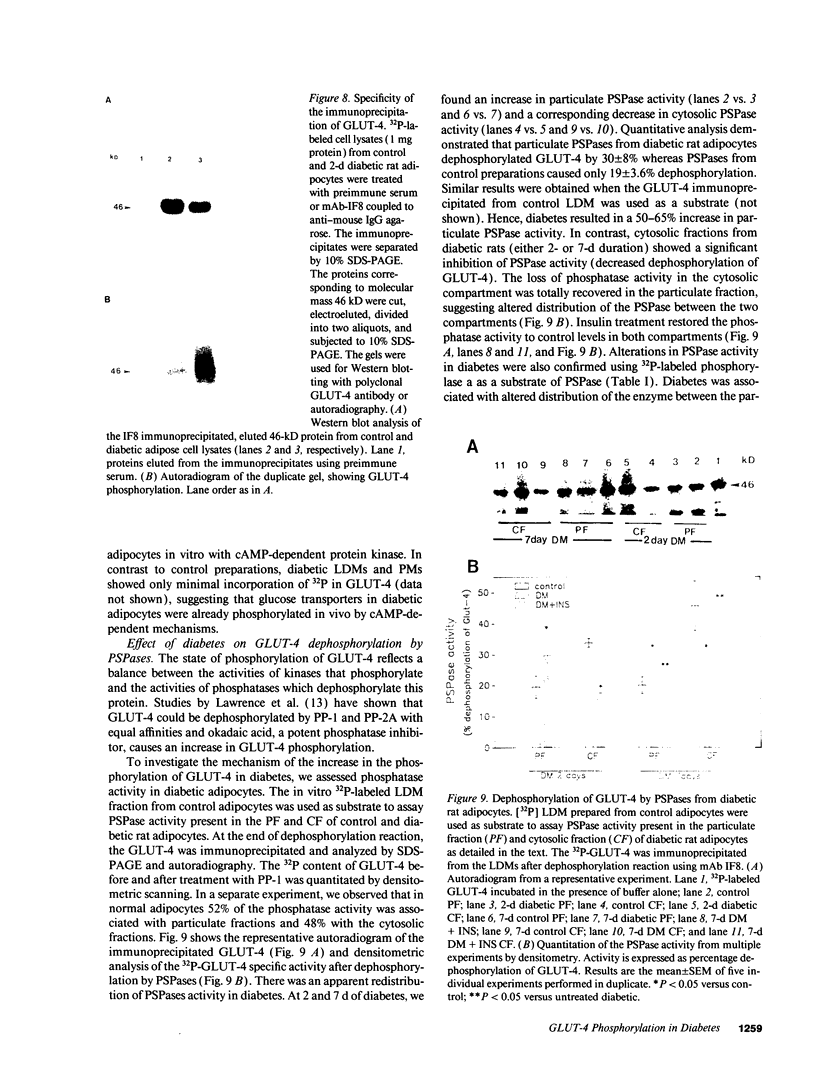
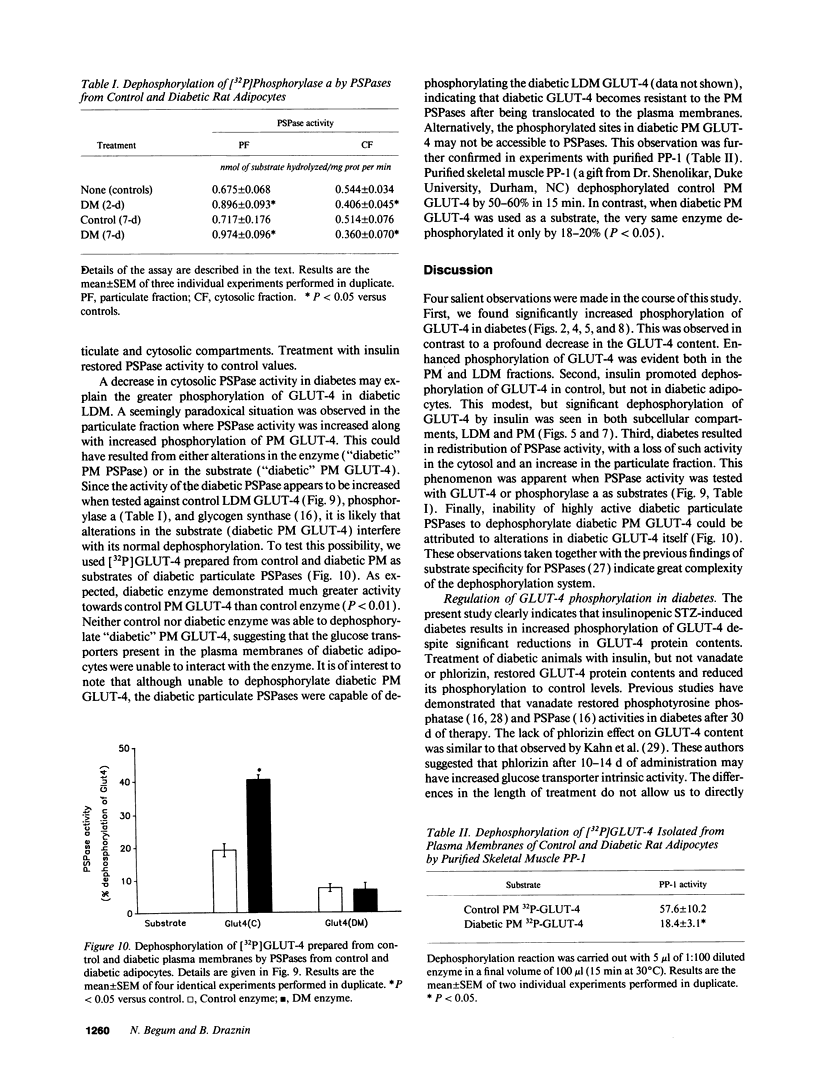
We have examined the regulation of GLUT-4 phosphorylation in adipocytes isolated from diabetic rats. Despite progressive (40-70%) reductions in GLUT-4 protein contents on the 2nd, 7th, and 14th day of diabetes, the phosphorylation of GLUT-4 was increased two- to fourfold. These alterations were accompanied by concomitant reductions (40-66%) in the insulin-stimulated 2-deoxyglucose transport. Insulin treatment of diabetic animals for 5 d restored glucose transport activity, GLUT-4 protein, and GLUT-4 phosphorylation to control levels whereas vanadate and phlorizin were ineffective. In control adipocytes, insulin promoted GLUT-4 translocation from the low density microsomal (LDM) pool to the plasma membranes (PM) and decreased the state of GLUT-4 phosphorylation. In adipocytes isolated from the diabetic rats, insulin failed to stimulate GLUT-4 translocation and to decrease GLUT-4 phosphorylation. To explore the mechanism of the diabetes-induced increases in the GLUT-4 phosphorylation, we investigated phosphoserine phosphatase (PSPase) activities using 32P-labeled GLUT-4 and phosphorylase "a" as substrates. Diabetes resulted in 50-60% increase in the particulate PSPase activity and concomitant reductions in cytosolic PSPase activities. Although reduced cytosolic PSPase activity correlated with an inadequate dephosphorylation of LDM GLUT-4, the existence of highly phosphorylated PM GLUT-4 in the presence of increased particulate PSPase activity required additional explanation. To address this problem, we used PM GLUT-4 from diabetic rats as a substrate of particulate PSPase. Highly active diabetic particulate PSPase, which dephosphorylated control GLUT-4 and phosphorylase a, failed to dephosphorylate PM GLUT-4 from diabetic rats. These data suggest that PM GLUT-4 from diabetic rats is unable to interact with PSPase or that its phosphorylation sites are not accessible to PSPase action. In summary, an induction of diabetes with streptozotocin resulted in significant increases in GLUT-4 phosphorylation. In contrast to normal cells, insulin failed to promote GLUT-4 recruitment to the plasma membranes and its dephosphorylation in diabetic adipocytes. At the same time, diabetes appears to induce redistribution of PSPases, resulting in lower cytosolic activity and higher particulate activity. It also appears that the existence of highly phosphorylated GLUT-4 in the plasma membranes of diabetic adipocytes resulted from its inability to interact with particulate PSPases.
Full text
PDF

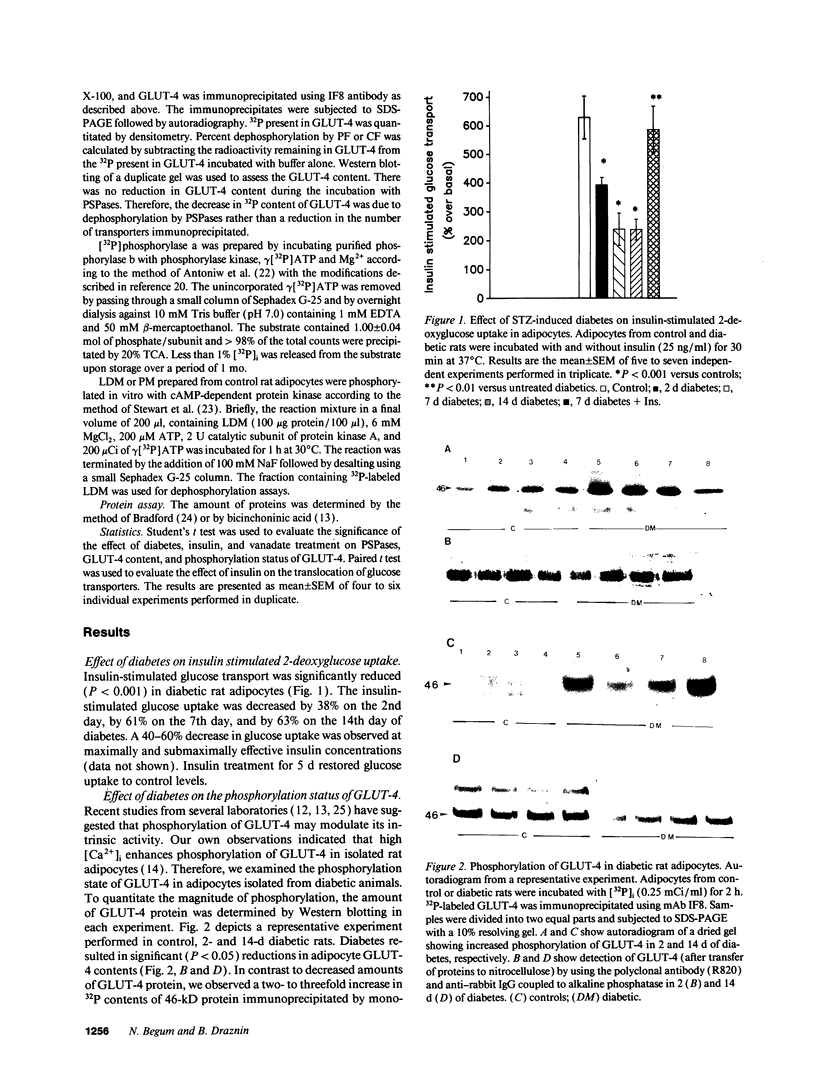


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniw J. F., Nimmo H. G., Yeaman S. J., Cohen P. Comparison of the substrate specificities of protein phosphatases involved in the regulation of glycogen metabolism in rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):423–433. doi: 10.1042/bj1620423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N., Sussman K. E., Draznin B. Differential effects of diabetes on adipocyte and liver phosphotyrosine and phosphoserine phosphatase activities. Diabetes. 1991 Dec;40(12):1620–1629. doi: 10.2337/diab.40.12.1620. [DOI] [PubMed] [Google Scholar]
- Begum N., Sussman K. E., Draznin B. High levels of cytosolic free calcium inhibit dephosphorylation of insulin receptor and glycogen synthase. Cell Calcium. 1991 Jun;12(6):423–430. doi: 10.1016/0143-4160(91)90068-p. [DOI] [PubMed] [Google Scholar]
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clari G., Bordin L., Marzaro G., Moret V. Effect of intracellular pH changes on the distribution of tyrosine- and serine/threonine-protein kinase activities in human erythrocytes. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1021–1027. doi: 10.1016/0006-291x(91)90994-i. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Corvera S., Czech M. P. Mechanism of insulin action on membrane protein recycling: a selective decrease in the phosphorylation state of insulin-like growth factor II receptors in the cell surface membrane. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7314–7318. doi: 10.1073/pnas.82.21.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S., Jaspers S., Pasceri M. Acute inhibition of insulin-stimulated glucose transport by the phosphatase inhibitor, okadaic acid. J Biol Chem. 1991 May 15;266(14):9271–9275. [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Denton R. M., Yorke R. E., Randle P. J. Measurement of concentrations of metabolites in adipose tissue and effects of insulin, alloxan-diabetes and adrenaline. Biochem J. 1966 Aug;100(2):407–419. doi: 10.1042/bj1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draznin B., Lewis D., Houlder N., Sherman N., Adamo M., Garvey W. T., LeRoith D., Sussman K. Mechanism of insulin resistance induced by sustained levels of cytosolic free calcium in rat adipocytes. Endocrinology. 1989 Nov;125(5):2341–2349. doi: 10.1210/endo-125-5-2341. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Matthaei S., Olefsky J. M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest. 1988 May;81(5):1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs E. M., Allard W. J., Lienhard G. E. The glucose transporter in 3T3-L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J Biol Chem. 1986 Dec 15;261(35):16597–16603. [PubMed] [Google Scholar]
- James D. E., Hiken J., Lawrence J. C., Jr Isoproterenol stimulates phosphorylation of the insulin-regulatable glucose transporter in rat adipocytes. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8368–8372. doi: 10.1073/pnas.86.21.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Kahn B. B., Shulman G. I., DeFronzo R. A., Cushman S. W., Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest. 1991 Feb;87(2):561–570. doi: 10.1172/JCI115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Armoni M., Cohen P., Kanter Y., Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes. 1987 Aug;36(8):925–931. doi: 10.2337/diab.36.8.925. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Dephosphorylation of insulin-receptor autophosphorylation sites by particulate and soluble phosphotyrosyl-protein phosphatases. Biochem J. 1990 Feb 15;266(1):251–259. doi: 10.1042/bj2660251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J. F., James D. E. Phosphorylation of the glucose transporter in rat adipocytes. Identification of the intracellular domain at the carboxyl terminus as a target for phosphorylation in intact-cells and in vitro. J Biol Chem. 1990 Feb 5;265(4):2324–2332. [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J. F., James D. E. Stimulation of glucose transport and glucose transporter phosphorylation by okadaic acid in rat adipocytes. J Biol Chem. 1990 Nov 15;265(32):19768–19776. [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J Clin Invest. 1989 Sep;84(3):976–983. doi: 10.1172/JCI114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reusch J. E., Begum N., Sussman K. E., Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology. 1991 Dec;129(6):3269–3273. doi: 10.1210/endo-129-6-3269. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S., Ingebritsen T. S. Protein (serine and threonine) phosphate phosphatases. Methods Enzymol. 1984;107:102–129. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- Shibata H., Robinson F. W., Soderling T. R., Kono T. Effects of okadaic acid on insulin-sensitive cAMP phosphodiesterase in rat adipocytes. Evidence that insulin may stimulate the enzyme by phosphorylation. J Biol Chem. 1991 Sep 25;266(27):17948–17953. [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Raineri-Maldonado C., Buchanan C., Pories W. J., Carter-Su C., Pilch P. F., Caro J. F. Adipose tissue glucose transporters in NIDDM. Decreased levels of muscle/fat isoform. Diabetes. 1991 Apr;40(4):472–477. doi: 10.2337/diab.40.4.472. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Hemmings B. A., Cohen P., Goris J., Merlevede W. The MgATP-dependent protein phosphatase and protein phosphatase 1 have identical substrate specificities. Eur J Biochem. 1981 Mar 16;115(1):197–205. doi: 10.1111/j.1432-1033.1981.tb06217.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Simpson I. A., Rechler M. M., Cushman S. W. Potential mechanism of the stimulatory action of insulin on insulin-like growth factor II binding to the isolated rat adipose cell. Apparent redistribution of receptors cycling between a large intracellular pool and the plasma membrane. J Biol Chem. 1984 Jul 10;259(13):8378–8383. [PubMed] [Google Scholar]