Abstract
Objective
To determine if SMAD4 expression is associated with recurrence pattern after resection for pancreatic ductal adenocarcinoma (PDA)
Introduction
SMAD4 expression status has been reported to be associated with patterns of failure in PDA, but studies have not examined recurrence patterns after resection.
Methods
A tissue microarray was constructed including 127 patients with resected PDA and either short (<12 months) or long (>30 months) survival. SMAD4 expression was evaluated by immunohistochemistry and categorized as present or lost in tumor cells. Conventional pathologic features (lymph node metastases, positive resection margin, poor grade, tumor size) were recorded, and disease-specific outcomes (e.g. recurrence pattern and early cancer-specific mortality) determined.
Results
Loss of SMAD4 expression in pancreatic adenocarcinoma was identified in 40 of 127 patients (32 %). SMAD4 loss occurred in 27% of patients who experienced isolated local recurrence, 33% of patients with a distant recurrence, 33% of patients who recurred locally and at distant sites, and 25% of patients who were without evidence of recurrence (Fisher's exact, p=0.9). In a multivariate analysis, the presence of regional lymph node metastases was the only factor associated with the development of distant metastases (odds ratio, OR=4.7, p=0.02). SMAD4 was neither associated with recurrence pattern (OR=0.9, p=0.9), nor early death (OR=0.5, p=0.15).
Conclusion
Primary tumor SMAD4 expression status was not a predictor of recurrence pattern in a large cohort of patients with resected PDA.
Introduction
The management approach for pancreatic ductal adenocarcinoma has not changed significantly over the past two decades, aside from more frequent use of neoadjuvant treatment at some centers[1] and the use of the multi-drug regimen FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) in good performance status patients with advanced disease [2]. Most patients are treated similarly using an empiric gemcitabine-based approach, despite the fact that pancreatic adenocarcinoma is a heterogeneous disease with significant molecular differences between tumors [3]. In the modern era of molecular profiling, there has been a push to identify molecular signatures that could be used to predict tumor biology, and perhaps tailor treatment [4-8].
In this context, two intriguing studies have recently been published which suggest that the SMAD4 expression pattern in pancreatic cancer is associated with disease distribution and eventual pattern of failure [9, 10]. One study analyzed SMAD4 expression patterns in pancreatic cancer in autopsies [10], and a second study focused on patients with locally advanced disease [9]. The studies reached similar conclusions in two distinct patient populations- that SMAD4 protein expression was associated with a locally predominant progression pattern (with treatment implications for local therapies such as radiation) while loss of SMAD4 was associated with distant metastases (with adjuvant radiation less likely to impact outcome). Validation in patients with resectable pancreatic cancer would support a treatment paradigm based on SMAD4 expression status, where chemoradiation would be favored in patients harboring tumors with retained SMAD4 expression. On the other hand, patients with absent SMAD4 expression would be reasonably spared the toxic effects of such treatment. In this study, we assessed the utility of SMAD4 expression status as a biomarker of recurrence pattern in a large cohort of patients with resectable pancreatic cancer.
Methods
Patients
This study was approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) institution review board. Patients underwent a pancreaticoduodenectomy or a distal pancreatectomy for ductal adenocarcinoma at MSKCC after the year 2000. Patients were selected based on survival, and included if they suffered a cancer-specific death within 1 year of resection (short survivors) or survived at least 30 months (long survivors). The TMA was constructed as part of a separate study of prognostic factors (manuscript in submission). As described elsewhere, survival cutoffs were selected to achieve a time gap between study groups (>18 months) that adequately distinguished aggressive and less aggressive tumor biology, and still yielded sample sizes with sufficient power for statistical analyses. In the present study, the study design which included dichotomous groups based on survival enabled comparisons of SMAD4 expression at two ends of the biological spectrum; the more aggressive variety has a biological phenotype that more closely approximates the phenotypes of PDAs included in the two aforementioned studies of SMAD4 in advanced pancreatic cancer [9, 10].
Clinicopathologic Information
Clinicopathologic data were extracted from the prospectively maintained MSKCC Pancreatic Surgery Database and from review of electronic medical records. Collected pathologic variables included resection margin status, lymph node status, tumor size, and histologic grade. Clinical information included recurrence pattern and the use of adjuvant chemotherapy or radiation. The recurrence pattern was determined through careful examination of medical records and follow-up imaging. Both synchronous and metachronous sites of recurrence were recorded. A local recurrence was defined as a retroperitoneal recurrence that occurred either in the resection bed or in the retroperitoneal lymph nodes. A distant recurrence was defined as recurrence at any other site, such as in the peritoneum, liver, lungs, or other solid organ. For patients who had not reached the endpoint of death, the pattern of failure at the time of the last patient encounter was recorded.
Tissue Preparation
A tissue microarray (TMA) was constructed from tissue cores obtained from formalin-fixed, paraffin-embedded tissue blocks in 151 patient samples. A single representative block was selected from each patient, and areas with the highest tumor density on a corresponding H & E stained section were marked under the microscope. TMA's were then constructed on an automated tissue array machine (ATA-27, Beecher Instruments, Silver Spring, MD). Triplicate cores of 0.6 mm in diameter were punched from each block and transferred to a virgin TMA block. Cores were placed on the block in no particular order so that immunohistochemical review of stained TMA slides could be performed in an unbiased fashion. Four micron thick sections were prepared from the TMA blocks for H & E stains and used for SMAD4 immunohistochemistry.
Immunohistochemical Analysis
Immunohistochemical stains were performed using a standard streptavidin-biotinperoxidase procedure. Thin 4 μm paraffin sections were deparaffinized and hydrated with distilled water. Heat-induced epitope retrieval with citric acid buffer (pH 6, 30 minutes, at 97°C) was performed with a steamer. Slides were cooled to 60°C and washed in running water for 2 minutes, and transferred to PBS buffer. A primary antibody against SMAD4 (1:800, Santa Cruz Bio, Santa Cruz, CA) was applied overnight at 4°C. The slides were washed with PBS, followed by a secondary antibody (1:500, biotinylated anti-mouse, Vector Labs, Burlingame, CA) for 60 minutes at room temperature. After additional washing, the slides were incubated for 60 minutes with streptavidin, washed, and developed with DAB for 5 minutes. The slides were washed and counterstained with Harris hematoxylin.
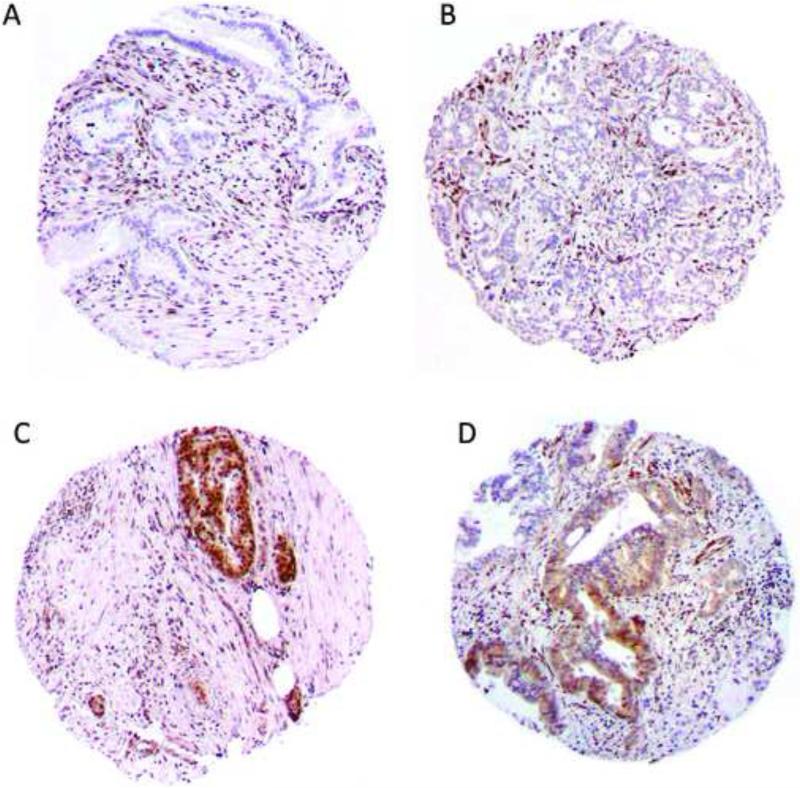
Immunohistochemical review was performed by a senior pancreatic pathologist (L.H.T.). A second pathologist scored the TMAs to test for inter-rater reliability (W.L.). Pancreatic ductal adenocarcinomas were considered to have absent SMAD4 expression if neoplastic cells lacked immunohistochemical labeling but non-neoplastic cells (e.g. stromal cells) reacted positively as an internal control [9, 10]. Any convincing labeling was considered as positive for individual cores, and the predominant SMAD4 expression pattern in each triplicate set was recorded for analysis. Representative cores labeled with an antibody to SMAD4 are provided in Figure 1.
Figure 1.
SMAD4 immunohistochemistry in representative cores of pancreatic ductal adenocarcinoma. Neoplastic epithelial cells lack SMAD4 expression in contrast to nonneoplastic stromal cells in a) and b). SMAD4 expression is observed in cancer cells as well as the non-neoplastic stromal cells in c) and d).
Statistical Analysis
The analysis was performed using Intercooled Stata 8.2. Categorical variables were tested by the Fisher's exact test in the univariate analysis, and with logistic regression in the multivariate analysis. Inter-rater agreement of SMAD4 immunohistochemistry was assessed with the kappa statistic, and interpreted according to the following scale: κ<0 shows poor agreement, 0 to 0.20 shows slight agreement, 0.21 to 0.40 shows fair agreement, 0.41 to 0.60 shows moderate agreement, 0.61-0.80 shows substantial agreement, and >0.80 shows almost perfect agreement [11]. All statistics were two-tailed with a p value<0.05 indicating statistical significance.
Results
The TMA included 151 different patient samples. There was insufficient neoplastic cellularity for proper assessment of SMAD4 expression in 9 sample sets and the recurrence pattern could not be determined using available follow-up documentation in 15 patients. The final analysis therefore included 127 patients, including 110 right sided lesions and 17 left sided lesions. The specimens were analyzed in aggregate, as well as in subgroups stratified by survival after resection (i.e. short and long survivors). A total of 56 patients had a short cancer-specific survival after resection (survival<12 months) and 71 patients had a relatively long survival (survival>30 months). The endpoint of death occurred in 112 of the 127 patients (88%). Median follow-up in living patients was 48 months (range 31-108 months).
Patients were grouped into one of four categories based on their recurrence pattern. The distribution of patients by recurrence pattern is provided in Table 1; the data are presented for the total cohort, as well as each survival group. Recurrence patterns for the total cohort were as follows: local recurrence only (n=15, 12%), distant recurrence only (n=42, 33%), both local and distant recurrence (n=58, 46%), and no recurrence (n=12, 9%). The majority of patients failed outside of the retroperitoneum (n=100, 79%). Out of these patients, just over half (n=58, 58%) developed a local recurrence in addition to a distant metastasis. Virtually all of the patients who recurred locally in the retroperitoneum without distant failure (i.e. local recurrence only) had tumors with a relatively favorable biology (13 out of 15 patients were in the long survival group). Patients with distant recurrences are more or less equally divided between the short and long survivor groups, regardless of whether or not a local recurrence was also present.
Table 1.
Recurrence pattern, stratified by tumor biology (n=127)
| Short survival, <12 months, n=56 | Long survival, >30 months, n=71 | All patients, n=127 | |
|---|---|---|---|
| Local recurrence only | 2 (4%) | 13 (18%) | 15 (12%) |
| Distant recurrence only | 21 (38%) | 21 (30%) | 42 (33%) |
| Local and distant recurrence | 33 (59%) | 25 (35%) | 58 (46%) |
| No recurrence | 0 (0) | 12 (17%) | 12 (9%) |
Percentages are with respect to the specified cohort (i.e. each column)
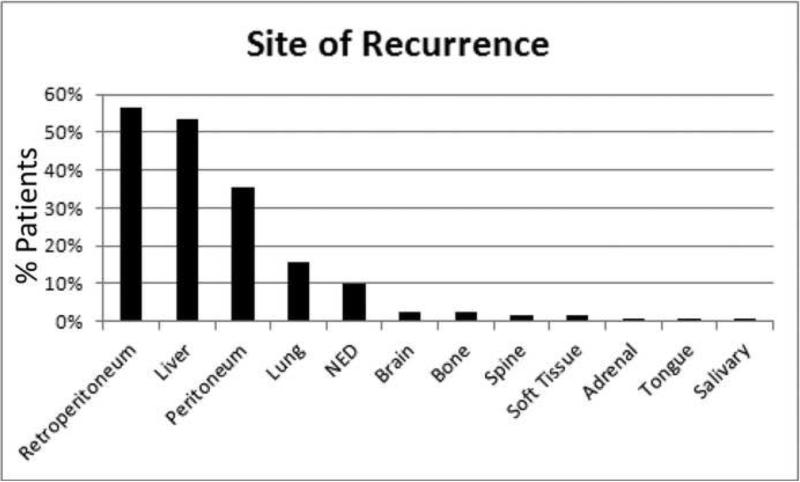
With regards to specific organ sites of recurrence, 73 of 127 patients (57%) had a retroperitoneal recurrence, 66 (52%) recurred in the liver, 44 (35%) elsewhere in the peritoneum, and 19 (15%) in the lung. Less common sites of metastases included the brain, bone, spine, soft tissue, adrenal gland, salivary gland, and tongue (Figure 2). Roughly half of the patients were noted to have recurred at multiple sites (n=62, 49%), and twelve patients (9%) had not experienced disease recurrence. At last follow-up, 9 of the 12 patients without disease remained alive with a median follow up of 60 months (range, 31-108 months). Of note, patients who died an early cancer-specific death recurred more frequently in the liver than patients with a prolonged survival (75% vs. 34%, p<0.0001). In contrast, lung metastases were more common in long-term survivors (21% vs. 7%, p=0.04).
Figure 2.
Percentage of patients (total, n=127) who recurred at the indicated sites. NED, no evidence of disease.
Next, we examined whether or not an association existed between SMAD4 expression and the pattern of failure (Table 2). Loss of SMAD4 expression in the tumor was observed in 32% of the total cohort, and ranged between 25% and 33% for each recurrence category (p=0.9). When the subgroup of patients with the most biologically aggressive tumors was examined (the short survivors share a similar aggressive phenotype with many patients in prior studies of SMAD4 and recurrence with advanced PDA), no difference amongst the recurrence patterns was observed (p=1.0, data not shown). Additionally, we analyzed the data according to anatomic or organ-specific site of metastasis (Figure 2). Since many patients recurred at multiple sites and therefore are included in multiple organ-specific subgroups, the data were not evaluated statistically. Loss of SMAD4 across the four most common metastatic sites were as follows: retroperitoneum, 32%; liver, 35%; peritoneum, 32%; and lung, 32%. With regards to primary tumor location, loss of SMAD4 was observed in 30% of right sided lesions and 41% of left sided lesions (p=0.2).
Table 2.
Recurrence pattern and SMAD4 expression status (n=127)
| SMAD4 Loss | SMAD4 Expression | |
|---|---|---|
| Total | 40 (31.5%) | 87 (68.5%) |
| Local recurrence only | 4 (27%) | 11 (73%) |
| Distant recurrence only | 14 (33%) | 28 (67%) |
| Local and distant recurrence | 19 (33%) | 39 (67%) |
| No recurrence | 3 (25%) | 9 (75%) |
Percentages are with respect to recurrence pattern (i.e. each row)
SMAD4 loss vs. SMAD4 expression, Fisher's exact, p=0.9
A multivariate analysis was performed to evaluate SMAD4 as a predictor of recurrence pattern after adjusting for conventional pathologic features and adjuvant treatment data. Only patients with documented recurrences were included in the subgroup analysis, and patients without any evidence of disease (n=12) at the time of the study were excluded. For simplicity, patients with distant metastases (including those with ‘distant-only recurrence,’ and those with ‘distant and local recurrence’) were collapsed into a single category for the regression model and compared to patients with a local-only recurrence (Table 3). Adjuvant therapy did not play a role in recurrence patterns, and this was consistent when either chemotherapy or radiation was factored into the model. Similarly, SMAD4 did not have any predictive value. Regional lymph node metastases were significantly associated with distant metastases, while other conventional pathologic features were not. Table 4 details the lymph node status according to recurrence pattern. While lymph node metastases were absent in 28% (36 of 127) of the total cohort, 60% (9 of 15 patients) of the patients in the local-only group were free of lymph node metastases (Fisher's exact= 0.04, Table 4).
Table 3.
Multivariate regression model of recurrence pattern: predictors of distant metastases (n=115)
| N (%) | Odds Ratio | P value | |
|---|---|---|---|
| Adjuvant therapy | 43 (39%) | 1.2 | 0.8 |
| SMAD4 | 37 (32%) | 1.2 | 0.8 |
| Positive lymph nodes | 81 (70%) | 6.0 | 0.001 |
| Positive resection margin | 16 (14%) | 1.3 | 0.8 |
| Poor differentiation | 39 (34%) | 1.1 | 0.9 |
| Size > 3 cm | 73(64%) | 1.9 | 0.3 |
The results are consistent when adjuvant chemotherapy or radiation are substituted for adjuvant therapy. The regressions estimate the risk of distant metastases. Patients in the 'distant recurrence only' category are grouped with patients who had both 'local and distant recurrence.' Patients with no evidence of disease (n=12) were excluded from the regression model, as many will recur at some point in the future.
Table 4.
Recurrence by site and lymph node status (n=127)
| Negative Lymph Nodes, n=36 | Positive Lymph Nodes N=91 | |
|---|---|---|
| Local recurrence only | 9 (60%) | 6 (40%) |
| Distant recurrence only | 9 (21%) | 33 (79%) |
| Local and distant recurrence | 16 (28%) | 42 (72%) |
| No recurrence | 2 (17) | 10 (83%) |
Percentages are with regards to recurrence pattern (i.e. each row) P=0.04
SMAD4 has been implicated as a prognostic marker, and therefore we evaluated the biomarker's ability to distinguish short- and long-term survivors in the present cohort. Loss of SMAD4 was observed in 22 of 56 patients (39%) in the short survivor group, as compared to 18 of 71 patients (25%) in the long survivor group (p=0.12). A multivariate regression analysis was performed which included conventional pathologic features and adjuvant treatment. In this model SMAD4 did not reach statistical significance: SMAD4, odds ratio (OR)=0.5, p=0.15; positive lymph nodes, OR=4.5, p=0.005; positive resection margin, OR=1.5, p=0.5; poor differentiation, OR=2.8, p=0.02; tumor more than 3 cm, OR=2.2, p=0.07; adjuvant treatment, OR=0.3, p=0.02.
SMAD4 expression was scored by a second pathologist to test inter-rater reliability. There was substantial agreement between pathologists, with 83% agreement and κ=0.6114 (p<0.0001). The entire analysis was repeated using scores from the second pathologist, and none of the findings changed (data not included).
Discussion
SMAD4 is a tumor suppressor gene involved in TGFβ signaling [12], and is inactivated by homozygous deletions or somatic mutations in over 50% of sporadic pancreatic adenocarcinomas [13-15]. In this study, primary tumor specimens from a large number of patients (n=127) with resected ductal adenocarcinoma of the pancreas were examined for SMAD4 expression and analyzed with respect to pattern of failure. SMAD4 expression was absent in approximately 1/3 of pancreatic adenocarcinomas, regardless of the survival outcome, general pattern of failure, or the specific organ site of recurrence. We believe this is an important finding in the context of two recent studies which observed that intact SMAD4 expression was associated with a local recurrence, while absent SMAD4 expression was associated with distant metastases [9, 10]. While we cannot definitively account for the different conclusions from our study, the most likely explanation relates to differences in patient selection.
Iacobuzio et al. profiled the pattern of failure in 76 autopsies performed on patients who died from pancreatic adenocarcinoma [10]. Less than 1/3 of the patients presented with resectable disease (Stage I or II), while the remaining patients presented with stage III (24%) or stage IV (47%) disease. In addition, 10% of patients had rare variants of non-conventional ductal (tubular) adenocarcinoma. The authors examined SMAD4 expression in tumors of 65 of the autopsy specimens, and observed loss of SMAD4 in 41 (63%) of the samples. Just 2 of 9 autopsies that exhibited a pattern of locally advanced pancreatic cancer without metastases had tumors with loss of SMAD4 (22%). However, 16 of 22 patients with numerous metastases had tumors that lacked SMAD4 expression (78%). SMAD4 expression status was then analyzed in individuals with intermediate phenotypes, but who had recurrence patterns that could nonetheless be classified as either locally destructive or locally confined. Again, a statistically significant association between the recurrence pattern and SMAD4 expression status was noted when all 65 individuals were analyzed together (p=0.007).
A recent phase two trial examining chemotherapy followed by chemoradiation in patients with locally unresectable pancreatic cancer included a biological correlative component that examined SMAD4 expression in cytologic samples and analyzed the findings with respect to disease pattern [9]. Out of 41 patients with available tissue, 15 (37%) recurred in a local predominant pattern, 14 (34%) with distant metastases, and 8 (20%) with an indeterminate pattern. Out of 15 patients with intact SMAD4 expression in their tumors, 11 (73%) of them progressed locally. On the other hand, 10 of the 14 (71%) patients with loss of SMAD4 in their tumors recurred predominantly at distant sites (p=0.016).
An important difference between the two aforementioned studies and the present one is that the entire cohort of patients in the current one underwent a pancreatic resection. In contrast, only 29% of patients included in the autopsy study and 10% in the study of locally advanced pancreatic cancer underwent resection. While resection is not curative in the majority of patients, the intervention usually renders patients free of gross disease at the primary site, and in doing so, can have a profound impact on the recurrence pattern of the cancer. It is also possible that the cancers examined from the present series have a slightly different biologic behavior as a group, since they generally presented at an earlier stage (AJCC 7th Ed/TNM stages I and II) than the cancers included in the previous SMAD4 studies (generally stages III or IV). Other distinguishing qualities of the present series include the relatively large samples size, the use of surgical pathology (as opposed to cytology) in all cases, and the uniformity with respect to tumor type (all ductal adenocarcinoma). Taken together, we believe that the findings herein comprise the most accurate and applicable published dataset of SMAD4 expression in surgical patients with pancreatic adenocarcinoma.
While previous studies classified recurrence patterns according to whether or not the tumor was locally destructive or locally predominant, we felt that a more clinically relevant classification scheme should be based on whether or not the recurrence was localized in the retroperitoneum. While this classification difference is largely semantic, a retroperitoneal recurrence, either in the resection bed or in the regional lymph nodes, is a local one. The recurrence can be treated by radiation with minimal acute toxicity to the intestinal tract. On the contrary, the therapeutic window with radiation therapy may be smaller for locally destructive tumors in other sites in the abdomen, such as the small bowel mesentery. Along these lines, recurrences limited to the retroperitoneum may be reduced, in theory, by adjuvant radiation.
Pathologic features of the tumor and treatment were included in a multivariate model to determine if these key variables influenced the study findings. SMAD4 failed to predict recurrence pattern in the multivariate model. Similarly radiation, chemotherapy, resection margin status, histologic grade, and tumor size were not associated with the pattern of failure. However, the absence of lymph node metastases in the resection specimen was associated with a local-only recurrence, while lymph node metastases predicted systemic recurrence. Patients with lymph node metastases in fact had an adjusted odds ratio for a systemic recurrence more than five-fold greater than patients without lymph node spread. Based on these findings, oncologists who selectively recommend adjuvant chemoradiation for ductal adenocarcinoma of the pancreas according to tumor related characteristics should give particular consideration for cancers without lymph node metastases. On the other hand, due to the high rate of systemic recurrences, resected patients with lymph nodes metastases may be less likely to benefit from adjuvant radiation therapy. In the present study, 9 patients without lymph node metastases in their resection specimen had a local-only recurrence. Of these patients, 6 (66%) did not receive adjuvant radiation therapy and in retrospect, may have benefited from additional local treatment.
Controversy exists in the literature regarding SMAD4 as a prognostic marker, in addition to a marker of recurrence pattern. For instance, SMAD4 expression was determined by direct sequencing in 89 patients with resected pancreatic ductal adenocarcinoma and inactivation was associated with worse survival (14 vs. 12 months, p=0.006) [16]. Interestingly, an older report described a population of 129 patients with pancreatic ductal adenocarcinoma (51 who underwent resections), in which loss of SMAD4 was associated with improved survival (9 vs. 6 months, p=0.009), resectability, and earlier stage [17]. The differences disappeared in a multivariate model adjusting for pathologic features. In the present study, there is a trend towards worse survival with loss of SMAD4, which did not reach statistical significance. Additional findings in the multivariate analysis were consistent with our overall experience of more than 1000 PDAs; lymph node metastases and poor histologic grade were statistically significant predictors of survival, while resection margin was not [18]. The consistency between the larger dataset and the present study with regards to standard pathologic features provides reassurance that the smaller group is representative in at least this one very important aspect. The present data, combined with prior studies, suggest that loss of SMAD4 may be associated with unfavorable prognosis in pancreatic cancer, although the connection is not robust.
The present study has certain limitations based on the study design that deserve mention. In contrast to the autopsy study, the ability to determine recurrence clinically and from imaging is difficult. The absence of a detected recurrence, local or distant, does not confirm the absence of disease. Furthermore, patients were included from two ends of the survival spectrum, and therefore we are required to extrapolate these results for patients with intermediate survivals. Finally, treatment regimens were variable. However, this last point can be viewed as an important requirement for a reliable biomarker to gain widespread acceptance, as these data reflect real world treatment patterns.
References
- 1.Varadhachary GR, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine- based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–95. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latil A, et al. Gene expression profiling in clinically localized prostate cancer: a four-gene expression model predicts clinical behavior. Clin Cancer Res. 2003;9(15):5477–85. [PubMed] [Google Scholar]
- 5.Yachida S, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeno A, et al. Gene expression profile prospectively predicts peritoneal relapse after curative surgery of gastric cancer. Ann Surg Oncol. 2010;17(4):1033–42. doi: 10.1245/s10434-009-0854-1. [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka S, et al. Molecular prediction of early recurrence after resection of hepatocellular carcinoma. Eur J Cancer. 2009;45(5):881–9. doi: 10.1016/j.ejca.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Wang DY, et al. A new gene expression signature, the ClinicoMolecular Triad Classification, may improve prediction and prognostication of breast cancer at the time of diagnosis. Breast Cancer Res. 2011;13(5):R92. doi: 10.1186/bcr3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane CH, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29(22):3037–43. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacobuzio-Donahue CA, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 12.Zhou S, et al. Targeted deletion of Smad4 shows it is required for transforming growth factor beta and activin signaling in colorectal cancer cells. Proc Natl Acad Sci U S A. 1998;95(5):2412–6. doi: 10.1073/pnas.95.5.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter JM, Maitra A, Yeo CJ. Genetics and pathology of pancreatic cancer. HPB (Oxford) 2006;8(5):324–36. doi: 10.1080/13651820600804203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn SA, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271(5247):350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 15.Hilgers W, et al. Novel homozygous deletions of chromosomal band 18q22 in pancreatic adenocarcinoma identified by STS marker scanning. Genes Chromosomes Cancer. 1999;25(4):370–5. doi: 10.1002/(sici)1098-2264(199908)25:4<370::aid-gcc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Blackford A, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15(14):4674–9. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biankin AV, et al. DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol. 2002;20(23):4531–42. doi: 10.1200/JCO.2002.12.063. [DOI] [PubMed] [Google Scholar]
- 18.Winter JM, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169–75. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]