Abstract
Objectives:
Infections caused by carbapenemase-producing Enterobacteriaceae are increasing worldwide, especially in ICUs, and have been associated with high mortality rates. However, unequivocally demonstrating causality of such infections to death is difficult in critically ill patients because of potential confounding and competing events. Here, we quantified the effects of carbapenemase-producing Enterobacteriaceae carriage on patient outcome in two Greek ICUs with carbapenemase-producing Enterobacteriaceae endemicity.
Design:
Observational cohort study.
Setting:
Two ICUs with carbapenemase-producing Enterobacteriaceae endemicity.
Patients:
Patients admitted to the ICU with an expected length of ICU stay of at least 3 days were included.
Interventions:
None.
Measurements and Main Results:
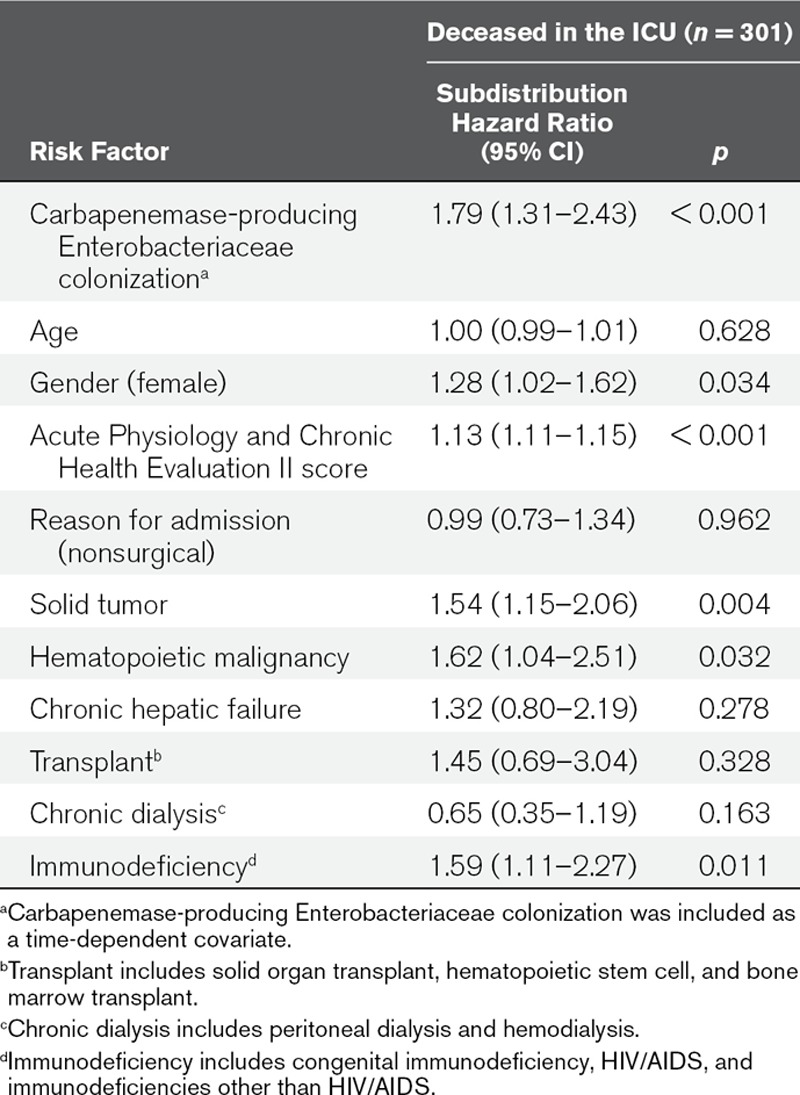
Carbapenemase-producing Enterobacteriaceae colonization was established through screening in perineum swabs obtained at admission and twice weekly and inoculated on chromogenic plates. Detection of carbapenemases was performed phenotypically, with confirmation by polymerase chain reaction. Risk factors for ICU mortality were evaluated using cause-specific hazard ratios and subdistribution hazard ratios, with carbapenemase-producing Enterobacteriaceae colonization as time-varying covariate. One thousand seven patients were included, 36 (3.6%) were colonized at admission, and 96 (9.5%) acquired carbapenemase-producing Enterobacteriaceae colonization during ICU stay, and 301 (29.9%) died in ICU. Of 132 carbapenemase-producing Enterobacteriaceae isolates, 125 (94.7%) were Klebsiella pneumoniae and 74 harbored K. pneumoniae carbapenemase (56.1%), 54 metallo-β-lactamase (40.9%), and four both (3.0%). Carbapenemase-producing Enterobacteriaceae colonization was associated with a statistically significant increase of the subdistribution hazard ratio for ICU mortality (subdistribution hazard ratio = 1.79; 95% CI, 1.31–2.43), not explained by an increased daily hazard of dying (cause-specific hazard ratio for death = 1.02; 95% CI, 0.74–1.41), but by an increased length of stay (cause-specific hazard ratio for discharge alive = 0.73; 95% CI, 0.51–0.94). Other risk factors in the subdistribution hazard model were Acute Physiology and Chronic Health Evaluation II score (subdistribution hazard ratio = 1.13; 95% CI, 1.11–1.15), female gender (subdistribution hazard ratio = 1.29; 95% CI, 1.02–1.62), presence of solid tumor (subdistribution hazard ratio = 1.54; 95% CI, 1.15–2.06), hematopoietic malignancy (subdistribution hazard ratio = 1.61; 95% CI, 1.04–2.51), and immunodeficiency (subdistribution hazard ratio = 1.59; 95% CI, 1.11–2.27).
Conclusions:
Patients colonized with carbapenemase-producing Enterobacteriaceae have on average a 1.79 times higher hazard of dying in ICU than noncolonized patients, primarily because of an increased length of stay.
Keywords: carbapenemase, Enterobacteriaceae, intensive care units, Klebsiella pneumonia, mortality, proportional hazards models
Carbapenemase-producing bacteria are mostly Enterobacteriaceae (carbapenemase-producing Enterobacteriaceae [CPE]), Pseudomonas aeruginosa, and Acinetobacter species (1, 2); and over the last decade, CPE have been reported with increasing frequency in all parts of the world. Klebsiella pneumoniae carbapenemase (KPC) producers were first described in the United States, and endemicity in hospitals has been established in many countries (3–5). Metallo-β-lactamase (MBL)-producing CPE are endemic in large regions of Asia or some parts of Southern Europe, and have been reported worldwide, often owing to international travel (4). CPE with OXA-48 type carbapenemases have been identified mostly in east and south Mediterranean countries, India, and all over Europe upon transfers from endemic regions (4).
CPE pose major challenges in patient management. Resistance is not consistently phenotypically expressed, hampering identification by routine microbiological laboratory methods (1, 6, 7). Failure to aggressively implement infection control measures has lagged behind the spread of CPE, and there are limited options for effective antibiotic treatment of infections (7–9). Several factors have been associated with increased risks of acquisition of CPE or carbapenem-resistant Enterobacteriaceae such as prior exposure to antibiotics, age, and severe illness (10–12).
Infections caused by CPE, mainly bloodstream infections by K. pneumoniae, have been associated with increased mortality as compared with infections caused by Enterobacteriaceae susceptible to carbapenems (10–12), with in-hospital mortality of carbapenem-resistant K. pneumoniae (CRKP) infections ranging between 27% and 61% (10, 12–16). Colonization precedes infection in most instances, and it, therefore, is a marker of a patient being at risk of a poorer outcome. Colonization with multidrug-resistant Gram-negative bacteria (e.g., Acinetobacter species, P. aeruginosa, and extended-spectrum β-lactamase-producing Enterobacteriaceae) has been associated with an increased risk of death of ICU patients (17, 18), and we wish to examine this relationship within the context of CPE endemicity.
MATERIALS AND METHODS
Study Design and Participants
We conducted a post hoc analysis of data from two Greek ICUs (Attikon University Hospital and Laikon General Hospital), that participated in an international interrupted time series and cluster randomized controlled trial, in which different infection control interventions were evaluated (8). These interventions were not associated with statistically significant effects on acquisition of highly resistant Enterobacteriaceae. A waiver for informed consent was granted for all participating centers in this trial by the institution’s review board or national ethics committee. Patients 18 years old or older admitted to the ICU with an expected length of stay (LOS) of 3 days or longer were included in the current study. Patients in the current study were included between August 2008 and January 2011. Patient characteristics were recorded at time of admission. Disposition at ICU discharge was defined as either deceased or alive.
Microbiology
Swabs from perineum and wounds (if present) were obtained within 2 days of admission and twice weekly thereafter. Culture frequency was reduced to once per week in patients staying longer than 3 weeks. All swabs were analyzed in the local laboratory according to a standardized protocol. Swabs were plated onto the Brilliance ESBL 2 Agar and colonies from the groups of Escherichia coli, Klebsiella/Enterobacter/Serratia/Citrobacter, and Proteus/Providencia/Morganella were selected (8). One colony of each morphotype per patient was selected for further analysis, and shipped to a central laboratory. Species identification was done with the Vitek 2 system (bioMérieux, Marcy l’Etoile, France). Isolates with nonsusceptibility to at least one of the carbapenems ertapenem, imipenem, and meropenem according to European Committee on Antimicrobial Susceptibility Testing criteria (http://www.eucast.org) were subjected to phenotypic detection of MBLs (double disk synergy test with disks containing imipenem, ceftazidime, and EDTA), KPCs (combined-disk test with disks containing imipenem and meropenem, and both supplemented with phenylboronic acid), and OXA-48 (temocillin disk test) (6, 19, 20). All of the isolates with phenotypes indicative of being CPE were subjected to polymerase chain reactions specific for blaKPC-, blaIMP-, blaVIM-, blaNDM-, and blaOXA-48-like genes (21–24).
Definitions
A patient was defined as being CPE colonized, if the patient had at least one CPE positive culture during ICU stay. The patient was assumed to have colonization at admission if the first culture was positive with CPE, and in analyses patients were assumed to be colonized from the date of admission. Colonization was considered acquired in case of a positive culture preceded by a negative one, and start of colonization was assumed to be in-between the last negative culture and the first positive culture. It was assumed that colonized patients remained colonized until death or discharge.
Statistical Analyses
The association between CPE carriage and ICU mortality was investigated by competing risk models with CPE colonization as a time-dependent covariate (25, 26). Patients admitted to ICU either die or are discharged alive after a period of ICU stay. Discharged patients are usually in a better health condition than patients in ICU. Therefore, discharge should not be treated as censored, and it is recommended to treat it as a competing event for ICU mortality (27). Further, admitted patients may be colonized with CPE at admission, or acquire CPE carriage during ICU stay. In case of acquisition of colonization, patients have an additional transient state before discharge, corresponding to a time-dependent binary covariate (CPE colonization). Not taking timing of the colonization into account would cause time-dependent bias, causing artificial inflation of a protective effect, or dampening or even reversal of a harmful effect (26, 28, 29).
Cause-specific hazard ratios (CSHRs) for ICU death and discharge alive were analyzed in multivariable analysis to estimate the direct effects of CPE colonization and other characteristics, and subdistribution hazard ratios (SHRs) to assess the overall effects on ICU mortality (26). CPE colonization was included as a time-varying covariate in these analyses. Results are presented graphically in cause-specific cumulative hazard plots, as CPE colonization is modeled as a time-varying risk factor, and cumulative incidence functions cannot be interpreted for time-varying risk factors (25).
To investigate the assumption that acquired colonization starts halfway between the last negative and first positive culture, we performed sensitivity analyses using the first and the last possible moments of CPE colonization, being just after the last negative culture and just before the first positive culture respectively, resembling a best and a worst-case scenario. In both scenarios, patients colonized at admission were considered to be colonized from day 1.
A post hoc analysis was performed to investigate possible differences between the effect of KPC and MBL on ICU mortality, using two dichotomous variables and their interaction (for patients colonized with both carbapenemases) in the models described above (CSHRs and SHRs). To investigate generalizability of the results, a post hoc analysis was performed for both ICUs separately.
Analyses were performed using R version 2.15.1 (The R Foundation for Statistical Computing; www.r-project.org/), using the survival package for CSHRs and SHRs, the mvna package for the cumulative hazard plots, and the clos function of the etm package for calculation of extra LOS, and p values less than 0.05 were considered statistically significant.
RESULTS
A total of 1,007 patients with an expected LOS greater than or equal to 3 days and culture results available were included, of whom 36 (3.6%) were colonized with CPE at admission, 96 (9.5%) acquired colonization during their ICU stay, and 301 (29.9%) died in ICU (Fig. 1). Missed cultures more often occurred at the beginning or the end of study periods (so most likely independent of patient characteristics), and patients with a short length of ICU stay were more likely to be excluded because of missed cultures. These patients were typically more often surgical patients, with lower Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and lower mortality (21%). Admission swabs were correctly taken in 93.5% of included patients, and 90.4% and 96.3% of included patients had a culture within 4 and 7 days before discharge (death or alive), respectively (for patients staying longer than 3 wk culture frequency was weekly according to protocol).
Figure 1.
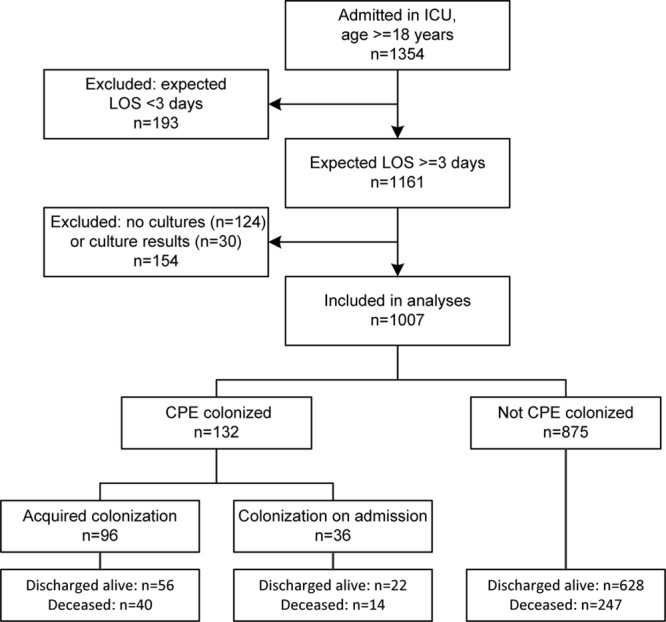
Inclusion of patients, patient selection, and outcomes. CPE = carbapenemase-producing Enterobacteriaceae, LOS = length of stay, n = number of patients.
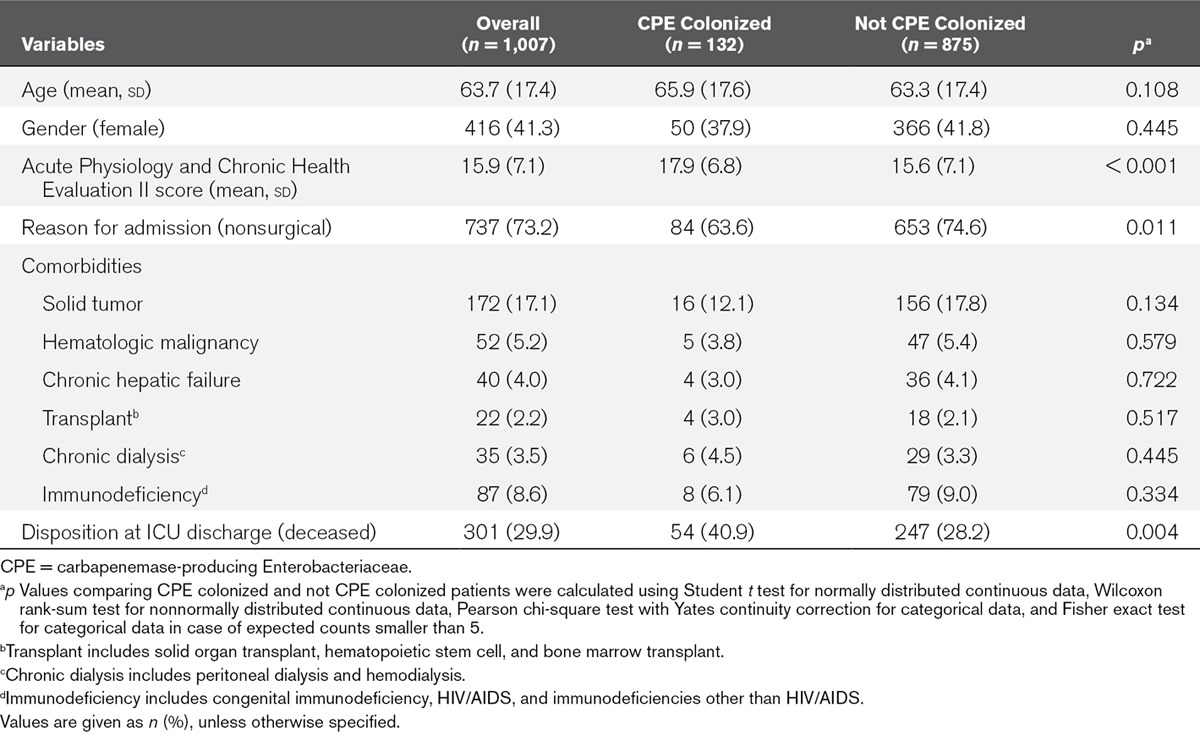
There were 132 CPE isolates, of which 125 (94.7%) were K. pneumoniae, others were Citrobacter freundii, Enterobacter cloacae, E. coli, Enterobacter species other than E. cloacae, Proteus mirabilis, and Providencia stuartii (Table 1). In 74 (56.1%) isolates KPC was present, in 54 (40.9%) isolates VIM type carbapenemases, and 4 (3.0%) isolates harbored both KPC and Verona integron-encoded metallo-β-lactamase (Table 1). No OXA-48 was detected. Mean age of included patients was 63.7 years, 58.7% was male, and mean APACHE II score was 15.9 (Table 2).
TABLE 1.
Bacterial Species and Carbapenemase Genotypes
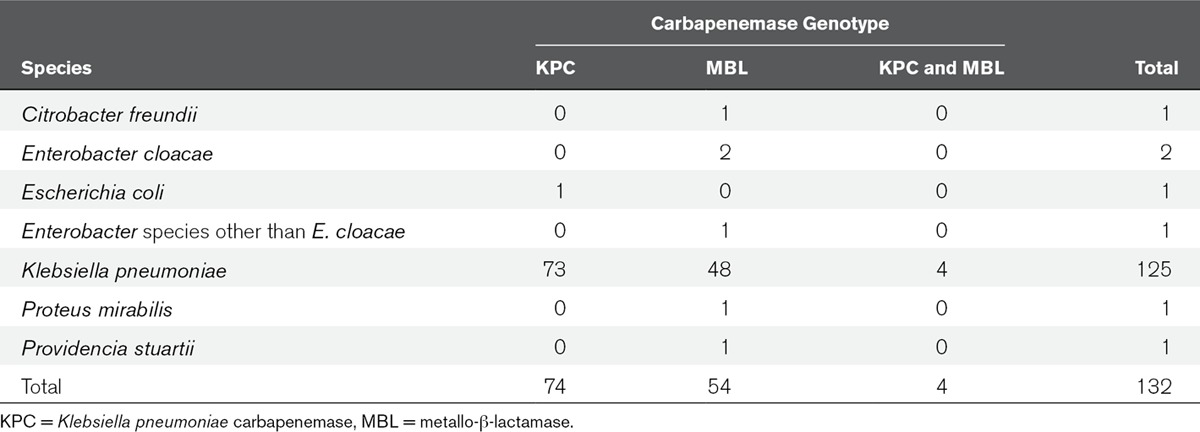
TABLE 2.
Descriptive Results of Potential Risk Factors and Outcomes
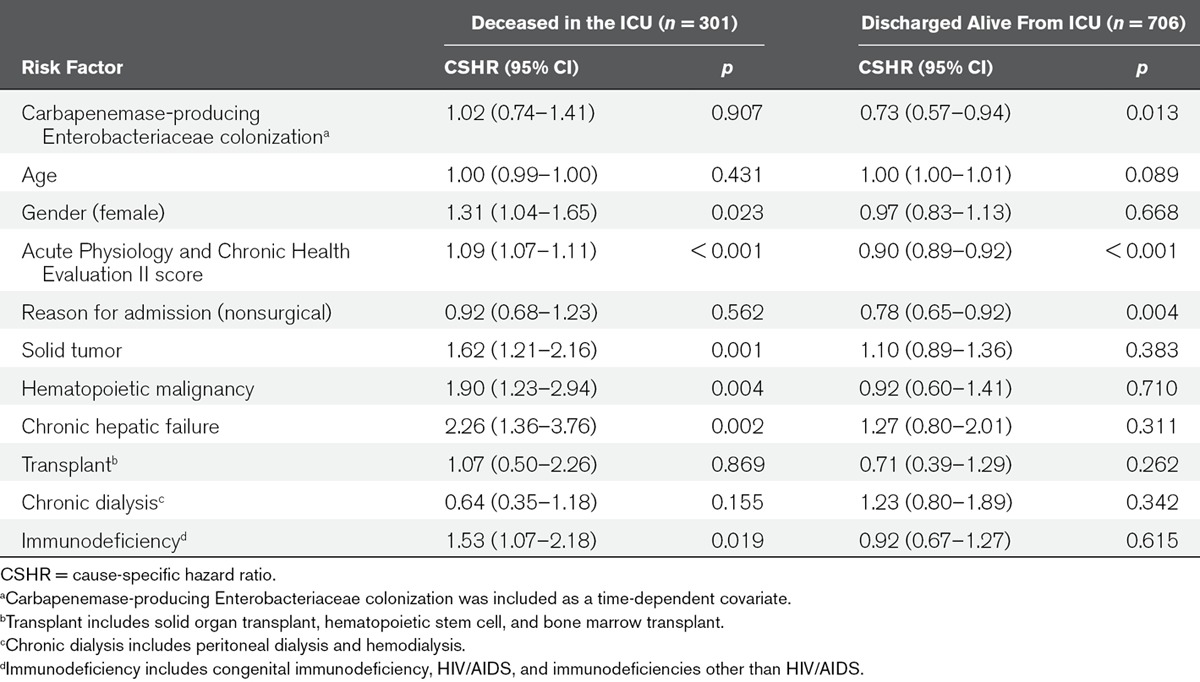
In multivariable analysis, female gender, APACHE II score, presence of solid tumor, hematopoietic malignancy, chronic hepatic failure, and immunodeficiency significantly increased the CSHR for dying in ICU. There was no statistically significant difference between CPE colonized and uncolonized patients in CSHR for death (CSHR = 1.02; 95% CI, 0.74–1.41), so the daily hazard of dying was not increased for CPE colonized patients. However, CPE colonization was associated with a statistically significant reduction in the CSHR for being discharged alive (CSHR = 0.73; 95% CI, 0.51–0.94) (Table 3), so the daily hazard of being discharged alive was lower for CPE colonized patients, leading to a longer LOS after being colonized with CPE, compared with patients that were not colonized with CPE. For some of the covariates in the cause-specific hazard analyses the proportionality assumption was violated, as happens often (26, 30), and these estimates should, therefore, be interpreted as the weighted average over follow-up. However, allowing the effects of the covariates to change over time did not change the estimate for CPE colonization.
TABLE 3.
Multivariable Analysis of Cause-Specific Hazard Ratios for ICU Mortality and Discharge Alive From ICU, With Carbapenemase-Producing Enterobacteriaceae Colonization as a Time-Dependent Covariate
Six variables were associated with a statistically significant increase of the SHR for ICU mortality: CPE colonization (SHR = 1.79; 95% CI, 1.31–2.43), female gender (SHR = 1.28; 95% CI, 1.02–1.62), APACHE II score (SHR = 1.13; 95% CI, 1.11–1.15), presence of solid tumor (SHR = 1.54; 95% CI, 1.15–2.06), hematopoietic malignancy (SHR = 1.62; 95% CI, 1.04–2.51), and immunodeficiency (SHR = 1.59; 95% CI, 1.11–2.27) (Table 4). This reflects an overall increase in mortality for patients colonized with CPE, taking into account the competing event of being discharged alive. The computed unadjusted expected change in LOS for CPE colonization was 3.2 days (95% CI, 2.1–4.3 d).
TABLE 4.
Multivariable Analysis of Subdistribution Hazard Ratios for ICU Mortality, With Carbapenemase-Producing Enterobacteriaceae Colonization as a Time-Dependent Covariate
In sensitivity analyses, in which either the day of the last negative culture or the day of the first day positive culture were used as proxy for the start of colonization, similar results were found, with point estimates for the SHR of death of 1.67 (95% CI, 1.23–2.27) and 1.95 (95% CI, 1.43–2.66) for the first and last possible moment of colonization, respectively.
The observed effects of CPE carriage were comparable for KPC and MBL (SHR KPC = 1.65; 95% CI, 1.11–2.46; SHR MBL = 2.00; 95% CI, 1.30–3.07; interaction term nonsignificant). In separate analyses for both ICUs, SHR with overlapping CIs were found (odds ratio [OR], 2.12 [95% CI, 1.28–3.51] and 1.46 [95% CI, 0.90–2.35]).
DISCUSSION
In this study, performed in two Greek ICUs with CPE endemicity, CPE colonization was associated with a 1.79 times higher overall hazard of mortality in ICU, which was caused by an increased LOS in ICU. Other independent risk factors that were associated with increased ICU mortality in multivariable subdistribution analysis were female gender, APACHE II score at ICU admission, presence of solid tumor, hematologic malignancy, and immunodeficiency.
The crude association between CPE colonization and death (40.9% mortality in CPE colonized vs 28.2% in uncolonized patients) was explored in the competing events multivariate analysis which incorporated potential confounding factors such as severity scores and comorbidities. The CSHRs for ICU death and discharge alive from ICU can be interpreted as two independent standard survival analyses disregarding the fact that they are related to each other. The CSHR for ICU death was not increased for patients colonized with CPE, implying that the daily risk of dying was not increased for CPE colonized patients. However, the CSHR for discharge alive was decreased for patients colonized with CPE, implying that colonized patients had a lower daily probability of being discharged alive from ICU after colonization. This resulted in a longer exposure to the daily risk of dying, thus increasing the total cumulative risk of dying in ICU. This was confirmed in the SHR that does take into account the competing event of being discharged alive, which revealed a significant increase in ICU death for CPE colonized patients. From the CSHRs, it can be concluded that the increased risk of dying in ICU is the result of a longer LOS in ICU after acquiring colonization. This is also illustrated in the cumulative hazard plots (Fig. 2). These results are in line with the calculated – however unadjusted – expected change in LOS of 3.2 days.
Figure 2.
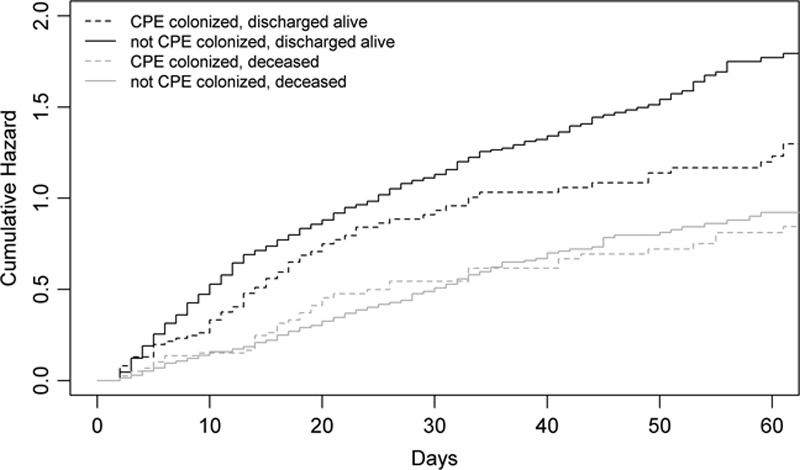
Cause-specific cumulative hazard of ICU mortality, being discharged alive, colonized versus not colonized. Patients acquiring colonization during their hospital stay contribute the not carbapenemase-producing Enterobacteriaceae (CPE) colonized curve up to the moment of colonization acquisition, after which they switch to the CPE colonized curve.
Results were comparable for both ICUs, suggesting the detected effect of CPE colonization is generalizable to other settings with CPE endemicity. Aggressiveness of therapy and chosen regimens may depend on CPE prevalence, so effects of CPE colonization may be different in low endemic settings.
In contrast to other studies that focused on infections caused by CPE, we investigated the effects of colonization, irrespective if it was associated with infection or not. It is still uncertain (though easy imaginable) that mortality can be attributed to CPE infections, as some studies investigating the effect of infection with CRKP compared with carbapenem susceptible Klebsiella pneumoniae on in-hospital mortality, not specifically focusing on ICU patients, reported statistically significant (10, 12, 15) or absence of differences in mortality (13). In a prospective observational study of 226 ICU patients (31) in a setting of high KPC endemicity, mortality was—in univariate analysis—not significantly higher in KPC-producing K. pneumoniae (KPC-Kp) colonized patients compared with KPC-Kp-negative patients (38.4 vs 27.4%; p = 0.160), but length of ICU stay was (25.8 d for KPC-Kp-positive and 12.8 d for KPC-Kp-negative patients; p < 0.001). In multivariate analysis, KPC-Kp colonization was not an independent risk factor for mortality, however, KPC-Kp infection was an independent risk factor (OR, 2.8; 95% CI, 1.1–6.9). In another study, both ICU and in-hospital mortality rates did not differ significantly between patients that acquired CRKP in ICU and CRKP-noncolonized patients, but the length of ICU stay was significantly longer for patients acquiring CRKP colonization (24.8 vs 13.7 d; p < 0.0001) (32). Yet, not accounting competing events and time-dependent covariates may have introduced bias in both studies.
APACHE II score at ICU admission was an independent risk factor for ICU mortality. The effect of CPE colonization on ICU mortality is adjusted for this effect. The association between female gender and ICU mortality has been reported before, but not consistently (33–37).
This study has several limitations, for instance because some assumptions were needed in our analyses. The exact moment of CPE colonization was unknown, as cultures were taken twice weekly. We assumed colonization to occur midway between the last negative and first positive culture. However, sensitivity analyses using the first or the last possible moment of colonization as time of colonization gave similar results, strengthening the validity of this assumption. Compliance to microbiological culture protocol was high, minimizing the risk of bias. Further, we assumed that CPE colonized patients remain colonized until the end of ICU stay. As there were no attempts for decolonization we consider this a valid assumption.
The use of ESBL-selective chromogenic medium may have induced missing ESBL-negative OXA-48 isolates. Yet, no ESBL and OXA-48 coproducing isolates were detected, thus the probability of missing significant amounts of colonized patients seems low. The first reported OXA-48 outbreak in Greece occurred between December 2011 and March 2012, which was after the current study (38).
Finally, the potential confounders that could be adjusted for was restricted, and did not include time-varying confounders, such as disease severity scores. Additionally, all-cause mortality could have been confounded by diagnosis at ICU admission and more specifically defined underlying comorbidities. Therefore, residual confounding may still be present. Additionally, we did not assess antibiotic use. Although results of screening for CPE were not known to treating physicians, patients with clinical cultures with CPE might have been treated with antibiotics for this, influencing chances of dying or discharge alive. Furthermore, we could not explore whether patients who were colonized by CPE developed an infection and if they died due to that infection.
ACKNOWLEDGMENTS
We thank E. D. Papadomichelakis for his contribution to data collection, A. Baraniak and M. Herda for help in the microbiological analysis, and C. Spitoni for assistance with data analysis.
Footnotes
This work was performed at University Medical Center Utrecht, Utrecht, The Netherlands; National Medicines Institute, Warsaw, Poland; University General Hospital Attikon, Athens, Greece; Laikon General Hospital, Athens, Greece; and APHP GH Henri Mondor, Creteil, France.
Drs. Dautzenberg and Wekesa contributed equally to this article.
The Mastering hOSpital Antimicrobial Resistance in Europe Work Package 3 Study Team consists of: E. Aires, D. Annane, A. Antoniadou, I. Aragão, A. Armaganidis, F. Blairon, M.J.M. Bonten, C. Brun-Buisson, J. Carneiro, A. Chalfine, D. Chaskou, B.S. Cooper, P. Coppadoro, M.J.D. Dautzenberg, L.P.G. Derde, A.-P. Dias, I. Drinovec, U. Dumpis, M. Elia, J. Empel, F. Esteves, V. Exarchou, A. Flet, J. Fournier, H. Giamarellou, N. Gillet, M. Gniadkowski, H. Goossens, M. Hopman, W. Hryniewicz, A. Jaklič, M. Jereb, A. Kane, E. Karkali, J. Kieffer, P. Kirpach, C. Landelle, F. Landercy, C. Lawrence, P. Legrand, F. Leus, V. Lopes, E. Magira, F. Marco, S. Malhotra-Kumar, J.A. Martinez, A. Melbārde-Kelmere, B. Misset, R. Monte, E. Moreno, I. Muzlovic, G. Nardi, J.C. Nguyen, M. Novak, D. Orazi, E. Papadomichelakis, J. Papaparaskevas, M. Paris, J. Pavleas, G.L. Petrikkos, F. Pimenta, R. Piner, A. Radouan, M.-G. Ramunno, M. Reis, I. Rinaldi, E. Ronco, A. San Jose, J. Schotsman, K. Seme, A. Skiada, P. Stammet, V. Tomic, A. Torres Martí, S. Trapassi, M. Tronci, M. Verachten, J. Vila, K. Vrankar, R.J.L. Willems, M. Winkler, S. Zagavierou, J. Zwerver.
Supported, in part, by the European Commission under the Life Science Health Priority of the 6th Framework Program (MOSAR network contract (LSHP-CT-2007-037941).
Presented, in part, at the 24th European Congress of Clinical Microbiology and Infectious Diseases (poster P0557), Barcelona, Spain.
Dr. Dautzenberg’s institution received grant support from the European Commission (EC) under the Life Science Health Priority of the 6th Framework Program (Mastering hOSpital Antimicrobial Resistance in Europe [MOSAR] network contract LSHP-CT-2007-037941). Dr. Gniadkowski consulted for European Centre for Disease Prevention and Control and European Society of Clinical Microbiology and Infectious Diseases (Dr. Gniadkwoski is a member of the Scientific Advisory Board of the EuSCAPE Surveillance project. He is a member of the Subcommittee for Scientific Affairs of the European Society of Clinical Microbiology and Infectious Disesease [travel costs]), provided expert testimony for the National Research Centre, Poland (Dr. Gniadkwoski is a member of the Expert Board reviewing grant applications for the National Research Centre), lectured for Liofilchem (support for travel and honorarium), received support for travel from bioMerieux, and received support for article research from the EC grant by MOSAR. His institution received grant support from the EC (institution participated in the EC project MOSAR within which this study was performed), National Research Center Poland, and Liofilchem (Dr. Gniadkowski’s team ran/is running a number of research grants from different entities). Dr. Giamarellou consulted for Astellas, Novartis, and Pfizer and lectured for Pfizer and Novartis. Dr. Petrikkos’ institution received grant support from the National and Kapodistrian University of Athens. Dr. Skiada received support for travel from the MOSAR study. Dr. Skiada and her institution received grant support from the MOSAR study. Dr. Brun-Buisson’s institution received grant support from the EC (MOSAR Integrated Project). Dr. Bonten’s institution received grant support from the EU 7th Framework Program. Dr. Derde is employed by UMC Utrecht. Her institution received grant support from the EC under the Life Science Health Priority of the 6th Framework Program (MOSAR network contract LSHP-CT-2007-037941). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Gupta N, Limbago BM, Patel JB, et al. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis. 2011;53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TR. Emerging carbapenemases: A global perspective. Int J Antimicrob Agents. 2010;36(Suppl 3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20:821–830. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 6.Nordmann P, Gniadkowski M, Giske CG, et al. European Network on Carbapenemases. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2012;18:432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 8.Derde LP, Cooper BS, Goossens H, et al. MOSAR WP3 Study Team. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: An interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasink LB, Edelstein PH, Lautenbach E, et al. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchaim D, Navon-Venezia S, Schwaber MJ, et al. Isolation of imipenem-resistant Enterobacter species: Emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob Agents Chemother. 2008;52:1413–1418. doi: 10.1128/AAC.01103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel G, Huprikar S, Factor SH, et al. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 13.Kofteridis DP, Valachis A, Dimopoulou D, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: A case-case-control study. J Infect Chemother. 2014;20:293–297. doi: 10.1016/j.jiac.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Lai CC, Wu UI, Wang JT, et al. Prevalence of carbapenemase-producing Enterobacteriaceae and its impact on clinical outcomes at a teaching hospital in Taiwan. J Formos Med Assoc. 2013;112:492–496. doi: 10.1016/j.jfma.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to beta-Lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: Molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50:364–373. doi: 10.1086/649865. [DOI] [PubMed] [Google Scholar]
- 17.Lortholary O, Fagon JY, Hoi AB, et al. Nosocomial acquisition of multiresistant Acinetobacter baumannii: Risk factors and prognosis. Clin Infect Dis. 1995;20:790–796. doi: 10.1093/clinids/20.4.790. [DOI] [PubMed] [Google Scholar]
- 18.Maragakis LL, Perl TM. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 19.Gazin M, Paasch F, Goossens H, et al. MOSAR WP2 and SATURN WP1 Study Teams. Current trends in culture-based and molecular detection of extended-spectrum-β-lactamase-harboring and carbapenem-resistant Enterobacteriaceae. J Clin Microbiol. 2012;50:1140–1146. doi: 10.1128/JCM.06852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazin M, Lammens C, Derde LPG, et al. External Quality Assessment of Culture-Based Detection of Extended Spectrum Beta Lactamase Producing Gram-Negative Bacteria by a Network of European Laboratories. London: ECCMID; 2012. [Google Scholar]
- 21.Fiett J, Baraniak A, Mrówka A, et al. Molecular epidemiology of acquired-metallo-beta-lactamase-producing bacteria in Poland. Antimicrob Agents Chemother. 2006;50:880–886. doi: 10.1128/AAC.50.3.880-886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mushtaq S, Irfan S, Sarma JB, et al. Phylogenetic diversity of Escherichia coli strains producing NDM-type carbapenemases. J Antimicrob Chemother. 2011;66:2002–2005. doi: 10.1093/jac/dkr226. [DOI] [PubMed] [Google Scholar]
- 23.Navon-Venezia S, Chmelnitsky I, Leavitt A, et al. Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob Agents Chemother. 2006;50:3098–3101. doi: 10.1128/AAC.00438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel L, Héritier C, Tolün V, et al. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyersmann J, Schumacher M. Time-dependent covariates in the proportional subdistribution hazards model for competing risks. Biostatistics. 2008;9:765–776. doi: 10.1093/biostatistics/kxn009. [DOI] [PubMed] [Google Scholar]
- 26.Beyersmann J, Allignol A, Schumacher M. Competing Risks and Multistate Models With R. New York, Springer; 2008. [Google Scholar]
- 27.Wolkewitz M, Vonberg RP, Grundmann H, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: Application of competing risks models. Crit Care. 2008;12:R44. doi: 10.1186/cc6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyersmann J, Gastmeier P, Wolkewitz M, et al. An easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimation. J Clin Epidemiol. 2008;61:1216–1221. doi: 10.1016/j.jclinepi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Beyersmann J, Wolkewitz M, Schumacher M. The impact of time-dependent bias in proportional hazards modelling. Stat Med. 2008;27:6439–6454. doi: 10.1002/sim.3437. [DOI] [PubMed] [Google Scholar]
- 30.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadimitriou-Olivgeris M, Marangos M, Fligou F, et al. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: The significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis. 2013;77:169–173. doi: 10.1016/j.diagmicrobio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31:1811–1817. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 33.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 34.Nachtigall I, Tafelski S, Rothbart A, et al. Gender-related outcome difference is related to course of sepsis on mixed ICUs: A prospective, observational clinical study. Crit Care. 2011;15:R151. doi: 10.1186/cc10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Combes A, Luyt CE, Trouillet JL, et al. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. 2009;37:2506–2511. doi: 10.1097/CCM.0b013e3181a569df. [DOI] [PubMed] [Google Scholar]
- 36.Adrie C, Azoulay E, Francais A, et al. OutcomeRea Study Group. Influence of gender on the outcome of severe sepsis: A reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 37.Epstein SK, Vuong V. Lack of influence of gender on outcomes of mechanically ventilated medical ICU patients. Chest. 1999;116:732–739. doi: 10.1378/chest.116.3.732. [DOI] [PubMed] [Google Scholar]
- 38.Voulgari E, Zarkotou O, Ranellou K, et al. Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. J Antimicrob Chemother. 2013;68:84–88. doi: 10.1093/jac/dks356. [DOI] [PubMed] [Google Scholar]


