Abstract
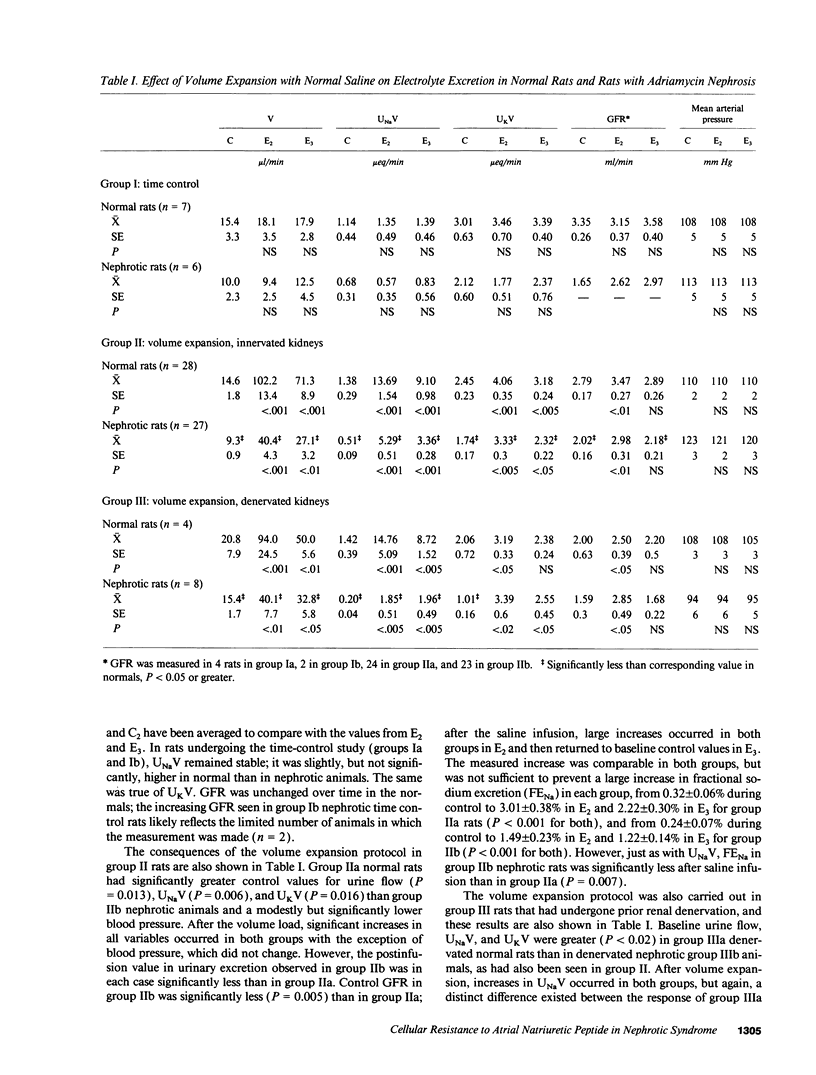
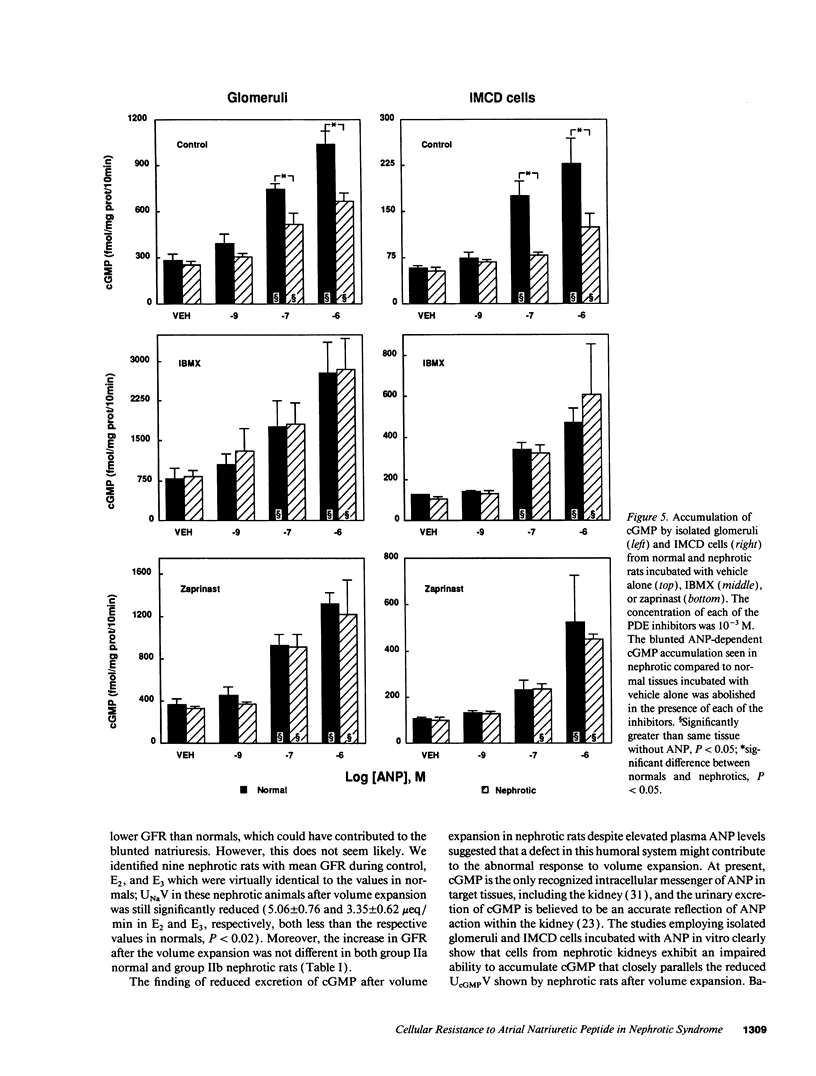
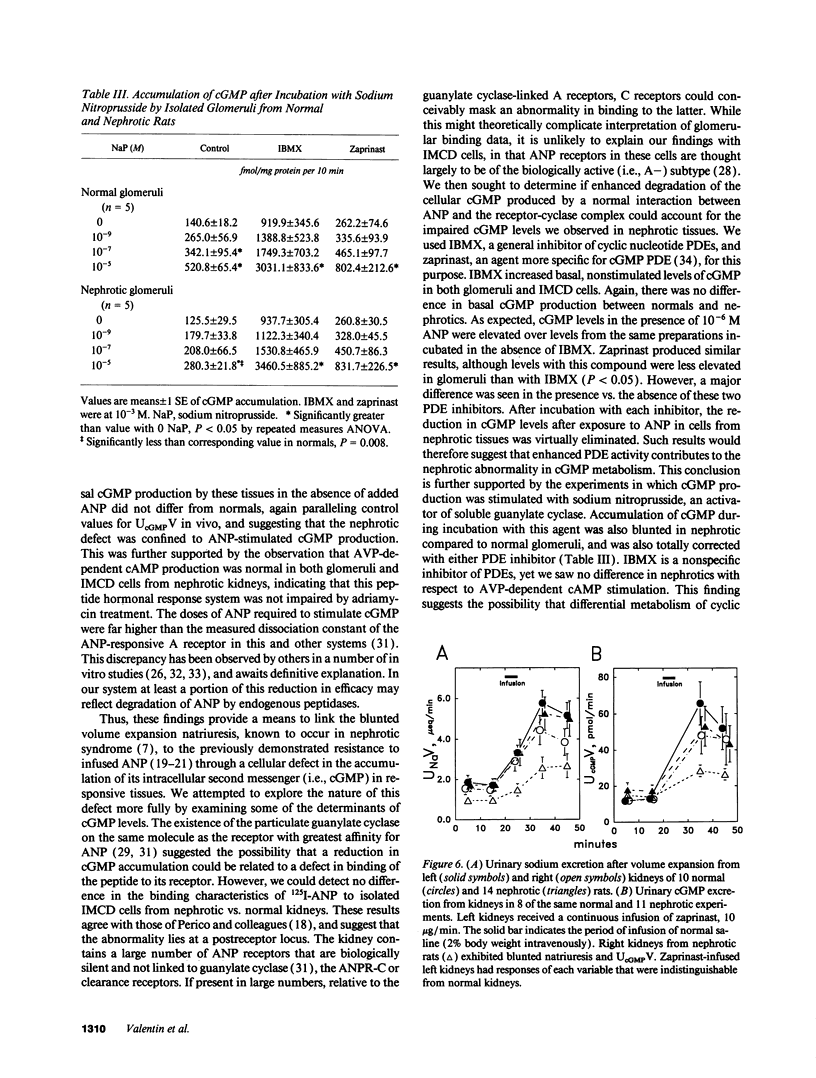
Experimental nephrotic syndrome results in sodium retention, reflecting, at least in part, an intrinsic defect in renal sodium handling in the distal nephron. We studied the relationships among plasma atrial natriuretic peptide (ANP) concentration, sodium excretion (UNaV), and urinary cyclic GMP excretion (UcGMPV) in vivo, and the responsiveness of isolated glomeruli and inner medullary collecting duct (IMCD) cells to ANP in vitro, in rats with adriamycin nephrosis (6-7 mg/kg body weight, intravenously). 3-5 wk after injection, rats were proteinuric and had a blunted natriuretic response to intravenous infusion of isotonic saline, 2% body weight given over 5 min. 30 min after onset of the infusion, plasma ANP concentrations were elevated in normals and were even higher in nephrotics. Despite this, nephrotic animals had a reduced rate of UcGMPV after the saline infusion, and accumulation of cGMP by isolated glomeruli and IMCD cells from nephrotic rats after incubation with ANP was significantly reduced compared to normals. This difference was not related to differences in binding of 125I-ANP to IMCD cells, but was abolished when cGMP accumulation was measured in the presence of 10(-3) M isobutylmethylxanthine or zaprinast (M&B 22,948), two different inhibitors of cyclic nucleotide phosphodiesterases (PDEs). Infusion of zaprinast (10 micrograms/min) into one renal artery of nephrotic rats normalized both the natriuretic response to volume expansion and the increase in UcGMPV from the infused, but not the contralateral, kidney. These results show that, in adriamycin nephrosis, blunted volume expansion natriuresis is associated with renal resistance to ANP, demonstrated both in vivo and in target tissues in vitro. The resistance does not appear related to a defect in binding of the peptide, but is blocked by PDE inhibitors, suggesting that enhanced cGMP-PDE activity may account for resistance to the natriuretic actions of ANP observed in vivo. This defect may represent the intrinsic sodium transport abnormality linked to sodium retention in nephrotic syndrome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard D. B., Alexander E. A., Couser W. G., Levinsky N. G. Renal sodium retention during volume expansion in experimental nephrotic syndrome. Kidney Int. 1978 Nov;14(5):478–485. doi: 10.1038/ki.1978.152. [DOI] [PubMed] [Google Scholar]
- Bernard D. B. Extrarenal complications of the nephrotic syndrome. Kidney Int. 1988 Jun;33(6):1184–1202. doi: 10.1038/ki.1988.129. [DOI] [PubMed] [Google Scholar]
- Bertani T., Poggi A., Pozzoni R., Delaini F., Sacchi G., Thoua Y., Mecca G., Remuzzi G., Donati M. B. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982 Jan;46(1):16–23. [PubMed] [Google Scholar]
- Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990 Jul;70(3):665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- Brown E. A., Markandu N. D., Sagnella G. A., Squires M., Jones B. E., MacGregor G. A. Evidence that some mechanism other than the renin system causes sodium retention in nephrotic syndrome. Lancet. 1982 Dec 4;2(8310):1237–1240. doi: 10.1016/s0140-6736(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Chaumet-Riffaud P., Oudinet J. P., Sraer J., Lajotte C., Ardaillou R. Altered PGE2 and PGF2 alpha production by glomeruli and papilla of sodium-depleted and sodium-loaded rats. Am J Physiol. 1981 Nov;241(5):F517–F524. doi: 10.1152/ajprenal.1981.241.5.F517. [DOI] [PubMed] [Google Scholar]
- Chonko A. M., Bay W. H., Stein J. H., Ferris T. F. The role of renin and aldosterone in the salt retention of edema. Am J Med. 1977 Dec;63(6):881–889. doi: 10.1016/0002-9343(77)90541-1. [DOI] [PubMed] [Google Scholar]
- Cogan M. G. Atrial natriuretic factor can increase renal solute excretion primarily by raising glomerular filtration. Am J Physiol. 1986 Apr;250(4 Pt 2):F710–F714. doi: 10.1152/ajprenal.1986.250.4.F710. [DOI] [PubMed] [Google Scholar]
- Conti M., Jin S. L., Monaco L., Repaske D. R., Swinnen J. V. Hormonal regulation of cyclic nucleotide phosphodiesterases. Endocr Rev. 1991 Aug;12(3):218–234. doi: 10.1210/edrv-12-3-218. [DOI] [PubMed] [Google Scholar]
- DiBona G. F., Herman P. J., Sawin L. L. Neural control of renal function in edema-forming states. Am J Physiol. 1988 Jun;254(6 Pt 2):R1017–R1024. doi: 10.1152/ajpregu.1988.254.6.R1017. [DOI] [PubMed] [Google Scholar]
- Don B. R., Blake S., Hutchison F. N., Kaysen G. A., Schambelan M. Dietary protein intake modulates glomerular eicosanoid production in the rat. Am J Physiol. 1989 Apr;256(4 Pt 2):F711–F718. doi: 10.1152/ajprenal.1989.256.4.F711. [DOI] [PubMed] [Google Scholar]
- Dorhout E. J., Roos J. C., Boer P., Yoe O. H., Simatupang T. A. Observations on edema formation in the nephrotic syndrome in adults with minimal lesions. Am J Med. 1979 Sep;67(3):378–384. doi: 10.1016/0002-9343(79)90782-4. [DOI] [PubMed] [Google Scholar]
- FRENK S., ANTONOWICZ I., CRAIG J. M., METCOFF J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955 Jul;89(3):424–427. doi: 10.3181/00379727-89-21833. [DOI] [PubMed] [Google Scholar]
- Geers A. B., Koomans H. A., Roos J. C., Boer P., Dorhout Mees E. J. Functional relationships in the nephrotic syndrome. Kidney Int. 1984 Sep;26(3):324–330. doi: 10.1038/ki.1984.176. [DOI] [PubMed] [Google Scholar]
- Grausz H., Lieberman R., Earley L. E. Effect of plasma albumin on sodium reabsorption in patients with nephrotic syndrome. Kidney Int. 1972;1(1):47–54. doi: 10.1038/ki.1972.7. [DOI] [PubMed] [Google Scholar]
- Guesry P., Kaufman L., Orloff S., Nelson J. A., Swann S., Holliday M. Measurement of glomerular filtration rate by fluorescent excitation of non-radioactive meglumine iothalamate. Clin Nephrol. 1975;3(4):134–138. [PubMed] [Google Scholar]
- Gur A., Adefuin P. Y., Siegel N. J., Hayslett J. P. A study of the renal handling of water in lipoid nephrosis. Pediatr Res. 1976 Mar;10(3):197–201. doi: 10.1203/00006450-197603000-00011. [DOI] [PubMed] [Google Scholar]
- Hildebrandt D. A., Banks R. O. Effect of atrial natriuretic factor on renal function in rats with nephrotic syndrome. Am J Physiol. 1988 Feb;254(2 Pt 2):F210–F216. doi: 10.1152/ajprenal.1988.254.2.F210. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Rennke H. G., Hoyer J. R., Badr K. F., Schor N., Troy J. L., Lechene C. P., Brenner B. M. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983 Jan;71(1):91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch R. C., Light G. S., Oliver W. J. The effect of albumin infusion upon plasma norepinephrine concentration in nephrotic children. J Lab Clin Med. 1972 Apr;79(4):516–525. [PubMed] [Google Scholar]
- Koepke J. P., DiBona G. F. Blunted natriuresis to atrial natriuretic peptide in chronic sodium-retaining disorders. Am J Physiol. 1987 May;252(5 Pt 2):F865–F871. doi: 10.1152/ajprenal.1987.252.5.F865. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., Goeddel D. V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991 Apr 5;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- Krishna G. G., Danovitch G. M. Effects of water immersion on renal function in the nephrotic syndrome. Kidney Int. 1982 Feb;21(2):395–401. doi: 10.1038/ki.1982.35. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin E. R., Lewicki J. A., Scarborough R. M., Ballermann B. J. Expression and regulation of ANP receptor subtypes in rat renal glomeruli and papillae. Am J Physiol. 1989 Oct;257(4 Pt 2):F649–F657. doi: 10.1152/ajprenal.1989.257.4.F649. [DOI] [PubMed] [Google Scholar]
- Meltzer J. I., Keim H. J., Laragh J. H., Sealey J. E., Jan K. M., Chien S. Nephrotic syndrome: vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Ann Intern Med. 1979 Nov;91(5):688–696. doi: 10.7326/0003-4819-91-5-688. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Knepper M. A., Manganiello V. C. Effects of atrial natriuretic factor on cyclic guanosine monophosphate and cyclic adenosine monophosphate accumulation in microdissected nephron segments from rats. J Clin Invest. 1987 Feb;79(2):500–507. doi: 10.1172/JCI112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico N., Delaini F., Lupini C., Benigni A., Galbusera M., Boccardo P., Remuzzi G. Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int. 1989 Jul;36(1):57–64. doi: 10.1038/ki.1989.161. [DOI] [PubMed] [Google Scholar]
- Perico N., Delaini F., Lupini C., Remuzzi G. Renal response to atrial peptides is reduced in experimental nephrosis. Am J Physiol. 1987 Apr;252(4 Pt 2):F654–F660. doi: 10.1152/ajprenal.1987.252.4.F654. [DOI] [PubMed] [Google Scholar]
- Peterson C., Madsen B., Perlman A., Chan A. Y., Myers B. D. Atrial natriuretic peptide and the renal response to hypervolemia in nephrotic humans. Kidney Int. 1988 Dec;34(6):825–831. doi: 10.1038/ki.1988.256. [DOI] [PubMed] [Google Scholar]
- Stokes T. J., Jr, McConkey C. L., Jr, Martin K. J. Atriopeptin III increases cGMP in glomeruli but not in proximal tubules of dog kidney. Am J Physiol. 1986 Jan;250(1 Pt 2):F27–F31. doi: 10.1152/ajprenal.1986.250.1.F27. [DOI] [PubMed] [Google Scholar]
- Wagnild J. P., Gutmann F. D. Functional adaptation of nephrons in dogs with acute progressing to chronic experimental glomerulonephritis. J Clin Invest. 1976 Jun;57(6):1575–1589. doi: 10.1172/JCI108428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins M. R., Settle S. L., Needleman P. Augmentation of the natriuretic activity of exogenous and endogenous atriopeptin in rats by inhibition of guanosine 3',5'-cyclic monophosphate degradation. J Clin Invest. 1990 Apr;85(4):1274–1279. doi: 10.1172/JCI114564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. R., Cogan M. G. Comparison of the natriuresis and chloruresis associated with glomerular hyperfiltration induced by atrial natriuretic factor or glucagon. Life Sci. 1987 Apr 20;40(16):1595–1600. doi: 10.1016/0024-3205(87)90125-1. [DOI] [PubMed] [Google Scholar]
- Wong K. R., Xie M. H., Shi L. B., Liu F. Y., Huang C. L., Gardner D. G., Cogan M. G. Urinary cGMP as biological marker of the renal activity of atrial natriuretic factor. Am J Physiol. 1988 Dec;255(6 Pt 2):F1220–F1224. doi: 10.1152/ajprenal.1988.255.6.F1220. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Brenner B. M., Seifter J. L. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol. 1987 Mar;252(3 Pt 2):F551–F559. doi: 10.1152/ajprenal.1987.252.3.F551. [DOI] [PubMed] [Google Scholar]



