Summary
HSV-1 continues to be the leading cause of infectious corneal blindness. Clinical trials for vaccines against genital HSV infection have been ongoing for more than three decades. Despite this, no approved vaccine exists, and no formal clinical trials have evaluated the impact of HSV vaccines on eye health. We review here the current state of development for an efficacious HSV-1 vaccine and call for involvement of ophthalmologists and vision researchers.
Keywords: HSV-1, vaccine, cornea, correlates of protection, immunopathology
Prologue
The merits of an efficacious vaccine to protect against ocular herpes simplex virus (HSV) infection and resultant sequelae are often overlooked. Though HSV vaccines have been reviewed extensively, much focus has been directed towards the development of a tenable vaccine for genital herpes, specifically HSV-2 [1]. What is often lacking in the design of clinical trials and discussions about HSV vaccines is practical relevance to arguably the most routine yet clinically serious complication resulting from HSV infection—herpetic stromal keratitis (HSK) [2].
Compared to other external anatomic sites, tissue pathology and healing in the cornea following HSV infection is complex and clinically problematic due to the need to preserve corneal clarity and sensation [3–6]. Corneal inflammation, scar formation, and neovascularization are hallmarks of HSK, yet these pathologies are intimately linked to the immune response elicited by recurrent corneal HSV infection [7,8]. Developing a vaccine for HSV that confers immunologic protection without eliciting irreversible corneal immunopathology is critically important for both clinicians and patients. Moreover, this conundrum is precisely why the insights of ophthalmologists and vision scientists may definitively contribute to HSV vaccine research.
In the current review, our goal is to highlight strategies that will integrate ongoing vaccine research with the medical discipline of ophthalmology. Clinical trials for HSV vaccines have been ongoing for more than three decades, yet none have reported data on the eye. We advocate herein for ophthalmologist involvement in HSV vaccine research and the implementation of clinically relevant, outcome-based experimental methodologies by researchers engaged in HSV vaccine studies using animal models. This thorough approach will effectually streamline translational research and vaccine design as well as diminish the limitations of animal models by pairing clinically relevant evaluations of corneal pathology with immunologic investigations of vaccine efficacy.
Introduction
Vaccines have been in use for more than two centuries to protect against infectious diseases. Herpes simplex virus type 1 (HSV-1) is among the most prominent pathogens threatening vision [2]. Despite aggressive focused research, multiple clinical trials, and significant funding in pursuit of a HSV vaccine, no approved vaccine exists to date [1,2,9]. Here, we provide an overview of HSV-1 virology, epidemiology, ocular infection, and interventions with emphasis on connections to clinically pertinent aspects of corneal pathophysiology and implications for vaccine research. HSV and varicella zoster virus (VZV) are contrasted throughout this review to differentiate how the approaches that led to profound successes in vaccinating against the latter may be applied in the pursuit of a safe and efficacious vaccine for its counterpart, HSV-1. The overall purpose of our review is to bridge the scientific disciplines of HSV vaccinology and ophthalmic medicine in order to initiate a methodological framework to guide research investigating the efficacy of emerging HSV vaccines with respect to herpetic eye disease.
For clarity, distinctions are made herein between ophthalmologists and vision scientists to address common perspectives and problems encountered in the eye clinic and laboratory, respectively. When both clinicians and laboratory scientists are intended, the colloquialism ‘vision professionals’ is used. The terms ophthalmologist and clinicians in ophthalmic medicine are used interchangeably and are not intended to exclude optometrists.
Though HSV-1 is an etiological agent responsible for a variety of sequelae including acute retinal necrosis, anterior uveitis, conjunctivitis, and keratitis in the eye alone [2], we will primarily focus on HSK for the intent of this review due to its established prevalence in visual impairment. We anticipate that an efficacious HSV-1 vaccine that protects against HSK would also protect against other herpetic sequelae in the eye and beyond. This includes devastating diseases such as frank sporadic encephalitis and genital herpes caused by HSV-1.
Basic Virology of HSV-1
Of the human herpesviruses, HSV-1 along with HSV-2 and VZV constitute the neurotropic taxonomic subfamily Alphaherpesvirinae. The alphaherpesviruses are relatively homologous structurally and, to a lesser extent, genetically. Their virions are comprised of a linear double-stranded DNA genome protected by a capsid (collectively, nucleocapsid), tegument proteins, and an envelope [10]. While an in-depth discussion of molecular virology is beyond the scope of this manuscript, basic information is provided to resolve immunologic concepts encompassed by this review.
Genomes of the human alphaherpesviruses contain seventy or more open reading frames [11,12]. Expression of the encoded products is regulated in several phases in concert with lytic and latent cycles or host immune-pressure [13]. During the lytic cycle, gene products are utilized in many processes such as viral entry, immune evasion, intracellular motility, and virion replication/assembly [10]. From the perspective of vaccine immunologists, the rich antigenic complexity of these viruses makes selecting appropriate components for inclusion in a vaccine difficult [1]. While HSV-1 is more closely related to HSV-2 than VZV, the success of vaccination against VZV primary infection and reactivation—i.e. varicella and herpes zoster (HZ), respectively—gives us hope that paralleled success is on the horizon for vaccines directed against HSV.
Initial host cell entry by HSV-1 is mediated via virion attachment to and fusion with host cell membranes. This process is dynamic with respect to the entry pathways (i.e. plasma membrane or endocytic vesicle fusion) exploited by HSV-1 as well as the broad range of host cell types susceptible to infection [14–16]. Viral entry of HSV-1 involves multiple interactions between surface receptors decorating the host cell membrane and glycoproteins decorating the virion envelope. An increasing number of host cell surface macromolecules are recognized as entry receptors for HSV-1 including: herpesvirus entry mediator (HVEM), nectin-1/2, 3-O-sulfated heparin sulfate (3-OS HS), and paired immunoglobulin-like 2 receptor alpha (PILRα)—all of which are expressed in various cells in the cornea [16].
Consistent with the rich antigenic complexity of HSV-1, virion entry involves the coordinated actions of multiple glycoproteins on the envelope to mediate attachment and fusion. The fusion process is a mechanism necessary to deposit viral tegument proteins and the nucleocapsid into host cells, which jointly mediate the fate of progeny virions. HSV-1 surface glycoproteins that comprise the molecular machinery necessary for host cell attachment and fusion include glycoprotein -B (gB), -D (gD), and a heterodimer of glycoprotein-H (gH) and -L (gL). Of these, gB and gD are directly associated with binding the aforementioned host cell entry receptors. Comparing HSV-1 and HSV-2, gB and gD are relatively homologous in structure and function [15]. From a vaccine standpoint, generating an immune response against gB and gD, the viral glycoproteins responsible for host cell attachment and fusion, is logical.
Central to the pathogenesis of the alphaherpesviruses is their ability to spread via mucocutaneous secretions or lesions, replicate at the site of infection, and invade autonomic ganglia. These viruses enter neuronal sensory fibers innervating infected external mucosal sites and subsequently shuttle their nucleocapsid to the ganglion-associated soma via retrograde axonal transport [17,18]. HSV and VZV persist for the life of the host in immune privileged neuronal niches by establishing an episomal latent infection characterized by suppression of viral gene expression [19]. Differences arise in the molecular mechanisms known to govern viral latency or promote reactivation between VZV and HSV-1/2. For HSV, latency is classically distinguished by the production of a non-coding latency associated transcript (LAT), some micro-RNAs, and absence of viral replication within the infected nerve. Latency is not maintained by LAT expression in VZV [19]. Upon reactivation, progeny virus particles are generated and shuttled to external sensory fibers via anterograde transport for egress into the external mucosae [18].
HSV-1 is most commonly associated with the formation of orolabial herpetic lesions or ‘cold sores’ on the vermilion border resulting from periodic reactivation of latent virus within neurons of the trigeminal ganglia (TG). With the exception of corneal infection in neonates, primary HSV-1 keratitis is thought to be caused by HSV-1 reactivation within the TG with subsequent anterograde transport through the ophthalmic branch of the trigeminal nerve to facilitate viral access to the cornea. Ocular HSV-1 infection can also result from direct exposure [2]. In stark contrast to varicella resulting from VZV infection in unvaccinated individuals, primary orofacial HSV-1 infection is generally thought to manifest as a subclinical pediatric condition [20]. However, epidemiologic studies have uncovered changing trends that vaccine researchers can capitalize on.
Epidemiologic Insights
Samuel Theobald, an ophthalmologist in Baltimore circa 1916, noted: “Unless my experience has been exceptional, herpetic keratitis is a more common affection than it is usually supposed to be” [21]. Despite diagnostic ambiguity a century ago, Theobald’s observation certainly remains true today: vision professionals recognize HSV-1 as the leading cause of infectious corneal blindness in the developed world. Current projections suggest 1.5 million cases of HSK occur annually worldwide. Among these, forty thousand cases of severe monocular visual impairment (acuity < 20/200) or blindness (acuity < 20/400) are estimated [2]. Nevertheless, the public at large remains relatively unfamiliar with HSK, as this condition has not crossed into the realm of common knowledge.
Meta-analysis of longitudinal data showing age of exposure to HSV-1 may be strategically useful in the design and implementation of a HSV-1 vaccine. The HSV-1 seroprevalence among 14–49-year-olds in the United States has declined steadily over the past 20–25 years according to National Health and Nutrition Examination Surveys (NHANES) data from highly sensitive and specific solid phase immunodot assays. Analysis of NHANES data comparing HSV-1 seroprevalence spanning 1988–1994, 1999–2004, and 2005–2010 reveal a progressive seven percent relative decrease in the number of seropositive individuals between each interval [22,23]. A highly significant relative decrease in seroprevalence was observed among 14–19-year-olds: ~23% between 1999–2004 and 2005–2010, and ~29% relative to NHANES data from 1976–1980 [23].
Seroprevalence data gained from clinical trials for the GlaxoSmithKline Herpevac vaccine in women of childbearing age in the United States supported established NHANES findings. However, this Herpevac cohort was nearly five times larger and was composed entirely of individuals denying any previous symptomatic HSV infection [24]. Collectively, these studies indicate that fewer children and adolescents are infected with HSV-1 today than in previous decades possibly due to delayed exposure to the virus. On one hand, trends in delayed exposure evidenced by seroconversion rates may be taken advantage of to facilitate prophylactic childhood vaccination for HSV-1; on the other hand, these trends may correlate with increased clinical severity and complication frequency resulting from primary infection of immunologically naive individuals later in life.
Seroconversion is only one indicator of a patient’s history of HSV-1 infection. Moreover, evidence suggests that seroconversion status is not always a conclusive indication of HSV-1 infection. One meta-analysis study evaluating HSV-1 shedding in the oral cavity of over 3500 individuals from multiple case reports indicates that the virus is shed asymptomatically by upwards of 70% of the population at least once a month [25]. Asymptomatic shedding is accepted to be the presence of HSV-1 virions or DNA within mucosal secretions in the absence of clinically evident viral lesions. Another study evaluating HSV-1 shedding in the saliva and tears found similar results with no overall difference in the viral load by PCR on HSV-1 DNA comparing tears and saliva [26]. Interestingly, asymptomatic shedding was observed in healthy adults who were reported as seronegative for HSV-1 by ELISA or viral serum neutralization assay [25,26]. Thus it is important for those interested in vaccine development for HSV-1 to recognize that transmission of HSV-1 may occur by asymptomatic individuals as well as by individuals with a seroconversion status below the limit of detection for the method used to determine the HSV-1 specific antibody titer.
In addition to seroprevalence data and virus shedding, PCR-based postmortem studies evaluating HSV-1 DNA in the TG also offer valuable epidemiologic information on the prevalence of HSV-1. Data from a recent large study (n = 414 TG from 207 cadavers) conducted in Japan show that the incidence of HSV-1 infection increases with age. Furthermore, this study revealed that nearly all people greater than 60 years old harbor latent HSV-1 in the TG [27] and complements an earlier study (n = 242 TG from 121 cadavers) indicating similar age-based trends in HSV-1 infection in the TG in Japan [28]. One large study from Germany (n = 109 cadavers) also supports age-based trends in the incidence of HSV-1 infection [29].
However, the only large postmortem study (n = 174 TG) identified by the authors that encompassed a broad age range and was conducted in the U.S. suggests that neither age nor gender have a significant impact on the prevalence of HSV-1 DNA in human TG [30]. One caveat to the cited U.S. study is that the tissue was procured from one tissue bank in Oregon and may not be representative of the entire U.S. population. Furthermore, an independent study of adults 56 years and older in the U.S. (n = 47 TG) revealed that 68% of the samples evaluated contained HSV-1 DNA. Many other detailed studies assessing latent HSV-1 in human cadavers have been conducted, but the data cannot be effectively utilized in a discussion regarding epidemiological aspects of HSV-1 due to small sample sizes and/or limited age distribution. It is clear from serological as well as PCR-based cadaver studies that the prevalence of HSV-1 is high.
All HSV vaccines tested in clinical trials to date have focused on HSV-2-derived antigens, as HSV-2 has historically been the chief causative agent of genital herpes and predisposes individuals to be more susceptible to infection by HIV [31]. Clinical trials for HSV vaccines have failed to produce protection against HSV-2. One recent Herpevac study shows partial protection against genital HSV-1 infection and disease, though only in women who were seronegative for HSV-1 and HSV-2 prior to vaccination [32]. The proportion of genital herpes caused by HSV-1 is on the rise, and HSV-1 is now the leading cause of primary genital herpes in young women [32,33], possibly due to delayed exposure or lack of HSV-1 specific antibodies upon sexual debut [23].
Due to these epidemiological trends in HSV acquisition, focus on the development of vaccines targeting HSV-1 specifically is likely to increase in the near future. The risk of clinical severity during primary VZV infection is known to increase with age [34], but the clinical course of disease can be eliminated or drastically limited by prophylactic vaccination [35]. Age of primary infection may have implications for trends seen in ocular (and genital) HSV-1 involvement as well, and particularly when age of the first clinical episode of ocular infection is evaluated [2,36].
While the epidemiology of HSV-1 infection varies among nations and demographic subpopulations, one trend is clear: HSV-1 seroconversion is happening later in life than in previous decades, and this may contribute to an increase in frequency and severity of clinically important complications—including HSK. However, the authors believe that the trend of delayed seroconversion rates may pave the way for successful prophylactic HSV-1 vaccine campaigns in children.
Herpetic Keratitis and the Prospects for a HSV-1 Vaccine
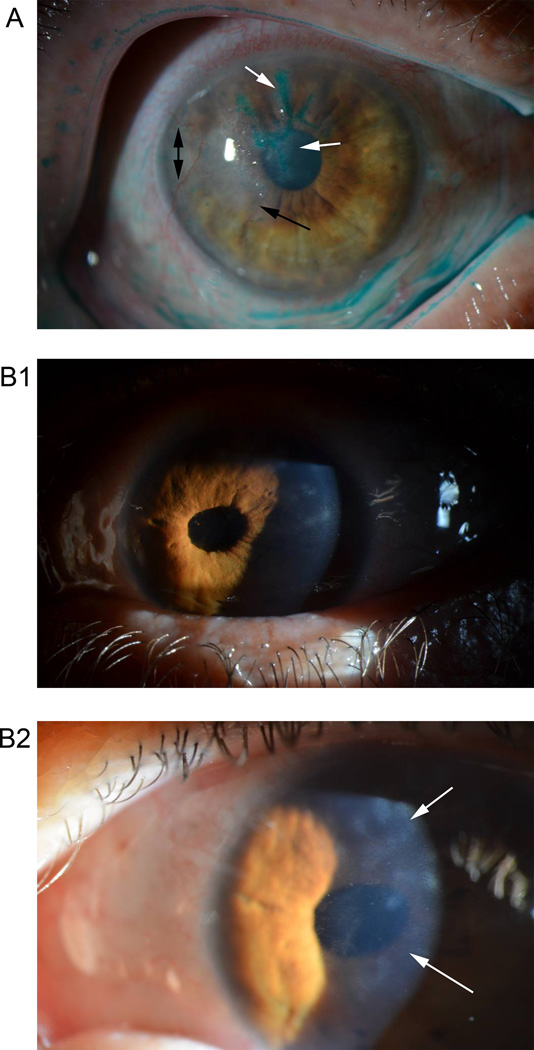
The prospects of developing an effective vaccine against ocular HSV have been reviewed previously [37] and mentioned in epidemiologic [2] and treatment-focused reviews on ocular HSV-1 infections [38]. Typical presentations of active HSV-1 keratitis include the formation of dendritic ulcers in the corneal epithelium. Visual impairment stemming from HSK involves stromal opacification. Figure 1 shows classic examples of each of these pathologies. HSK is believed to affect roughly 500,000 individuals in the United States alone, with an associated annual economic burden of nearly $18 million in costs associated with disease management [2,39,40]. The utility of a vaccine that prevents visual morbidity resulting from recurrent ocular HSV-1 infection is evident.
Figure 1.
Clinical manifestations of ocular HSV-1 in two patients. Patient A: Lissamine green stain on cornea with active herpetic keratitis (HK) highlights dendritic ulcers (white arrows) in the corneal epithelium. Stromal disease is also evidenced by corneal haze (black arrow) and neovascularization (black double arrow). Patient B1: Correctopia (abnormal pupil) resultant from prior HSV-1 iridocyclitis (anterior uveitis). The disease is currently quiet. B2: Closer image shows corneal haze (lower white arrow) and scarring (upper white arrow) from prior HK.
Clinical presentation and management of HSV-1 infection in the human cornea is generally understood by ophthalmologists, and has been reviewed extensively [2,8,38,41]. One conundrum in the field is that HSK is a secondary immune-mediated pathology and not the direct result of clinically active ocular HSV-1 infection. Therefore, it stands to reason that increasing the immune response to HSV-1 could potentially exacerbate the severity of HSK. Insights from VZV, another important viral cause of herpetic keratitis, will be evaluated in the next section to determine the impact vaccination may have on exacerbation of immune-mediated ocular disease.
Several challenges have arisen in the clinical management of HSK. These issues include: topical drug toxicity, acyclovir-resistant infections, failed cornea transplants, and immune-driven pathology occurring beyond the resolution of detectable infection [8,38]. The frequency and clinical severity of ocular HSV-1 reactivation in patients with a history of corneal involvement increases over time and ultimately contributes to HSK [42,43]. Concern about LASIK surgery in individuals with a recent history of ocular HSV-1 infection has been advised due to reports of reactivation following the procedure; however, the incidence is rare [44–46]. Such challenges emphasize the need for an efficacious vaccine to prevent HSK, but they can also be used to inform the design and implementation of a vaccine aimed to prevent active HSV-1 keratitis in the first place. Future vaccine studies must rigorously examine the extent of vaccine-mediated protection against herpetic eye disease in light of what is known about progression of HSK.
Previous and ongoing clinical trials for HSV vaccines have virtually ignored the problem of ocular infection due to their focus on genital herpes caused by HSV-2. It is known that HSV-2 contributes to ocular disease, but it is a minor player in the development of HSK relative to HSV-1 and VZV [2]. As of the last week of July 2014, sixteen formal clinical trials investigating vaccines against genital HSV infection were documented by the National Institutes of Health. These studies were selected by the authors based on relevance to the current vaccine review from among twenty-five results returned using the search query “herpes simplex virus” and “vaccine” at the National Clinical Trials registry accessible at ClinicalTrials.gov (see Table 1). None of these studies propose to investigate changes in HSV-related phenomena in the eye relative to vaccination. Furthermore, half of them specifically exclude patients with a history of ocular HSV from enrollment. The reasoning for this exclusion criterion is likely due to long-held concerns that boosting immunity to HSV may exacerbate HSK [47]. As outlined by Pepose and others, the occurrence of primary genital HSV-1 now greater than HSV-2 in some demographics, thus emerging HSV vaccines should be designed to target genital HSV-1 and HSV-2 [47].
Table 1.
National Clinical Trial (NCT) registry listings for HSV vaccines Information is accessible at ClinicalTrials.gov
| NCT Registry ID | Study Title | Status | Phase | Vaccine Composition |
Investigates the eye? (Y/N) |
Enrollment status |
|---|---|---|---|---|---|---|
| NCT01915212 | Study of the Safety of a Particular Herpes Vaccine in Adults With or Without Herpes Infection | Active, recruiting | I | HSV529 replication disabled live virus | N; PTs with history of ocular HSV excluded | PTs may be HSV-1/2± seroreactive |
| NCT00698893 | Evaluation of Safety of Candidate gD Vaccine, With or Without MPL in Healthy Herpes Simplex Virus-Positive Adults | Complete July 1992 | I | GSK208141 HSV-2 glycoprotein D subunit ± MPL | N | PTs must be seropositive for HSV-1/2 |
| NCT00057330 | HerpeVac Trial for Young Women | Complete August 2009 | III | GSK 208141 HSV-2 glycoprotein D subunit with MPL | N | PTs must be seronegative for HSV |
| NCT02030301 | Safety and Efficacy Trial of DNA Vaccines to Treat Genital Herpes in Adults | Active, recruiting | I / II | Vical DNA-based plasmid vaccine encoding two HSV-2 proteins(gD + tegument) with Vaxfectin® | N | PTs must be seropositive for HSV-2 |
| NCT00698490 | Humoral and Cellular Immune Response of Herpes Simplex (gD) Candidate Vaccines From 2 Different Cell Lines | Complete January 1997 | I / II | GSK 208141 HSV-2 glycoprotein D subunit | N | PTs may be HSV-1/2± seroreactive |
| NCT00274300 | Safety Study of HSV2 DNA Vaccine to Treat Patients With Recurrent Genital Herpes Caused by HSV-2 | Complete July 2005 | I | PowderMed DNA-based plasmid vaccine (Pfizer) | N | PTs must have recurrent genital HSV-2 infections |
| NCT00138320 | Herpevac Neonatal Substudy | Terminated | N/A | GSK 208141 HSV-2 glycoprotein D subunit with MPL | N | Mothers must be HSV seronegative |
| NCT00698568 | Safety Evaluation of Herpes Simplex Candidate Vaccine (gD2t) With Adjuvant in HSV Seropositive / Seronegative Subjects | Complete April 1999 | III | GSK 208141 HSV-2 glycoprotein D subunit with MPL | N; PTs with history of ocular HSV excluded | PTs may be HSV-1/2± seroreactive with no history or evidence of genital HSV |
| NCT02114060 | Dose Ranging Safety and Efficacy of Therapeutic HSV-2 Vaccine | Active, recruiting | II | Genocea Biosciences HSV-2 glycoprotein D and ICP4 subunits with Matrix-M2 | N; PTs with history of ocular HSV excluded | PTs must be HSV-2 seropositive with history of genital HSV-2 |
| NCT01687595 | Biological Efficacy Study of HerpV Vaccine With QS-21 to Treat Subjects With Recurrent Genital Herpes | Active, not recruiting | II | Agenus HerpV HSV-2 polypeptide subunit with QS-21 | N; PTs with history of ocular HSV excluded | PTs must be HSV-2 seropositive with history of genital HSV-2 |
| NCT01667341 | Safety and Immunogenicity Study of Therapeutic HSV-2 Vaccine | Complete April 2014 | I / II | Genocea Biosciences HSV-2 glycoprotein D and ICP4 subunits ± Matrix-M2 | N; PTs with history of ocular HSV excluded | PTs must be HSV-2 seropositive with history of genital HSV-2 |
| NCT00231049 | Trial Evaluating Safety, Tolerability and Immune Response of AG-707 | Complete October 2008 | I | Agenus AG-707 (HerpV) HSV-2 polypeptide subunit ±QS-21 | N; PTs with history of ocular HSV excluded | PTs must be HSV-1±/2+ seropositive with history of genital HSV-2 |
| NCT00699764 | Safety of a Herpes Simplex Candidate Vaccine (gD2t) With MPL and Its Efficacy to Prevent Genital Herpes Disease | Complete October 2009 | III | GSK 208141 HSV-2 glycoprotein D subunit with MPL | N; PTs with history of ocular HSV excluded | PTs may be HSV-1/2± seroreactive with no history or evidence of genital HSV |
| NCT00697567 | Evaluation of Immunogenicity, Reactogenicity and Safety of Herpes Simplex (gD) Candidate Vaccine With/Without Adjuvant | Complete December 1997 | II | GSK208141 HSV-2 glycoprotein D subunit ± MPL | N | PTs may be HSV-1/2± seroreactive |
| NCT00224471 | Study to Compare Safety and Immunogenicity of Commercial Scale Consistency Lots of Herpes Simplex Vaccine | Complete January 2006 | III | GSK208141 HSV-2 glycoprotein D subunit | N | PTs must be HSV-1/2 seronegative |
| NCT00224484 | Safety Study of Herpes Simplex Vaccine in HSV Seronegative and Seropositive Females Between 10 and 17 Years Old | Complete July 2007 | III | GSK208141 HSV-2 glycoprotein D subunit | N; PTs with history of ocular HSV excluded | PTs may be HSV-1/2± seroreactive |
Ophthalmologists must become proactive in HSV vaccine research to ensure that boosting immunity to HSV is protective and does not have a reciprocal effect on eye health by exacerbating HSK immunopathology. One article has validated that a heat-killed HSV-1 vaccination delivered subcutaneously in the deltoid region can cause a significant drop in frequency and duration of ocular HSV-1 reactivations in a small clinical cohort (n = 10 vaccinated & 10 placebo) [48]. While a drop in frequency and duration is certainly beneficial, what is needed to abrogate eye disease is to diminish HSV-1 infection in the TG or reactivation in the eye. Many vision scientists have used animal models to imitate aspects of HSV-1 infection. This includes the potential for a vaccine-mediated boost in immunity to HSV-1 to exacerbate HSK, and these issues will be addressed later in the animal models section. It is unlikely that a HSV vaccine will ever eliminate latent ganglionic infection with current technologies being utilized in the field; however, data show symptomatic reactivation can be diminished [32,48].
Endpoint measurements of a successful prophylactic HSV-1 vaccine would include prevention of the establishment of viral latency in the TG among individuals vaccinated before exposure to HSV-1. Alternatively, endpoint measurements of a successful therapeutic vaccine for HSV-1 would include significant suppression of the latent reservoir of virus already established in the TG. This would be indicated by a significant drop in detectable reactivation via symptomatic or asymptomatic virus shedding in external mucosae. We will now look to data from several pilot studies and preliminary clinical evidence available for eye-related VZV complications following vaccination to evaluate the prospects of ocular protection and immunopathology following HSV vaccination.
Insight from VZV Vaccination
The live-attenuated prophylactic Varivax vaccine for varicella has been commercially available in the US since 1995, while its higher-dose, therapeutic counterpart Zostivax was introduced in 2006 for immunization against HZ. Varivax immunization has been demonstrated in multiple studies to be approximately 85% effective in preventing signs of varicella and almost entirely effective in preventing severe disease in children [35]. Zostivax has been shown to reduce the incidence of HZ by more than 50% in those age 60 years and older [49].
Data from one retrospective study on a single community over the course of 28 years show that HZ eye complications affect approximately 9% of patients with HZ in any dermatome, but the incidence is increasing among unvaccinated individuals [50]. This data projected to the United States population reveal that there may be up to 90,000 HZ eye complications annually [50]. Induction or exacerbation of VZV keratitis has been reported following Varivax or Zostivax administration in two children and one adult, leading some clinicians to speculate that persistent viral antigens in the cornea contribute to immune-mediated pathology arising from a vaccine-induced boost in cellular immunity [51–53]. Three patients experienced HZ following vaccination that led to retinal necrosis, and in at least one case, the vaccine strain was identified in the vitreous of the infected eye by PCR [54,55].
Acute posterior multifocal placoid pigment epitheliopathy, uveitis, and retinitis have also been reported following immunization for VZV [52,56–59]. Nonetheless, many of these reports of complications arose in severely ill or otherwise immunocompromised individuals, while several other events did not correspond closely with the time of vaccination. One recent study of 13,681 individuals emphasizes the general safety of Zostivax and shows that recent history of HZ is not a contraindication for vaccination [60]. Given the current national estimates of ocular HZ involvement, the isolated adverse reports likely reflect rare events. Collectively, there are no serious concerns about increased incidence of HZ eye disease resulting from vaccination using a live-attenuated virus. Understanding the immunologic impact of VZV vaccines on the incidence and severity of ocular HZ may give HSV-1 vaccine researchers discernment in the pursuit of an efficacious vaccine for HSV-1-related ocular sequelae. Specifically, the potential for a vaccine against HSV-1 to induce greater immunopathology in patients suffering from herpetic keratitis is a potential reality and necessitates the involvement of vision professionals in future vaccine trials for HSV-1.
Among other factors such as immunosenescence, differential coverage in vaccine-mediated protection against varicella and HZ is likely due to the respective immune correlates of protection. Prophylactic Varivax immunization elicits humoral and cell-mediated immunity. While the immune correlate of protection against signs of varicella is clearly humoral immunity, cell-mediated immunity expedites viral clearance and latency [61,62]. Therapeutic Zostivax immunization benefits patients by boosting cell-mediated responses responsible for maintaining viral latency [61,63]. This paradox highlights a relevant postulate for vaccine research: the particular immunologic mechanisms governing protection, resolution, and preservation of latency during the course of HSV infection may differ (adapted from Plotkin) [61].
Vaccination Strategies for HSV
While the high prevalence of HSV-1 indicates that a therapeutic vaccine would currently be most beneficial, a prophylactic vaccine would ultimately be more advantageous. This apparent contradiction invites an important question: Can an HSV-1 vaccine be implemented universally for prophylactic and therapeutic use to limit HSV-1 pathogenicity? Epidemiologic trends in the age of HSV-1 acquisition/seroconversion would suggest that a dual-use approach is plausible.
Clinical evidence supports the effectiveness of prophylactic HSV vaccination. In one Herpevac clinical trial cohort study in which HSV-1/2 seronegative women were vaccinated, 82% of the cohort was protected from HSV-1 genital disease [32]. Furthermore, patient antibody titer against gD elicited from vaccination but not T cell-mediated immune responses correlated with protection against HSV-1 infection such that antibody titers against gD were inversely proportional to HSV-1 seroconversion status and/or culture positive disease incidence among the cohort [64]. Based on the classic model of receptor-mediated virus entry, many subunit vaccines have been designed with HSV-2 glycoproteins that bind to the host viral entry receptors as target antigens, particularly gD and gB [1,14]. Suboptimal performance of the GlaxoSmithKline Herpevac gD subunit vaccines in clinical trials against genital HSV-2 suggest that broad protection against HSV may involve more antigenic targets [1,32].
It is critically important to evaluate both the safety and efficacy of any future HSV vaccine. Current conventional vaccine design largely capitalizes on modern advancements in technology that facilitate a rational, ground-up approach and the use of ‘safe’ DNA vector or protein-subunit vaccines due to their inability to cause infection [65].
Table 1 details the compositions of HSV vaccines used in human clinical trials to date. Of these, nine contain HSV-2 gD with various adjuvants to form monovalent protein subunit vaccines (GlaxoSmithKline). Four others combine HSV-2 gD with one or more other viral proteins and various adjuvants to form polyvalent protein subunit vaccines (Genocea Biosciences and Agenus). Two use DNA plasmid-based technologies to drive expression of multiple viral proteins following transfection/injection (Vical and Powdermed/Pfizer). Lastly, one ongoing clinical trial uses a replication disabled live virus (HSV529) to elicit a protective immune response.
Strengths and weaknesses of various HSV vaccine compositions and vaccination strategies have recently been reviewed in detail by Halford [1]. Halford’s underlying conclusion was that protein subunit vaccines being used in multiple clinical trials for HSV have demonstrated limited to no effectiveness in preventing HSV-2 genital herpes due to their miniscule representation (1%) of the antigenic breadth of the native virus. The same conclusion is likely to be true with respect to future vaccines specifically targeting HSV-1. Protein subunit vaccines have been used effectively against HPV and hepatitis B, although these are much smaller viruses such that the subunit vaccine covers 12–22% of the viral proteome. Halford argues that the implementation of live-attenuated HSV vaccines representing more than 99% of the native antigens is the most logical approach for vaccine development [1].
History suggests that the most efficacious vaccines for viral infections are live-attenuated strains [66,67]. These include vaccines for influenza, measles, mumps, rubella, polio (oral), rotavirus, VZV, yellow fever and rabies. Inactivated whole virus, virion derivatives, and protein subunit vaccines have shown success for hepatitis A, hepatitis B, human papilloma virus (HPV), influenza, and polio. Incidentally, the correlate of protection against primary infection involves humoral immunity for all of these vaccines [61]. Cell-mediated immunity generated from many of these vaccines is certainly involved in viral clearance and protection from recurrent disease following infection, but the correlate of protection with respect to disease prevention is a pre-existing pathogen-specific antibody response [61]. Moreover, the innate pathways activated by attenuated strains also contribute to the generation of protective adaptive immune responses [67,68].
It is important to discern what risks may be associated with vaccination using live-attenuated vaccines. While concern has been raised about the possibility of recombination with strains endemic among the human population when vaccinating using replication disabled or attenuated viruses, it is important to note the near ubiquitous nature and increasing clinical burden of HSV when considering acceptable risks [1]. Evidence for naturally occurring recombination between separate HSV-1 stains in vivo is lacking due to unperceivable changes in viral fitness or pathogenesis within the same host; although, the phenomenon has been observed and characterized in vitro [69–71]. From a clinical perspective, however, frame shift mutations in the viral gene encoding thymidine kinase are appreciable and problematic due to their association with acyclovir-resistance and recurrent keratitis [72,73].
Fortunately, the concept of a naturally occurring oncogenic alphaherpesvirus is not supported by peer-reviewed medical or scientific literature; therefore, the risk of a vaccine strain being oncogenic is unlikely. Instead, genetically engineered HSV-1 strains have been and are currently being used to fight cancer in human clinical trials [74,75]. Specifically, a therapeutic oncolytic live HSV-1 strain is slated for a phase I trial to treat inoperable gliomas (National Clinical Trial registry #02062827). Epstein-Barr virus (EBV/HHV-4) and Kaposi’s Sarcoma-associated herpesvirus (KSHV/HHV-8) are recognized as oncogenic; however, these two viruses are gammaherpesvirus family members and differ greatly in their composition and pathophysiology from HSV-1 [76].
It would be unreasonable to not take precautions to ensure the utmost safety when considering vaccine candidates for clinical trials. Nevertheless, ongoing clinical trials have not uncovered significant concerns about the clinical use of genetically modified HSV-1 strains for the treatment of cancer. While vaccine safety is a primary concern of vaccine immunologists, it is imperative that we address the clinically significant problem resulting from the lack of an efficacious HSV vaccine in a timely manner, because millions of people suffer complications arising from HSV infection.
Luckily, much research can be accomplished with HSV-1 outside of human cohorts due to the variety of suitable animal models and ease of in vitro viral propagation. This is not the case for VZV, due to in vitro propagation issues and absence of parallel disease characteristics in small animal models relative to humans [19,77]. Many unresolved questions regarding the composition and efficacy of HSV vaccines can be resolved with HSV vaccines in such animal models. Moreover, these models may help to uncover immune correlates of protection during various phases of disease as well as vaccine-mediated contributions to protection and pathology in the eye.
Some have made a case for symptomatic and asymptomatic immune responses based on human serum profiling of immunodominant antigens, and they suggest that vaccine design should be centered on antibody production against asymptomatic epitopes [78]. This clinical data is supported by experimental animal models showing that antibodies against HSV glycoprotein K (gK) exacerbate HSV infection leading to increased corneal pathology [79]. Independent clinical evidence suggests that antibodies directed against gK can enhance HSK in humans as well [80]. This particular study also showed that anti-gD antibody titer did not correlate with total HSV-1 neutralization titer among patients with HSK, leading us to question why one would immunize with this antigen alone [80]. Administration of acyclovir has also been noted to skew antigen-specific antibody titers in latently infected mice; overall, acyclovir had no impact on total anti-HSV-1 titer, but treatment significantly reduced gD- and gB-specific antibody titers by day 120 post infection [81]. These studies support the notion that animal models can be utilized to solve some immunologic concerns about HSV vaccines.
Animal Models and Ocular HSV-1 Vaccines
Various animal models for HSV-1 infection exist and are useful to discern different aspects of viral pathogenesis and correlates of vaccine mediated protection. These animal models spanning multiple species have been reviewed extensively over the past few years [82–85]. A fundamental concept vision professionals need to appreciate with respect to vaccine-induced protection in animal models is the postulate posed earlier: the particular immunologic mechanisms governing protection, resolution, and preservation of latency during the course of HSV-1 infection may differ. Furthermore, appreciating the continuum between these subjective phases outlined in the postulate provides a basis for comprehending the complexity of host-pathogen interactions during HSV-1 infection. Animal models utilized in HSV-1 vaccine research will be discussed relative to the postulate above.
The lines between immunologic protection and resolution may be blurred in immunologically naive animal models and humans in which prophylactic vaccination does not offer absolute protection against a challenge by primary infection. Such instances facilitate breakthrough disease. While breakthrough disease scenarios afford the host a limited degree of protection via preexisting vaccine-induced measures of adaptive immunity, it still allows for de novo activation and generation of further adaptive immune countermeasures to contribute to the resolution of infection and ultimately drive the virus into a latent state.
Importantly, breakthrough disease scenarios complicate how investigators may interpret data resolving correlates of protection with respect to prophylactic vaccines. Is the correlate of protection in such instances the result of vaccine-mediated or pathogen-induced immune responses? What is the immunologic correlate and threshold of vaccine-mediated protection required to prevent crossover between protection against and resolution of primary infection? Do these immune responses inhibit or exacerbate HSK? Data from experimental models suggest that these questions remain incompletely understood [82,86].
The most commonly utilized animal model to study HSK is a murine corneal infection model. While mouse models of primary ocular infection are inherently different than HSK in humans, many parallels can be made to clarify vaccine efficacy. One limitation of the mouse model is that primary infection elicits the generation of HSK and this phenomenon is not dependent upon virus reactivation as it is in humans. Despite this, mouse models are routinely used for basic research to assess ocular pathogenesis of acute HSV-1 infection largely due to the array of available commercial products and gene deficient mice that enable characterization of immune responses [87–89].
As previously discussed, the concept of asymptomatic and symptomatic T cell epitopes has been established to differentiate between protection against HSV-1 infection and exacerbation of HSK, respectively [79,80]. However, evidence from a prophylactic replication disabled whole virus vaccine in mice suggests that enhanced cell mediated immune responses not only resolve acute infection in the TG but also reduce the severity of HSK following challenge [90]. On the other hand, much literature has implicated CD4+ T cells in the pathogenesis of HSK in the mouse model of ocular HSV-1 infection [82].
Recent immunologic advances have resolved aspects of the cell-mediated immune response to HSV-1 infection. HSV-1 employs an apparent binary ‘life cycle’ reflecting lytic and latent phases. However, emerging evidence in mice at the single cell resolution suggests that latency is not merely a static process, but one of constant flux that permits low-level, asymptomatic shedding of infectious virus from individual neurons within the TG [91]. It is clear that CD8+ T cell responses are critical to resolve acute HSV-1 infection and maintain the suppression of latent infection in the TG [92].
The immunodominant HSV-1 antigenic targets in the CD8+ T cell repertoire are now known for C57BL/6 mice through the use of MHC tetramers and flow cytometry [93]. Furthermore, the breadth of this response correlates with the degree of suppression of virus reactivation in C57BL/6 mice [93]. However, HSV-1 specific T cells do not readily infiltrate the TG in latently infected mice from the peripheral circulation [94]. This notion makes the prospect of a protective T cell-based therapeutic vaccine seem limited.
The role of antibody derived from HSV-specific humoral immune responses in preventing primary infection is undervalued in the field. Clinical trials for the Herpevac vaccine introduced a paradigm shift for the field by showing that antibody titer against gD but not cell mediated immunity is the correlate of protection against genital HSV-1 infection [64]. Antibody responses specific for gB have been shown to successfully prevent cell-to-cell spread of HSV-1, a mechanism classically associated with evasion of humoral immune responses [95,96]. Additionally, antibody titers generated from vaccination with various live-attenuated HSV-2 strains in mice and guinea pigs correlates with protection against challenge infection in mucosal sites including the eye [97].
Data from vaccine studies in other animal models including rabbits and guinea pigs is particularly useful due to the ability of HSV-1 to spontaneously reactivate. Similarly to humans, spontaneous HSV-1 reactivation in rabbits includes viral shedding in the saliva and tears [98]. Humanized rabbits have been used as a predictive measure of HLA-restricted T cell responses elicited by subunit vaccination [99]. Humanized animal models are a welcome advancement in the field, as previous gD subunit vaccines showed promising results with respect to protection against HSV-1/2 in animals, but had no efficacy in human clinical trials [85]. Unfortunately, characterization of HSK in the eye of animal models for HSV vaccines often rely on superficial comparisons and only include data such as virus shedding, viral titers, and stand-alone slit lamp examinations [100].
Conclusion
Current evidence shows the need and benefits of an HSV-1 vaccine to prevent ocular pathology associated with recurrent HK. However, we advocate for ophthalmologist involvement in HSV vaccine research due to the potential for immunomodulation of HK mediated by HSV-1 vaccination. Clinical data from ocular VZV infection show rare instances of vaccine-mediated exacerbation of ocular HZ infection. The development of novel HSV-1 vaccines should be based on clinically relevant empirical evidence showing protection in vaccinated animals. Such approaches will allow for the elucidation of immune mechanisms associated with protection.
Expert commentary
HSV-1 remains a significant contributor to corneal blindness and is emerging as a significant cause of genital herpes. A vaccine that can successfully combat both of these sequelae is needed. Boosting immunity to HSV-1 may exacerbate the potential for the generation of HSK, thus it is critical that vision professionals pool their expertise and resources to faithfully characterize and link data from preclinical animal model studies with clinically relevant analyses of immune responses and detailed evaluations of ocular pathology.
It cannot be argued that the fields of HSV vaccine development and ophthalmology are changing quickly with the advancement of new technologies. Therefore, cooperation between vision scientists and ophthalmologists is important to move each field forward towards better insight and more effective treatments, respectively. We believe that such cooperation would enable researchers to better understand and record clinically relevant aspects during animal eye exams in order to publish specifics details of ocular pathology relevant to the genesis and severity of HSK that can be appreciated by scientists and clinicians alike.
Animal models permit extensive characterization of corneal pathology both in vivo and ex vivo using clinically relevant tools and techniques that are largely missing from the ocular HSV vaccine literature. While slit lamp examination can be valuable, other tools used clinically in diagnosis and evaluation of active ocular HSV-1 infection and HSK can be applied to animal models. Pachymetry can be used to measure corneal inflammation or thinning, and corneal sensitivity can be assessed using a Cochet-Bonnet esthesiometer [101,102]. Data showing preservation of corneal sensation and absence of edema following ocular HSV-1 challenge may be more convincing to ophthalmologists as a measure of vaccine-induced protection than a subjectively better slit lamp exam [4]. Overall visual acuity can also be quantified in mice [103,104]. Implementation of clinically relevant aspects of corneal pathology will improve the translational application and utility of animal studies conducted with HSV-1 vaccines.
Clinicians can appreciate and benefit from experimental evidence obtained using animal models to determine mechanisms of vaccine-mediated protection and pathology in the eye in order to provide invaluable data not obtainable in the clinic. For example, extracted animal corneas can be analyzed for leukocyte infiltration using flow cytometry to determine kinetics and characteristics of innate and adaptive immune responses with respect to HSK. Corneal whole mounts offer an excellent platform for the study of corneal neovascularization by confocal microscopy [89,105]. Tetramer profiling in the TG and corneas of mice in vaccine studies could be utilized to specifically define the antigenic breadth of cell-mediated responses in addition to serum neutralizing antibody profiling. Such measures would help to uncover mechanistic determinants involved in the development and progression of HSK.
Five-year view
Technological innovation and advances in research have informed vaccine design, and they are paving the road to rational vaccine design [65]. Over the next five years, we anticipate that immunologic insights from both the clinic and laboratory may optimize design of HSV-1 vaccines. Due to the lack of widespread success with subunit vaccines targeting HSV, we advocate for the use of live-attenuated vaccines against HSV at the moment to increase antigenic coverage. However, we also call on ophthalmologists to get involved in clinical trials with HSV-1 vaccines to define protection and pathology associated with HSV-1 vaccines. Cohorts of individuals with recurrent ocular HSV-1 infection may even benefit from an organized clinical trial. Nontraditional vaccine approaches or refinement in our knowledge of correlates of protection may pave the way to identifying protective epitopes and potentially defining reactogenic or symptomatic epitopes. Continued elucidation of the immune system’s functions may also lead to breakthroughs in targeted vaccine design or novel adjuvants to increase efficacy of glycoprotein subunits or identify T cell- and antibody-reactive epitopes that can be used as a cocktail to maximize the protective efficacy of a vaccine.
Key Issues.
The utility and need for an efficacious vaccine to protect against ocular HSV-1 infection is often overlooked.
HSV-1 is the leading cause of infectious corneal blindness, yet no NIH-sanctioned clinical trials for HSV vaccines are evaluating the impact on eye health.
Ophthalmologists and vision researchers have a unique role in the development and implementation of HSV-1 vaccines.
Changing epidemiological trends due to delayed HSV-1 exposure have created an environment where a vaccine is both timely and needed to combat increasing incidence of clinical complications arising from primary HSV-1 infection during later ages.
Success of vaccination against VZV can inform our design of HSV vaccines.
New evidence is challenging the static nature of the balance between lytic and latent HSV-1 infection and suggest that it is a dynamic process.
The immune correlates of protection against HSV-1 infection, resolution, and maintenance of latency may differ.
Improvement in our immunologic understanding of future HSV vaccines can be achieved in animal models of ocular infection.
Acknowledgements
This review was financially supported by NIH R01 EY021238 awarded to DJJC and an unrestricted grant from Research to Prevent Blindness. DJR is supported by NEI training grant T32EY023202. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial Disclosure
The authors declare no financial conflict of interest with the materials or subject matter discussed in this review.
References
References held by the authors to be especially noteworthy are indicated below:
• of interest
•• of considerable interest
- 1. Halford WP. Antigenic breadth: a missing ingredient in HSV-2 subunit vaccines? Expert Rev. Vaccines. 2014;13(6):691–710. doi: 10.1586/14760584.2014.910121. •• This is the first study to critically evaluate the concept and significance of antigenic valence in HSV vaccines.
- 2. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv. Ophthalmol. 2012;57(5):448–462. doi: 10.1016/j.survophthal.2012.01.005. •• Best current estimate of the incidence and impact of HSK in the United States.
- 3.Divito SJ, Hendricks RL. Activated Inflammatory Infiltrate in HSV-1-Infected Corneas without Herpes Stromal Keratitis. Invest. Ophthalmol. Vis. Sci. 2008;49(4):1488–1495. doi: 10.1167/iovs.07-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun H, Rowe AM, Lathrop KL, Harvey SAK, Hendricks RL. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J. Virol. 2014;88(14):7870–7880. doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallar J, Tervo TMT, Neira W, et al. Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest. Ophthalmol. Vis. Sci. 2010;51(9):4516–4522. doi: 10.1167/iovs.10-5225. [DOI] [PubMed] [Google Scholar]
- 7.Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J. Virol. 2009;83(5):2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Dujaili LJ, Clerkin PP, Clement C, et al. Ocular herpes simplex virus: how are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiol. 2011;6(8):877–907. doi: 10.2217/fmb.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alami Chentoufi A, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Towards a Rational Design of an Asymptomatic Clinical Herpes Vaccine: The Old, the New, and the Unknown. Clin. Dev. Immunol. 2012;2012:1–16. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arvin AM, editor. Human herpesviruses: biology, therapy, and immunoprophylaxis. New York: Cambridge University Press, Cambridge; [PubMed] [Google Scholar]
- 11.Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 1986;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald SJ, Mostafa HH, Morrison LA, Davido DJ. Genome sequence of herpes simplex virus 1 strain KOS. J. Virol. 2012;86(11):6371–6372. doi: 10.1128/JVI.00646-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halford WP, Weisend C, Grace J, et al. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol. J. 2006;3:44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campadelli-Fiume G, Menotti L, Avitabile E, Gianni T. Viral and cellular contributions to herpes simplex virus entry into the cell. Curr. Opin. Virol. 2012;2(1):28–36. doi: 10.1016/j.coviro.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 2011;9(5):369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karasneh GA, Shukla D. Herpes simplex virus infects most cell types in vitro: clues to its success. Virol. J. 2011;8:481. doi: 10.1186/1743-422X-8-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrady CD, Drevets DA, Carr DJJ. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing? J. Neuroimmunol. 2010;220(1–2):1–9. doi: 10.1016/j.jneuroim.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer T, Enquist LW. Directional spread of alphaherpesviruses in the nervous system. Viruses. 2013;5(2):678–707. doi: 10.3390/v5020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy PGE, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. J. Neurovirol. 2010;16(6):411–418. doi: 10.1007/BF03210846. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler CE. The herpes simplex problem. J. Am. Acad. Dermatol. 1988;18(1 Pt 2):163–168. doi: 10.1016/s0190-9622(88)70019-5. [DOI] [PubMed] [Google Scholar]
- 21.Theobald S. Observations upon Herpes Corneae “Febrilis,” with Reference, Especially, to Etiology. Trans. Am. Ophthalmol. Soc. 1916;14(Pt 2):520–527. [PMC free article] [PubMed] [Google Scholar]
- 22.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA J. Am. Med. Assoc. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 23. Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999–2010. J. Infect. Dis. 2014;209(3):325–333. doi: 10.1093/infdis/jit458. •• This study provides the most up-to-date, broad coverage information on seroprevalence and delayed exposure with respect to HSV-1 in the United States.
- 24.Schulte JM, Bellamy AR, Hook EW, et al. HSV-1 and HSV-2 seroprevalence in the united states among asymptomatic women unaware of any herpes simplex virus infection (Herpevac Trial for Women) South. Med. J. 2014;107(2):79–84. doi: 10.1097/SMJ.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 25.Miller CS, Danaher RJ. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;105(1):43–50. doi: 10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest. Ophthalmol. Vis. Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue H, Motani-Saitoh H, Sakurada K, et al. Detection of varicella-zoster virus DNA in 414 human trigeminal ganglia from cadavers by the polymerase chain reaction: a comparison of the detection rate of varicella-zoster virus and herpes simplex virus type 1. J. Med. Virol. 2010;82(2):345–349. doi: 10.1002/jmv.21687. [DOI] [PubMed] [Google Scholar]
- 28.Motani H, Sakurada K, Ikegaya H, et al. Detection of herpes simplex virus type 1 DNA in bilateral human trigeminal ganglia and optic nerves by polymerase chain reaction. J. Med. Virol. 2006;78(12):1584–1587. doi: 10.1002/jmv.20742. [DOI] [PubMed] [Google Scholar]
- 29.Liedtke W, Opalka B, Zimmermann CW, Lignitz E. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J. Neurol. Sci. 1993;116(1):6–11. doi: 10.1016/0022-510x(93)90082-a. [DOI] [PubMed] [Google Scholar]
- 30.Hill JM, Ball MJ, Neumann DM, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J. Virol. 2008;82(16):8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hook EW, Cannon RO, Nahmias AJ, et al. Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection in heterosexuals. J. Infect. Dis. 1992;165(2):251–255. doi: 10.1093/infdis/165.2.251. [DOI] [PubMed] [Google Scholar]
- 32.Belshe RB, Leone PA, Bernstein DI, et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N. Engl. J. Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013;56(3):344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tunbridge AJ, Breuer J, Jeffery KJM. British Infection Society. Chickenpox in adults - clinical management. J. Infect. 2008;57(2):95–102. doi: 10.1016/j.jinf.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Pediatrics. Committee on Infectious Diseases. Varicella vaccine update. Pediatrics. 2000;105(1 Pt 1):136–141. [PubMed] [Google Scholar]
- 36.Uchio E, Hatano H, Mitsui K, et al. A retrospective study of herpes simplex keratitis over the last 30 years. Jpn. J. Ophthalmol. 1994;38(2):196–201. [PubMed] [Google Scholar]
- 37.Koelle DM, Ghiasi H. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr. Eye Res. 2005;30(11):929–942. doi: 10.1080/02713680500313153. [DOI] [PubMed] [Google Scholar]
- 38.Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv. Ophthalmol. 2009;54(2):226–234. doi: 10.1016/j.survophthal.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Lairson DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch. Ophthalmol. 2003;121(1):108–112. doi: 10.1001/archopht.121.1.108. [DOI] [PubMed] [Google Scholar]
- 40.Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch. Ophthalmol. 1989;107(8):1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 41.Chentoufi AA, Benmohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin. Dev. Immunol. 2012;2012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Predictors of recurrent herpes simplex virus keratitis. Herpetic Eye Disease Study Group. Cornea. 2001;20(2):123–128. doi: 10.1097/00003226-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Chong E-M, Wilhelmus KR, Matoba AY, Jones DB, Coats DK, Paysse EA. Herpes simplex virus keratitis in children. Am. J. Ophthalmol. 2004;138(3):474–475. doi: 10.1016/j.ajo.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Levy J, Lapid-Gortzak R, Klemperer I, Lifshitz T. Herpes simplex virus keratitis after laser in situ keratomileusis. J. Refract. Surg. Thorofare NJ 1995. 2005;21(4):400–402. doi: 10.3928/1081-597X-20050701-17. [DOI] [PubMed] [Google Scholar]
- 45.Moshirfar M, Welling JD, Feiz V, Holz H, Clinch TE. Infectious and noninfectious keratitis after laser in situ keratomileusis Occurrence, management, and visual outcomes. J. Cataract Refract. Surg. 2007;33(3):474–483. doi: 10.1016/j.jcrs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 46.De Rojas Silva V, Rodríguez-Conde R, Cobo-Soriano R, Beltrán J, Llovet F, Baviera J. Laser in situ keratomileusis in patients with a history of ocular herpes. J. Cataract Refract. Surg. 2007;33(11):1855–1859. doi: 10.1016/j.jcrs.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am. J. Ophthalmol. 2006;141(3):547–557. doi: 10.1016/j.ajo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Pivetti-Pezzi P, Accorinti M, Colabelli-Gisoldi RA, Pirraglia MP, Sirianni MC. Herpes simplex virus vaccine in recurrent herpetic ocular infection. Cornea. 1999;18(1):47–51. [PubMed] [Google Scholar]
- 49.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 50.Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin. Proc. 2013;88(6):562–570. doi: 10.1016/j.mayocp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krall P, Kubal A. Herpes zoster stromal keratitis after varicella vaccine booster in a pediatric patient. Cornea. 2014;33(9):988–989. doi: 10.1097/ICO.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 52.Naseri A, Good WV, Cunningham ET. Herpes zoster virus sclerokeratitis and anterior uveitis in a child following varicella vaccination. Am. J. Ophthalmol. 2003;135(3):415–417. doi: 10.1016/s0002-9394(02)01957-8. [DOI] [PubMed] [Google Scholar]
- 53.Hwang CW, Steigleman WA, Saucedo-Sanchez E, Tuli SS. Reactivation of herpes zoster keratitis in an adult after varicella zoster vaccination. Cornea. 2013;32(4):508–509. doi: 10.1097/ICO.0b013e318277acae. [DOI] [PubMed] [Google Scholar]
- 54.Gonzales JA, Levison AL, Stewart JM, Acharya NR, Margolis TP. Retinal necrosis following varicella-zoster vaccination. Arch. Ophthalmol. 2012;130(10):1355–1356. doi: 10.1001/archophthalmol.2012.2255. [DOI] [PubMed] [Google Scholar]
- 55.Charkoudian LD, Kaiser GM, Steinmetz RL, Srivastava SK. Acute retinal necrosis after herpes zoster vaccination. Arch. Ophthalmol. 2011;129(11):1495–1497. doi: 10.1001/archophthalmol.2011.320. [DOI] [PubMed] [Google Scholar]
- 56.Fine HF, Kim E, Flynn TE, Gomes NL, Chang S. Acute posterior multifocal placoid pigment epitheliopathy following varicella vaccination. Br. J. Ophthalmol. 2010;94(3):282–283. 363. doi: 10.1136/bjo.2008.144501. [DOI] [PubMed] [Google Scholar]
- 57.Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul. Immunol. Inflamm. 2009;17(1):33–35. doi: 10.1080/09273940802491892. [DOI] [PubMed] [Google Scholar]
- 58.Esmaeli-Gutstein B, Winkelman JZ. Uveitis associated with varicella virus vaccine. Am. J. Ophthalmol. 1999;127(6):733–734. doi: 10.1016/s0002-9394(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 59.Ross A, McLean TW, Farber R, et al. Retinitis following varicella in a vaccinated child with acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2005;45(2):191–194. doi: 10.1002/pbc.20118. [DOI] [PubMed] [Google Scholar]
- 60.Morrison VA, Oxman MN, Levin MJ, et al. Safety of zoster vaccine in elderly adults following documented herpes zoster. J. Infect. Dis. 2013;208(4):559–563. doi: 10.1093/infdis/jit182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Plotkin SA. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. CVI. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. •• Provides information about immune correlates of protection across a broad range of pathogens. Original source of postulate adopted for evaluating HSV pathogenesis in the current review.
- 62.Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: Seventy-seven cases. Pediatrics. 1975;56(3):388–397. [PubMed] [Google Scholar]
- 63.Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 2008;197(6):825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belshe RB, Heineman TC, Bernstein DI, et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 2014;209(6):828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Gregorio E, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014;14(7):505–514. doi: 10.1038/nri3694. •• This paper highlights changing trends and new technologies available in vaccine development.
- 66.Malkevitch NV, Robert-Guroff M. A call for replicating vector prime-protein boost strategies in HIV vaccine design. Expert Rev. Vaccines. 2004;3(4 Suppl):S105–S117. doi: 10.1586/14760584.3.4.s105. [DOI] [PubMed] [Google Scholar]
- 67.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 68.Nesburn AB, Bettahi I, Zhang X, et al. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul. Surf. 2006;4(4):178–187. doi: 10.1016/s1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 69.Schumacher AJ, Mohni KN, Kan Y, Hendrickson EA, Stark JM, Weller SK. The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog. 2012;8(8):e1002862. doi: 10.1371/journal.ppat.1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkinson DE, Weller SK. The role of DNA recombination in herpes simplex virus DNA replication. IUBMB Life. 2003;55(8):451–458. doi: 10.1080/15216540310001612237. [DOI] [PubMed] [Google Scholar]
- 71.Schaffer PA, Tevethia MJ, Benyesh-Melnick M. Recombination between temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1974;58(1):219–228. doi: 10.1016/0042-6822(74)90156-1. [DOI] [PubMed] [Google Scholar]
- 72.Pan D, Coen DM. Quantification and analysis of thymidine kinase expression from acyclovir-resistant G-string insertion and deletion mutants in herpes simplex virus-infected cells. J. Virol. 2012;86(8):4518–4526. doi: 10.1128/JVI.06995-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Velzen M, van de Vijver DAMC, van Loenen FB, Osterhaus ADME, Remeijer L, Verjans GMGM. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J. Infect. Dis. 2013;208(9):1359–1365. doi: 10.1093/infdis/jit350. [DOI] [PubMed] [Google Scholar]
- 74.Todo T. Active immunotherapy: oncolytic virus therapy using HSV-1. Adv. Exp. Med. Biol. 2012;746:178–186. doi: 10.1007/978-1-4614-3146-6_14. [DOI] [PubMed] [Google Scholar]
- 75.Hersey P, Gallagher S. Intralesional immunotherapy for melanoma. J. Surg. Oncol. 2014;109(4):320–326. doi: 10.1002/jso.23494. [DOI] [PubMed] [Google Scholar]
- 76.Nicholas J. Evolutionary aspects of oncogenic herpesviruses. Mol. Pathol. MP. 2000;53(5):222–237. doi: 10.1136/mp.53.5.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steain M, Slobedman B, Abendroth A. Experimental models to study varicella-zoster virus infection of neurons. Curr. Top. Microbiol. Immunol. 2010;342:211–228. doi: 10.1007/82_2010_15. [DOI] [PubMed] [Google Scholar]
- 78.Dasgupta G, Chentoufi AA, Kalantari M, et al. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J. Virol. 2012;86(8):4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghiasi H, Perng GC, Nesburn AB, Wechsler SL. Antibody-dependent enhancement of HSV-1 infection by anti-gK sera. Virus Res. 2000;68(2):137–144. doi: 10.1016/s0168-1702(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 80.Mott KR, Osorio Y, Maguen E, et al. Role of anti-glycoproteins D (anti-gD) and K (anti-gK) IgGs in pathology of herpes stromal keratitis in humans. Invest. Ophthalmol. Vis. Sci. 2007;48(5):2185–2193. doi: 10.1167/iovs.06-1276. [DOI] [PubMed] [Google Scholar]
- 81.Halford WP, Gebhardt BM, Carr DJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238(1):53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 82.Stuart PM, Keadle TL. Recurrent herpetic stromal keratitis in mice: a model for studying human HSK. Clin. Dev. Immunol. 2012;2012:728480. doi: 10.1155/2012/728480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Webre JM, Hill JM, Nolan NM, et al. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J. Biomed. Biotechnol. 2012;2012:612316. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29(35):5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine. 2014;32(50):6733–6745. doi: 10.1016/j.vaccine.2014.10.002. •• This review provides an excellent and up-to-date review on various animal models used in HSV vaccine studies.
- 86.Khodai T, Chappell D, Christy C, et al. Single and combination herpes simplex virus type 2 glycoprotein vaccines adjuvanted with CpG oligodeoxynucleotides or monophosphoryl lipid A exhibit differential immunity that is not correlated to protection in animal models. Clin. Vaccine Immunol. CVI. 2011;18(10):1702–1709. doi: 10.1128/CVI.05071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012;5(2):173–183. doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conrady CD, Zheng M, Stone DU, Carr DJJ. CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J. Immunol. Baltim. Md 1950. 2012;189(1):425–432. doi: 10.4049/jimmunol.1200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wuest T, Zheng M, Efstathiou S, Halford WP, Carr DJJ. The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization. PLoS Pathog. 2011;7(10):e1002278. doi: 10.1371/journal.ppat.1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schrimpf JE, Tu EM, Wang H, Wong YM, Morrison LA. B7 costimulation molecules encoded by replication-defective, vhs-deficient HSV-1 improve vaccine-induced protection against corneal disease. PloS One. 2011;6(8):e22772. doi: 10.1371/journal.pone.0022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma JZ, Russell TA, Spelman T, Carbone FR, Tscharke DC. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS Pathog. 2014;10(7):e1004237. doi: 10.1371/journal.ppat.1004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322(5899):268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J. Immunol. Baltim. Md 1950. 2011;186(7):3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2(1):5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krawczyk A, Krauss J, Eis-Hübinger AM, et al. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J. Virol. 2011;85(4):1793–1803. doi: 10.1128/JVI.01924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krawczyk A, Arndt MAE, Grosse-Hovest L, et al. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci. U. S. A. 2013;110(17):6760–6765. doi: 10.1073/pnas.1220019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halford WP, Geltz J, Gershburg E. Pan-HSV-2 IgG antibody in vaccinated mice and guinea pigs correlates with protection against herpes simplex virus 2. PloS One. 2013;8(6):e65523. doi: 10.1371/journal.pone.0065523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hill JM, Nolan NM, McFerrin HE, et al. HSV-1 latent rabbits shed viral DNA into their saliva. Virol. J. 2012;9:221. doi: 10.1186/1743-422X-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chentoufi AA, Dasgupta G, Christensen ND, et al. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. Baltim. Md 1950. 2010;184(5):2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J. Virol. 1998;72(10):7715–7721. doi: 10.1128/jvi.72.10.7715-7721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flynn TH, Ohbayashi M, Dawson M, Siddique M, Ono SJ, Larkin DFP. Use of ultrasonic pachymetry for measurement of changes in corneal thickness in mouse corneal transplant rejection. Br. J. Ophthalmol. 2010;94(3):368–371. doi: 10.1136/bjo.2009.160671. [DOI] [PubMed] [Google Scholar]
- 102.Cortina MS, He J, Russ T, Bazan NG, Bazan HEP. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest. Ophthalmol. Vis. Sci. 2013;54(6):4109–4116. doi: 10.1167/iovs.13-12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40(16):2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 104.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 2004;45(12):4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 105.Wuest TR, Carr DJJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010;207(1):101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]