Abstract
In clinical trials, sofosbuvir showed high antiviral activity in patients infected with hepatitis C virus (HCV) across all genotypes. We aimed to determine the cost-effectiveness of sofosbuvir-based treatment compared to current standard treatment in mono-infected patients with chronic hepatitis C (CHC) genotypes 1–4 in Switzerland. Cost-effectiveness was modelled from the perspective of the Swiss health care system using a lifetime Markov model. Incremental cost-effectiveness ratios (ICERs) used an endpoint of cost per quality-adjusted life year (QALY) gained. Treatment characteristics, quality of life, and transition probabilities were obtained from published literature. Country-specific model inputs such as patient characteristics, mortality and costs were obtained from Swiss sources. We performed extensive sensitivity analyses. Costs and effects were discounted at 3% (range: 0–5%) per year. Sofosbuvir-containing treatment in mixed cohorts of cirrhotic and non-cirrhotic patients with CHC genotypes 1–4 showed ICERs between CHF 10,337 and CHF 91,570 per QALY gained. In subgroup analyses, sofosbuvir dominated telaprevir- and boceprevir-containing treatment in treatment-naïve genotype 1 cirrhotic patients. ICERs of sofosbuvir were above CHF 100,000 per QALY in treatment-naïve, interferon eligible, non-cirrhotic patients infected with genotypes 2 or 3. In deterministic and probabilistic sensitivity analyses, results were generally robust. From a Swiss health care system perspective, treatment of mixed cohorts of cirrhotic and non-cirrhotic patients with CHC genotypes 1–4 with sofosbuvir-containing treatment versus standard treatment would be cost-effective if a threshold of CHF 100,000 per QALY was assumed.
Introduction
Hepatitis C virus (HCV) is a ribonucleic acid (RNA) virus causing acute and chronic hepatitis [1]. Worldwide, the HCV prevalence is about 3% [2]. In Europe and the US, HCV infection through injection drug use has become the major transmission route [3]. Although most patients infected with HCV are symptomless, chronic hepatitis C (CHC) poses a significant risk of developing cirrhosis and hepatocellular carcinoma, if left untreated [4]. Thus, CHC is a cause of major health burden causing substantial morbidity and mortality [5]. In the US, costs of about 6.5 billion per year are estimated [6] despite the availability of antiviral therapy.
The aim of therapy in chronic hepatitis C is to achieve a sustained virological response (SVR). SVR is defined as undetectable serum HCV RNA after the end of treatment, signalling eradication of HCV infection [7]. SVR at 12 weeks has shown high concordance with SVR at 24 weeks [7] and has been accepted by regulators in the US and Europe as an appropriate endpoint indicating treatment success [8]. Response to HCV treatment differs according to HCV genotype, disease stage, and HCV treatment history [9]. Pegylated interferon alpha and ribavirin have long been considered standard of care [8] with SVR rates of 40–50% in genotypes 1 and 4 [10] and SVR rates up to 80% in patients with genotypes 2 and 3 [11]. Due to significant side effects and contraindications associated with pegylated interferon alpha and ribavirin therapy, direct-acting antivirals have been developed [9]. Protease inhibitors such as telaprevir and boceprevir have been licensed since 2011 for HCV genotype 1 and have increased SVR rates, but major safety and efficacy issues persist [9].
Sofosbuvir, a newly developed uridine nucleotide analogue HCV NS5B polymerase inhibitor, has shown high antiviral activity across genotypes and few severe side-effects in a range of clinical trials including various patient populations [12–16]. In treatment-naïve genotype 1 patients, triple therapy with sofosbuvir, pegylated interferon alpha and ribavirin for 12 weeks reached a SVR of 89% in a phase III trial [13]. SVR was 96% and 83% in a phase II trial enrolling treatment-experienced patients with genotypes 2 and 3 receiving 12 weeks of triple therapy with sofosbuvir, pegylated interferon alpha and ribavirin [16]. Treatment with sofosbuvir in combination with pegylated interferon alpha and/or ribavirin recently received approval for reimbursement by the Swiss statutory health insurance in patients with CHC and fibrosis stage 3 or 4, or symptomatic patients with extra hepatic manifestations [17].
The aim of this cost-effectiveness analysis was to estimate clinical effectiveness in terms of quality-adjusted life years (QALYs) gained, the direct medical cost, and the cost-effectiveness in terms of cost per QALY gained, of sofosbuvir-based treatment strategies compared with the current standard treatment of mono-infected patients with CHC genotypes 1–4. The article follows the CHEERS statement for reporting health economic evaluations [18].
Materials and Methods
Patient population and treatment strategies
We evaluated non-HIV-infected patients diagnosed with CHC genotypes 1–4. Patient groups were further subdivided into treatment-naïve versus treatment-experienced and interferon-eligible patients versus patients unsuitable for interferon (interferon-ineligible patients or patients unwilling to take interferon). To reflect real-life medical practice, we obtained average Swiss CHC patient characteristics, such as mean age, mean weight and percentage of cirrhotic patients by genotype, from the Swiss Hepatitis C Cohort Study (SCCS) [19]. This representative cohort study collects standardised prospective data on demographics, laboratory markers, HIV infection status, treatment and treatment results of HCV infected patients in Switzerland aged 18 years and over, and is fairly representative of the overall infected population in terms of age, sex distribution and risk factors for HCV acquisition [19]. The following data points were extracted from the SCCS in May 2014. The percentage of cirrhotic patients was 24% in genotype 1, 21% in genotype 2, 25% in genotype 3 and 22% in genotype 4. Mean age was 54, 53 and 51 years and mean body weight 73.5, 73.6 and 73.7 kilograms for genotype 1, genotypes 2 and 3, and genotype 4, respectively. The percentage of cirrhotic patients obtained from the SCCS was used for the base-case analysis.
Pairs of treatment strategies containing sofosbuvir and comparator strategies representing current standard of care were identified by experienced Swiss clinicians, based on their relevance for Switzerland and taking into account the local prevalence of different genotypes. No treatment was included as the comparator for CHC patients unsuitable for interferon treatment, in the absence of treatment alternatives. Treatment regimens were implemented in the model as per their marketing authorisations and according to the European Association for the Study of the Liver (EASL) guidelines [20].
Health economic model characteristics
We used a Markov state-transition model with a life-long time horizon implemented in MS Excel; the structure is shown in Fig 1. The model structure of two health economic models developed by the Southampton Health Technology Assessment Centre [21,22] that were submitted to the UK National Institute for Health and Care Excellence (NICE) served as basis and was adapted to achieve reconciliation with available data from clinical trials. Specifically, mild (METAVIR [23] score F0-F1, absent or portal fibrosis) and moderate HCV (METAVIR score F2 or F3, portal fibrosis with few or several septa) states included in the original models were combined to a non-cirrhotic state to reflect available data from clinical trials. In the resulting structure, virtual mixed cohorts of 10,000 patients with no cirrhosis or compensated cirrhosis entered the model at the beginning of CHC treatment. Possible health states included non-cirrhotic with SVR, cirrhosis with SVR, decompensated cirrhosis, hepatocellular carcinoma, liver transplant, post-liver transplant and death (Fig 1). Disease-specific mortality (excess mortality) and general mortality were included separately; patients were exposed to age-specific probabilities of death in the general population [24] in each health state. The possibility of recurrences and relapses were tested in sensitivity analysis (dotted arrow in Fig 1). The disease was assumed not to progress while patients were on treatment and 12 weeks after the end of treatment. Patients could not die during treatment. A half-cycle correction was used for costs as well as effects. Costs (Swiss Francs, CHF 2014) and effects were discounted at 3% (range in sensitivity analysis: 0–5%) as recommended in current literature [25]. We calculated the cost-effectiveness of sofosbuvir in terms of cost per quality-adjusted life year (QALY) from the perspective of the Swiss health care system (indirect costs not included). There is no published or generally accepted cost-effectiveness threshold for Switzerland but other Swiss cost-effectiveness studies have used CHF 100,000 per QALY gained to distinguish potentially favourable from unfavourable incremental cost-effectiveness ratios (ICERs) [26–28].
Fig 1. Structure of the Markov state transition model.
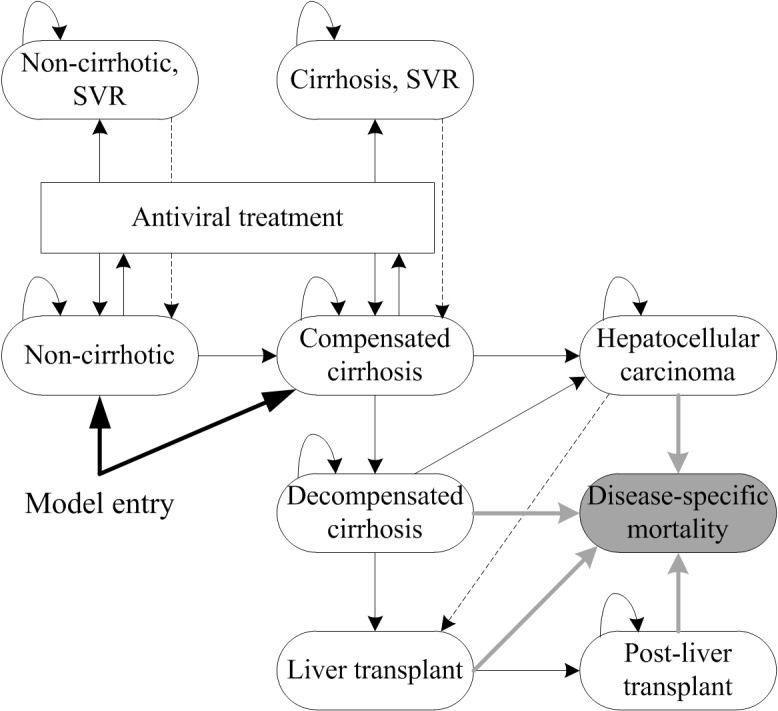
Patients can transition from each health state to death from any cause.
Model input parameters and assumptions
Transition probabilities, quality of life weights, treatment efficacy and safety data, resource use data, and treatment-related adverse event (AE) costs were obtained from published clinical trial reports, other peer-reviewed literature and treatment guidelines. Patient characteristics, all-cause mortality, treatment-related costs and health state costs were extracted from Swiss-specific sources.
Annual transition probabilities between health states were extracted from published literature as referenced in Table 1. Probabilities of death in the general population were obtained from the Swiss Federal Statistical Office [24].
Table 1. Base-case values for model input parameters and ranges used in the sensitivity analyses.
| Item | Source | Base-case | DSA ranges | PSA distribution and parameters | |||||
|---|---|---|---|---|---|---|---|---|---|
| Annual transition probabilities | From | To | GT1 | others | Lower -25% | Upper +25% | GT1 | others | |
| Non-cirrhotic 30 years | Compensated cirrhosis | [30], [31] | 0.006 | 0.009 | 0.0015 | 0.0105 | Beta, α = 9 β = 1481 | Beta, α = 20 β = 2209 | |
| Non-cirrhotic 40 years | Compensated cirrhosis | [30], [31] | 0.010 | 0.014 | 0.0025 | 0.0175 | Beta, α = 11 β = 1088 | Beta, α = 21 β = 1511 | |
| Non-cirrhotic 50 years | Compensated cirrhosis | [30], [31] | 0.016 | 0.025 | 0.0048 | 0.0280 | Beta, α = 10 β = 619 | Beta, α = 8 β = 316 | |
| Non-cirrhotic, SVR | Recurrence | Expert opinion | Not assessed in base-case | Not assessed in base-case | 0–0.01 | 0–0.01 | - | - | |
| Non-cirrhotic, SVR | Re-infection | Expert opinion | Not assessed in base-case | Not assessed in base-case | 0–0.01 | 0–0.01 | - | - | |
| Compensated cirrhosis | Decompensated cirrhosis | [29] | 0.039 | 0.039 | 0.0219 | 0.0608 | Beta, α = 15 β = 360 | Beta, α = 15 β = 360 | |
| Compensated cirrhosis | Hepatocellular carcinoma | [21,22,29] | 0.014 | 0.014 | 0.0016 | 0.0392 | Beta, α = 2 β = 136 | Beta, α = 2 β = 136 | |
| Compensated cirrhosis, SVR | Recurrence | Expert opinion | Not assessed in base-case | Not assessed in base-case | 0–0.01 | 0–0.01 | - | - | |
| Compensated cirrhosis, SVR | Re-infection | Expert opinion | Not assessed in base-case | Not assessed in base-case | 0–0.01 | 0–0.01 | - | - | |
| Decompensated cirrhosis | Hepatocellular carcinoma | [21,22,29] | 0.014 | 0.014 | 0.002 | 0.039 | Beta, α = 2 β = 136 | Beta, α = 2 β = 136 | |
| Decompensated cirrhosis | Liver transplant | [22] | 0.030 | 0.030 | 0.012 | 0.056 | Beta, α = 7 β = 211 | Beta, α = 7 β = 211 | |
| Decompensated cirrhosis | Death | [21,22,29] | 0.130 | 0.130 | 0.111 | 0.150 | Beta, α = 147 β = 984 | Beta, α = 147 β = 984 | |
| Hepatocellular carcinoma | Liver transplant | Expert opinion | 0–0.01 | 0–0.01 | - | - | - | - | |
| Hepatocellular carcinoma | Death | [21,22,29] | 0.43 | 0.43 | 0.37 | 0.49 | Beta, α = 117 β = 155 | Beta, α = 117 β = 155 | |
| Liver transplant | Death, year 1 | [22] | 0.21 | 0.21 | 0.13 | 0.31 | Beta, α = 16 β = 61 | Beta, α = 16 β = 61 | |
| Post-liver transplant | Death, year 2 | [22] | 0.057 | 0.057 | 0.037 | 0.082 | Beta, α = 23 β = 379 | Beta, α = 23 β = 379 | |
| Probability of death for the general population in 2003* | Age-group | Annual | 3-month | Source | Base-case | Lower -25% | Upper +25% | PSA | PSA |
| 15–24 | 0.000522 | 0.000130 | SFSO | 0.000522 | 0.0004 | 0.0007 | - | - | |
| 25–34 | 0.000660 | 0.000165 | SFSO | 0.000660 | 0.0005 | 0.0008 | - | - | |
| 35–44 | 0.001128 | 0.000282 | SFSO | 0.001128 | 0.0008 | 0.0014 | - | - | |
| 45–54 | 0.002758 | 0.000689 | SFSO | 0.002758 | 0.0021 | 0.0034 | - | - | |
| 55–64 | 0.006848 | 0.001711 | SFSO | 0.006848 | 0.0051 | 0.0086 | - | - | |
| 65–74 | 0.017697 | 0.004145 | SFSO | 0.017697 | 0.0133 | 0.0221 | - | - | |
| 77–84 | 0.054529 | 0.013540 | SFSO | 0.054529 | 0.0409 | 0.0682 | - | - | |
| 85+ | 0.165443 | 0.040517 | SFSO | 0.165443 | 0.1241 | 0.2068 | - | - | |
| Quality of life—utilities | Health state | Source | Base-case | Lower -25% | Upper +25% | PSA | PSA | ||
| Non-cirrhotic | [29] | 0.74 | 0.71 | 0.77 | Beta, α = 707 β = 248 | Beta, α = 707 β = 248 | |||
| Compensated cirrhosis | [29] | 0.55 | 0.44 | 0.65 | Beta, α = 47 β = 39 | Beta, α = 47 β = 39 | |||
| Patients on treatment with SOF (utility decrement) | [13] | -0.15 | -0.20 | +0.20 | Gamma, α = 63 β = 0.002 | Gamma, α = 63 β = 0.002 | |||
| Patients receiving comparator (utility decrement) | [13] | -0.15 | -0.12 | -0.18 | Gamma, α = 8 β = 0.02 (PegIFN+RBV) | Gamma, α = 8 β = 0.02 (PegIFN+RBV) | |||
| Patients receiving comparator (utility decrement) | [13] | -0.15 | -0.12 | -0.18 | Gamma, α = 204 β = 0.0007 (Telaprevir) | Gamma, α = 204 β = 0.0007 (Telaprevir) | |||
| Patients receiving comparator (utility decrement) | [13] | -0.15 | -0.12 | -0.18 | Gamma, α = 143 β = 0.0009 (Boceprevir) | Gamma, 03B1 = 143 β = 0.0009 (Boceprevir) | |||
| SVR (utility increment) | [29] | +0.05 | +0.002 | +0.17 | Gamma, α = 1.25 β = 0.04 | Gamma, α = 1.25 β = 0.04 | |||
| Non-cirrhotic, SVR | 0.74+0.05 | 0.79 | - | - | - | - | |||
| Cirrhotic, SVR | 0.55+0.05 | 0.60 | - | - | - | - | |||
| Decompensated cirrhosis | [29] | 0.45 | 0.39 | 0.51 | Beta, α = 124 β = 151 | Beta, α = 124 β = 151 | |||
| Hepatocellular carcinoma | [29] | 0.45 | 0.39 | 0.51 | Beta, α = 124 β = 151 | Beta, α = 124 β = 151 | |||
| Liver transplant | [29] | 0.45 | 0.39 | 0.51 | Beta, α = 124 β = 151 | Beta, α = 124 β = 151 | |||
| Post-liver transplant | [29] | 0.67 | 0.61 | 0.73 | Beta, α = 163 β = 80 | Beta, α = 163 β = 80 | |||
DSA, deterministic sensitivity analysis; GT, genotype; HCC, hepatocellular carcinoma; PegIFN, pegylated interferon; PSA, probabilistic sensitivity analysis; RBV, ribavirin; SFSO, Swiss Federal Statistical Office; SOF, Sofosbuvir; SVR, sustained virological response
*Obtained by converting mortality rates using the formulae probability = 1-exp(-rate*time)
Treatment duration, efficacy and safety were obtained from randomised and non-randomised phase II and phase III clinical trials as presented and referenced in Table 2. The percentages of patients and the time point at which these patients have discontinued treatment due to adverse events (AEs) or other reasons before the end of the planned treatment duration are provided in Table 2. Incidences of adverse events (AEs) such as anaemia, nausea, vomiting and rash are provided in S1 Table. Efficacy data were obtained from phase II trials if the corresponding phase III trials had missing data, e.g. did not report SVR rates according to cirrhosis status, or the treatment strategy did not match, e.g. SVR rates were reported for 12 instead of 24 weeks of treatment. There was one head-to-head trial available in genotypes 2 and 3 patients which compared a combination of sofosbuvir and ribavirin with a regimen consisting of pegylated interferon alpha and ribavirin [13]. Other efficacy and safety data were obtained from available randomised trials and single-arm studies with comparable patient characteristics.
Table 2. Genotype-specific parameters including treatment efficacy, duration and safety.
| Indication [Source] | SOF-based and comparator strategies | SVR | Discontinued# | Patients with adverse events | |||
|---|---|---|---|---|---|---|---|
| cirrhotic | Non-cirrhotic | Due to AEs | Other reasons | Any | Serious | ||
| GT1 TN IE [13,32,33] | SOF + PegIFN2a + RBV for 12 wks | 80.8% | 91.3% | 1.7% at 5.3 wks | 0.7% at 4.8 wks | 95% | 1% |
| PegIFN2a/2b + RBV for 48 wks | 23.6% | 43.6% | 7.0% at 24 wks | 24% at 24 wks | 96% | 1% | |
| TEL + PegIFN2a + RBV for 24/48 wks | 61.9% | 75.4% | NA | 23.3% at 18 wks | 100% | 21% | |
| BOC + PegIFN2b + RBV for 28/48 wks | 55.0% | 64.1% | NA | 26.1% at 24 wks | 99% | 13% | |
| GT1 TN II [34] | SOF + RBV for 24 wks | 53.3% | 68.3% | 1% at 12 wks | 0% | 89% | 0% |
| NT | 0% | 0% | 0% | 0% | 69% | 0% | |
| GT2 TN IE [13]* | SOF + RBV for 12 wks | 90.9% | 98.3% | 0% | 0% | 86% | 3% |
| PegIFN2a/2b + RBV for 24 wks | 61.5% | 81.5% | 12% at 14.9 wks | 6 | 96% | 1% | |
| GT2 TN II [12] | SOF + RBV for 12 wks | 93.3% | 91.8% | 1% at 0.9 wks | 1% at 1.3 wks | 89% | 5% |
| NT | 0% | 0% | 0% | 0% | 78% | 0% | |
| GT3 TN IE [13,16,35,36] | SOF + RBV for 24 wks | 92.3% | 93.5% | 0.4% at 21.5 wks | 1.2% at 21.5 wks | 92% | 4% |
| SOF + PegIFN2a + RBV for 12 wks | 83.3% | 100% | 0% | 0% | NA | 1% | |
| PegIFN2a/2b + RBV for 24 wks | 29.7% | 71.2% | 10.2% at 10.8 wks | 13.6% at 11.9 wks | 96% | 1% | |
| GT3 TN II [35] | SOF + RBV for 24 wks | 92.3% | 93.5% | 0.4% at 21.5 wks | 1.2% at 21.5 wks | 92% | 4% |
| NT | 0% | 0% | 0% | 0% | 71% | 0% | |
| GT3 TE IE [16,35,37,38] | SOF + RBV for 24 wks | 62% | 87% | 0.4% at 21.5 wks | 1.2% at 21.5 wks | 92% | 4% |
| SOF + PegIFN2a + RBV for 12 wks | 83.3% | 83.3% | 8% at 1 wk | 0% | 96% | 1% | |
| PegIFN2a/2b + RBV for 48 wks | 35% | 35% | 36.8% at 24 wks | 0% | 100% | 1% | |
| GT3 TE II [35] | SOF + RBV for 24 wks | 60% | 85% | 0.4% at 21.5 wks | 1.2% at 21.5 wks | 92% | 4% |
| NT | 0% | 0% | 0% | 0% | 71% | 0% | |
| GT4 TN [13,39] | SOF + PegIFN2a + RBV for 12 wks | 50% | 100% | 0% | 0% | 95% | 2% |
| PegIFN2a/2b + RBV for 48 wks | 38.6% | 50% | 14% at 24 wks | 26% at 24 wks | 96% | 1% | |
BOC, boceprevir; GT, genotype; IE, interferon eligible; II, interferon ineligible; NA, not available; PegIFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; TE, treatment-experienced; TEL, telaprevir; TN, treatment-naïve
* head-to-head comparison trial
# the percentages of patients and the time point at which these patients have discontinued treatment due to adverse events or other reasons before the end of the planned treatment duration
Quality of life weights (utilities) for the health states represented in the model, based on the European quality of life questionnaire (EQ-5D), were obtained from published literature (Table 1). For patients on treatment with sofosbuvir or a comparator, a utility decrement obtained in a sofosbuvir trial based on the short-form health survey (SF-6D) was applied [13]. When non-cirrhotic or cirrhotic patients reached SVR after treatment, utilities obtained in a UK randomised controlled trial of interferon and ribavirin based on the EQ-5D [29] were added to the utility of the corresponding health state (Table 1).
Resource use parameters such as tests and procedures related to CHC diagnosis and treatment were obtained from EASL Clinical Practice Guidelines [8,20] (S2 Table). Swiss clinical experts checked whether they reflected Swiss clinical practice. Experts were selected to represent all Swiss regions and university hospitals. In Swiss regions without a university hospital, clinical experts from hospitals experienced in the treatment of HCV patients were included.
For every resource use parameter, we obtained unit costs, if possible, using published Swiss sources or adjusting foreign sources to reflect Swiss practice. Drug unit costs for HCV treatment and treatment-related AEs were derived from the list of pharmaceutical specialties of the Swiss Federal Office of Public Health [40]. AE management costs were obtained by multiplying incidences of AEs such as anaemia, nausea, vomiting and rash with drug unit costs for their treatment. Unit costs for monitoring were obtained from Swiss tariff lists [41] or the analyses list of the Swiss Federal Office of Public Health [42]. If not otherwise indicated, unit costs were for the year 2014. Health state costs for the year 2012 were collected at the Gastroenterology and Hepatology clinic, University Hospital of Zurich. They thus represent a single centre experience. All cost parameters are shown in Table 3.
Table 3. Cost parameters and their ranges used in the deterministic and probabilistic sensitivity analyses.
| Item | Source | Base-case (CHF) | Sensitivity analysis | |||
|---|---|---|---|---|---|---|
| Treatment-related drug unit costs | Drug | Cost/pack or injection | Cost/unit (CHF) | PSA distribution and parameters (DSA ranges) | PSA distribution and parameters (DSA ranges) | |
| Sofosbuvir, 28x400mg | 19208.50 | FOPH | 1.72 | Uniform, α = 14406 β = 24011 | Uniform, α = 14406 β = 24011 | |
| Ribavirin, 56x400mg | 738.25 | FOPH | 0.03 | Uniform, α = 664 β = 812 | Uniform, α = 664 β = 812 | |
| Peg. Interferon 2a, 180 μg | 278.31 | FOPH | 1.55 | Uniform, α = 250 β = 306 | Uniform, α = 250 β = 306 | |
| Peg. Interferon 2b, 120 μg | 314.84 | FOPH | 2.62 | Uniform, α = 283 β = 346 | Uniform, α = 283 β = 346 | |
| Telaprevir, 42x375mg | 2948.70 | FOPH | 0.019 | Uniform, α = 2654 β = 3244 | Uniform, α = 2654 β = 3244 | |
| Boceprevir, 336x200mg | 4163.75 | FOPH | 0.06 | Uniform, α = 3747 β = 4580 | Uniform, α = 3747 β = 4580 | |
| Treatment-related adverse event costs | Item | Dosage | Source | Unit cost | Lower -25% | Upper +25% |
| AE costs | [43]* | 8938.40 | 6703.80 | 11173.00 | ||
| Nausea: 4 wks Metoclopramide | 30mg/day | FOPH | 0.019 | - | - | |
| Vomiting: 4 wks Metoclopramide | 30mg/day | FOPH | 0.019 | - | - | |
| Diarrhoea: 4.3 wks Loperamide | 2mg/day | FOPH | 0.241 | - | - | |
| Pruritus: 4wks Piriton | 16mg/day | FOPH | 0.200 | - | - | |
| Anaemia: 4 wks Binocrit | 40,000/ wk | FOPH | 0.010 | - | - | |
| Anaemia: blood transfusion | FOPH | 3,325.00 | - | - | ||
| Rash: 4 wks Hydrocortisone | 1% 15g | FOPH | 7.90 | - | - | |
| Thrombocytopenia: 4 wks Revolade | 50mg/day | FOPH | 1.94 | - | - | |
| Neutropenia: 2 wks Filgrastim | 5 μg/kg/ day | FOPH | 0.999 | - | - | |
| Depression: 4 wks Citalopram | 20mg/day | FOPH | 0.064 | - | - | |
| Treatment-related monitoring unit costs | Item | Source | Unit cost | - | - | |
| Nurse | 1 hour | Spitex | 79.80 | - | - | |
| Physician | 1 hour | Tarmed | 181.77 | - | - | |
| Inpatient care | 1 hour | Tarmed | 23.71 | - | - | |
| HCV screen (RNA), viral load, genotype, SVR test, HIV RNA | FOPH | 180.00 | - | - | ||
| HBV, Anti-HIV | FOPH | 20.00 | - | - | ||
| Liver function test | FOPH | 5.00 | - | - | ||
| Alfa-fetoprotein | FOPH | 19.30 | - | - | ||
| Alfa-antitrypsin | FOPH | 23.00 | - | - | ||
| Thyrotrophic, Free T4 | FOPH | 9.00 | - | - | ||
| Caeruloplasmin | FOPH | 19.90 | - | - | ||
| Iron | FOPH | 2.80 | - | - | ||
| Urea and electrolytes | FOPH | 11.20 | - | - | ||
| Glucose, Alanine aminotransferase | FOPH | 2.50 | - | - | ||
| Pregnancy test | FOPH | 12.00 | - | - | ||
| Thyroid function tests | FOPH | 18.00 | - | - | ||
| Full blood count, blood clotting factors | FOPH | 4.20 | - | - | ||
| Ferritin | FOPH | 7.90 | - | - | ||
| Blood group | FOPH | 17.10 | - | - | ||
| Autoantibodies | FOPH | 37.00 | - | - | ||
| Immunoglobulins | FOPH | 6.20 | - | - | ||
| Ultrasound scan of liver | Tarmed | 113.69 | - | - | ||
| Chest X-ray | Tarmed | 75.62 | - | - | ||
| Ultrasound guided biopsy | Tarmed | 179.05 | - | - | ||
| Ultrasound of liver. Fibroscan | Tarmed | 77.77 | - | - | ||
| Electrocardiography | Tarmed | 55.67 | - | - | ||
| Magnetic resonance imaging of liver | Tarmed | 390.14 | - | - | ||
| Pulmonary function test | Tarmed | 62.15 | - | - | ||
| Liver biopsy | Tarmed | 255.95 | - | - | ||
| Fibrotest | FOPH | 69.10 | - | - | ||
| Endoscopy diagnosis | Tarmed | 337.90 | - | - | ||
| Health state costs | Item | Source | Base-case (CHF) | DSA ranges (CHF) | PSA distribution and parameters | |
| Non-cirrhotic | Calc.# | 479 | 64–1301 | Gamma, α = 2 β = 224 | ||
| Non-cirrhotic, mild | Expert opinion§ | 283 | - | - | ||
| Non-cirrhotic, moderate | Expert opinion§ | 1138 | - | - | ||
| Non-cirrhotic, SVR | Calc.# | 366 | 275–458 | Gamma, α = 8 β = 47 | ||
| Non-cirrhotic, mild, SVR | [31]& | 348 | - | - | ||
| Non-cirrhotic, moderate, SVR | [31]& | 426 | - | - | ||
| Compensated cirrhosis | Expert opinion§ | 2,715 | 1,357–4,535 | Gamma, α = 11 β = 246 | ||
| Compensated cirrhosis, SVR | [31]& | 754 | 282–779 | Gamma, α = 5 β = 161 | ||
| Decompensated cirrhosis | Expert opinion§ | 20,347 | 16,561–24,517 | Gamma, α = 13 β = 1510 | ||
| Hepatocellular carcinoma | Expert opinion§ | 16,944 | 6,163–33,082 | Gamma, α = 6 β = 2865 | ||
| Liver transplant | Expert opinion§ | 125,102 | 93,827–156,378 | - | ||
| Post-liver transplant | Expert opinion§ | 19,323 | 14,492–24,154 | Gamma, α = 4 β = 4471 | ||
AE, adverse event; Calc., calculation; FOPH, Federal Office of Public Health; GP, general practitioner; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; mg, milligram; Peg., pegylated; wks, weeks; RNA, Ribonucleic acid; SOF, Sofosbuvir; SVR, sustained virological response; TE, treatment-experienced; wks, weeks
# weighted average: 77% mild, 23% moderate
§ provided by one of the authors (BM), Gastroenterology and Hepatology clinic, University Hospital of Zurich
& inflated by 1.14 to £ 2012 and converted to Swiss Francs (exchange rate 1.52)
Subgroup analyses
In subgroup analyses, the cost-effectiveness of sofosbuvir was calculated separately for non-cirrhotic (fibrosis stage 0–3) and cirrhotic patients (fibrosis stage 4) based on the METAVIR classification [23].
Sensitivity analyses
We performed extensive deterministic (DSA) and probabilistic sensitivity analysis (PSA) to explore the impact of parameter uncertainty on the cost-effectiveness results. The ranges of variation for DSA and the distributional assumptions and parameters used for PSA are listed in Tables 1 and 3. SVR rates were varied by ±25% of the base-case SVR rate.
To gain a better understanding of the impact of AEs on the cost-effectiveness of sofosbuvir-based strategies versus current standard of care, we performed a scenario analysis including total cost of AE management. The incidence of severe AEs was obtained from published clinical trials (Table 2). Costs of AE management were obtained from Bichoupan et al. [43]. The authors reported the total treatment costs for AE management in CHC patients treated with a combination of telaprevir, pegylated interferon alpha and ribavirin in the USA. We assumed that 80% of these total treatment costs were due to severe AEs. This cost parameter was adjusted for differences in the amount of services used, approximated by health care expenditure per head of the population [46]. Foreign prices were additionally adjusted using purchasing power parities [45]; prices were further adjusted to the increase in health expenditure using national statistical data [44].
Results
Base-case analysis
In the base-case analysis including a mixed population of about 25% cirrhotic and 75% non-cirrhotic CHC patients as seen in the SCCS, the incremental cost-effectiveness of therapy with sofosbuvir depended on genotype, treatment history, and interferon tolerability (Table 4). Sofosbuvir-containing treatment regimens in patients with CHC genotype 1–4 led to base-case ICERs between CHF 10,337 and CHF 91,570 per QALY gained. ICERs are below CHF 50,000 per QALY in treatment-naïve interferon eligible genotype 1 patients and in treatment-naïve interferon ineligible genotype 2 and 3 patients.
Table 4. Summary of base-case, sensitivity and subgroup analysis results.
| Indication | Treatment and comparator strategies | Base-case (21–25% cirrhotic*) ICERs | DSA ranges | PSA | Subgroup (100% cirrhotic) ICERs | Subgroup (100% non-cirrhotic) ICERs |
|---|---|---|---|---|---|---|
| GT1 TN IE | SOF + PegIFN2a + RBV for 12 wks vs. PegIFN2a/2b + RBV for 48 wks | 19.474 | 6,339–31,668 | 100% of ICERs<100,000 | 1,565 | 36,501 |
| SOF + PegIFN2a + RBV for 12 wks vs. TEL + PegIFN2a + RBV for 24/48 wks | 10,337 | SOF dominant-44,639 | 98.2% of ICERs<100,000 | SOF dominant | 28,608 | |
| SOF + PegIFN2a + RBV for 12 wks vs. BOC + PegIFN2b + RBV for 28/48 wks | 13,276 | SOF dominant-35,975 | 100% of ICERs<100,000 | 963 | 26,579 | |
| GT1 TN II | SOF + RBV for 24 wks vs. NT | 86,648 | 42,713–148,297 | 50.8% of ICERs<100,000 | 75,799 | 95,741 |
| GT2 TN IE | SOF + RBV for 12 wks vs. PegIFN2a/2b + RBV for 24 wks | 76,526 | 40,468–138,263 | 58.7% of ICERs<100,000 | 35,302 | 115,138 |
| GT2 TN II | SOF + RBV for 12 wks vs. NT | 10,471 | 1,080–19,273 | 100% of ICERs<100,000 | SOF dominant | 17,808 |
| GT3 TN IE | SOF + RBV for 24 wks vs. PegIFN2a/2b + RBV for 24 wks | 91,570 | 47,672–130,036 | 48.9% of ICERs<100,000 | 28,384 | 189,063 |
| SOF + PegIFN2a + RBV for 12 wks vs. PegIFN2a/2b + RBV for 24 wks | 38,512 | 17,109–57,694 | 97.4% of ICERs<100,000 | 8,491 | 74,341 | |
| GT3 TN II | SOF + RBV for 24 wks vs. NT | 34,826 | 14,649–53,365 | 99.5% of ICERs<100,000 | 17,275 | 46,900 |
| GT3 TE IE | SOF + RBV for 24 wks vs. PegIFN2a/2b + RBV for 48 wks | 74,805 | 38,237–112,762 | 65.8% of ICERs<100,000 | 90,653 | 76,064 |
| SOF + PegIFN2a + RBV for 12 wks vs. PegIFN2a/2b + RBV for 48 wks | 16,235 | 4,314–26,946 | 99.7% of ICERs<100,000 | 4,382 | 24,957 | |
| GT3 TE II | SOF + RBV for 24 wks vs. NT | 45,935 | 20,783–68,920 | 96.6% of ICERs<100,000 | 36,189 | 52,720 |
| GT4 TN | SOF + PegIFN2a + RBV for 12 wks vs. PegIFN2a/2b + RBV for 48 wks | 36,108 | 17,738–87,678 | 83.9% of ICERs<100,000 | 89,131 | 33,054 |
BOC, boceprevir; DSA, deterministic sensitivity analysis; GT, genotype; ICER, incremental cost-effectiveness ratio; IE, interferon eligible; II, interferon ineligible; PegIFN, pegylated interferon; PSA, probabilistic sensitivity analysis; RBV, ribavirin; SOF, sofosbuvir; TE, treatment-experienced; TEL, telaprevir; TN, treatment-naïve; wks, weeks
* percentage of cirrhotic patients depends on genotype: 24% in genotype 1, 21% in genotype 2, 25% in genotype 3 and 22% in genotype 4
ICERs for treatment-naïve genotype 1 and 4 patients receiving sofosbuvir, pegylated interferon alpha and ribavirin for 12 weeks ranged from CHF 10,337 to CHF 36,108 per QALY. The lowest ICERs for genotype 1 were obtained against telaprevir-containing regimens (CHF 10,337 per QALY) and boceprevir-containing regimens (CHF 13,276 per QALY). Underlying drug cost differences were smaller in these cases than in comparisons of sofosbuvir, pegylated interferon alpha and ribavirin with pegylated interferon alpha and ribavirin alone (Table 5). In treatment-naïve, interferon-ineligible genotype 1 patients, the treatment-related and total costs for a sofosbuvir-based treatment compared to no treatment were higher, as reflected in a higher ICER (CHF 86,648 per QALY).
Table 5. Cost (CHF) per patient and cost-effectiveness results in genotype 1.
| Indication | Parameter | Intervention | Comparator | Δ | Comparator | Δ | Comparator | Δ | Comparator | Δ |
|---|---|---|---|---|---|---|---|---|---|---|
| GT1 TN IE | SOF+PaR 12 wks | PaR 48 wks | PbR 48 wks | TEL+PaR 48 wks | BOC+PbR 48 wks | |||||
| Drug costs | 62,862 | 20,454 | 42,408 | 20,875 | 41,987 | 49,333 | 13,529 | 45,135 | 17,727 | |
| AE costs | 15 | 2,064 | -2,049 | 2,064 | -2,049 | 3,086 | -3,071 | 56 | -41 | |
| Monitoring costs | 3,279 | 4,650 | -1,371 | 4,650 | -1,371 | 4,115 | -836 | 4,404 | -1,125 | |
| Total treatment costs | 66,156 | 27,168 | 38,988 | 27,589 | 38,567 | 56,534 | 9,622 | 49,595 | 16,561 | |
| Health state costs | 8,646 | 24,403 | -15,757 | 24,398 | -15,752 | 13,986 | -5,340 | 16,897 | -8,251 | |
| Total cost per patient | 74,802 | 51,571 | 23,231 | 51,987 | 22,815 | 70,520 | 4,282 | 66,492 | 8,310 | |
| QALYs per patient* | 13.3 | 12.1 | 1.2 | 12.1 | 1.2 | 12.9 | 0.4 | 12.7 | 0.6 | |
| GT1 TN II | SOF+RBV 24 wks | NT | Δ | |||||||
| Drug costs | 120,184 | 0 | 120,184 | |||||||
| AE costs | 40 | 0 | 40 | |||||||
| Monitoring costs | 3,502 | 1,472 | 2,030 | |||||||
| Total treatment costs | 123,726 | 1,472 | 122,254 | |||||||
| Health state costs | 19,070 | 35,964 | -16,894 | |||||||
| Total cost per patient | 142,796 | 37,436 | 105,360 | |||||||
| QALYs per patient* | 12.6 | 11.4 | 1.2 |
AE, adverse event; BOC, boceprevir; GT, genotype; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon alpha+ribavirin; PbR, pegylated interferon beta+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TEL, telaprevir; TN, treatment-naïve
* values are rounded to one decimal place
In genotype 2, treatment-naïve patients we observed a substantial difference in the ICERs (Table 4) for interferon-eligible and interferon-ineligible patients (CHF 76,526 vs. CHF 10,471 per QALY). With a sofosbuvir-based treatment, interferon-ineligible patients gained 2.6 QALYs compared to no treatment, whereas interferon-eligible patients gained 0.6 QALYs compared to treatment with pegylated interferon alpha and ribavirin (S3 Table).
In genotype 3, ICERs of interferon-ineligible patients were in the same range for treatment-naïve (CHF 34,826 per QALY) and treatment-experienced (CHF 45,935 per QALY) patients (Table 4). The limited difference in ICERs was due to moderately lower QALY gains in treatment-experienced patients (2.1 vs. 2.5 QALY). We also noted a large difference in the cost per QALY gained of two different sofosbuvir-containing regimens, versus the same comparator, for the treatment of genotype 3, interferon-eligible patients (S4 Table). A treatment strategy of sofosbuvir in combination with pegylated interferon alpha and ribavirin for 12 weeks was clearly more cost effective than a 24 week-alternative without interferon in treatment-experienced (ICERs CHF 16,235 per QALY vs. CHF 74,805 per QALY, respectively) and treatment-naïve patients (ICERs CHF 38,512 per QALY vs. CHF 91,570 per QALY) (Table 4).
Total and disaggregated costs and QALYs gained per patient are presented in Table 5 for genotype 1. Cost and QALY results for the other HCV genotypes are presented in S3–S5 Tables. In genotype 1 treatment-naïve patients, drug costs for sofosbuvir-containing regimens were substantially higher than drug costs for comparator strategies (e.g. CHF 62,862 for sofosbuvir+pegylated interferon alpha+ribavirin vs. CHF 20,454 for pegylated interferon alpha+ribavirin). Monitoring costs were generally lower for sofosbuvir-containing regimens than comparator strategies (CHF 3,279 for sofosbuvir+ pegylated interferon alpha+ribavirin vs. CHF 4,650 for pegylated interferon alpha+ribavirin, respectively), except when the comparator strategy was no treatment. Health state costs were usually higher in comparator strategies than sofosbuvir-containing regimens (e.g. CHF 24,403 for pegylated interferon alpha+ribavirin vs. CHF 8,646 for sofosbuvir+ pegylated interferon alpha+ribavirin). QALYs per patient were always higher with treatment regimens including sofosbuvir, by 0.4 to 2.6 QALYs.
Subgroup analysis
Subgroup-analyses were performed for cirrhotic and non-cirrhotic patients separately. Generally, costs per QALY gained were lower in cirrhotic patients than in non-cirrhotic patients (Table 4). In genotype 1 treatment-naïve, interferon-eligible patients with cirrhosis, sofosbuvir dominated (was clinically more advantageous and less expensive than) telaprevir- and boceprevir-containing treatment regimens. The cost per QALY was more than CHF 100,000 in genotypes 2 and 3 treatment-naïve, interferon-eligible, non-cirrhotic patients.
Sensitivity analyses
The majority of the results of the DSA and PSA were robust and remained below CHF 100,000 per QALY gained (Table 4). Discount rates, the utility gain after treatment, and differences in SVR probabilities (SVR rates were varied by either ±20% of the base-case SVR rate or by estimating 95% confidence intervals assuming a beta distribution) were the most influential parameters. When we took into account an approximated total cost for severe AE management, ICERs of sofosbuvir-based treatment versus current standard care generally changed by less than 5% from the base-case ICERs. In genotype 1, treatment-naïve, interferon-eligible patients, AE management costs per patient for telaprevir-containing regimens (CHF 1,877) and boceprevir-containing regimens (CHF 1,162) were higher than AE management costs per patient for sofosbuvir-based treatment (CHF 89). Hence, the corresponding ICERs of sofosbuvir-based treatment versus telaprevir-containing regimens (CHF 13,425 per QALY) and boceprevir-containing regimens (CHF 11,633 per QALY) increased by 30% and decreased by 12% from base-case, respectively. The ICER for telaprevir-containing regimens increased, because the incidence of severe AEs was lower than the cumulative incidence of AEs such as anaemia, nausea, vomiting and rash used in the base-case analysis.
Discussion
Although the initial costs of sofosbuvir-containing regimens were high, AE costs, monitoring costs and health state costs were generally lower than in active comparator strategies. Additionally, QALYs gained per patient were consistently higher in sofosbuvir-containing regimens. In the base-case representing a mixed cirrhotic and non-cirrhotic cohort, ICERs for sofosbuvir-containing treatment regimens were better than CHF 100,000 per QALY, for all comparisons. In the majority of sensitivity analyses, results were robust. ICERs of sofosbuvir-based regimens were also lower than CHF 100,000 per QALY in cirrhotic patients, but were above CHF 100,000 per QALY for treatment-naïve, interferon eligible genotype 2 and 3 non-cirrhotic patients.
SVR, the goal of antiviral therapy, can be achieved with a shorter duration of sofosbuvir-containing treatment than with other treatment regimens. For patients who are ineligible, intolerant or unwilling to take interferon-based regimens, the combination of sofosbuvir and ribavirin addresses an unmet medical need. There has been a shift in European and American guidelines in the way that EASL [20] and the American Association for the Study of Liver Diseases (AASLD/IDSA/IAS-USA) [47] no longer recommend treatment with telaprevir or boceprevir in genotype 1 patients. Given the recent marketing authorisation of a combination pill of sofosbuvir and ledipasvir for the treatment of HCV genotype 1 patients in October 2014 by the Food and Drug Administration [48] and more recently by the European Medicines Agency [49], further guideline adaptations may follow.
In our modelling study, sofosbuvir-containing treatment regimens compared to standard regimens led to gains in QALYs. This is in contrast to the German Institute for Quality and Efficiency in Health Care's (IQWiG) conclusion [50] that “there is no data documenting an additional benefit of sofosbuvir-containing regimens compared to other treatment regimens because no appropriate analyses were provided by the pharmaceutical company”. IQWiG’s position was partially due to a lack of head-to-head trials and the use of mixed treatment comparisons. Heterogeneity between patient characteristics may limit the validity of mixed treatment comparisons [51]. As patient characteristics such as age and genotype were comparable between studies, we regard mixed treatment comparisons as an acceptable approach to integrate available evidence, in the present case.
Leleu et al. published a cost-effectiveness study of sofosbuvir in mono-infected and co-infected CHC patients in France [52]. The resulting ICERs were better than in our study. Leleu et al. used a lower utility decrement for patients during treatment with sofosbuvir than for patients during treatment with comparators [52]. We used the same utility decrement for all treatments. In the French study, the percentages of cirrhotic patients were up to twice as high as in the base-case of our study. We showed that ICERs for cirrhotic patients were better than ICERs for non-cirrhotic patients. Hence, these differences in model input parameters may explain the variation in the magnitude of the ICERs. Another cost-effectiveness study in France by Deuffic-Burban et al. [53] compared sofosbuvir-containing treatment regimens and standard treatments in genotype 1 treatment-naïve CHC patients. The authors concluded that treatment with sofosbuvir is cost-effective in CHC patients with fibrosis stage 2 or higher [53]. An Italian cost-effectiveness study by Petta et al. [54] compared triple therapies with sofosbuvir, boceprevir, and telaprevir. Sofosbuvir-based regimens were cost-effective compared to boceprevir, except in cirrhotic and IL28B CC patients, and mostly cost-effective compared to telaprevir. A third cost-effectiveness study in the US concluded that sofosbuvir-based treatment regimens generally dominated telaprevir or boceprevir-based regimens [55]. The authors of a Spanish cost-effectiveness study reported that treatment regimens with sofosbuvir, pegylated interferon alpha and ribavirin for 12 weeks were below the considered cost-effectiveness threshold [56]. Overall, the results of the international cost-effectiveness studies were similar to the present analysis. However, transferability of cost-effectiveness results between different countries is limited due to e.g. differences in epidemiology of the disease, clinical practice, consumer preferences and price levels [57].
In Switzerland, there is no formally accepted cost-effectiveness threshold. In 2010, the Swiss Federal Court of Justice decided that treating a very rare orphan disease at CHF 500,000 per year was too expensive for the compulsory health insurance to cover, given its small health benefits [58]. The Court further stated that beyond a threshold of CHF 100,000 per QALY, health insurers cannot be obliged to pay for a treatment [58]. This was the first time that a formal cost-effectiveness threshold has been suggested for Switzerland to distinguish favourable from unfavourable ICERs. On this basis, sofosbuvir-containing regimens would be cost-effective compared to standard treatment. However, this remains arguable and there will be further discussion, also given widely varying approaches in other countries. NICE's acceptable cost-effectiveness thresholds for the UK are in the range of GBP 20,000 to 30,000 per QALY gained and are intended to represent the opportunity cost to a fixed National Health Service budget [59]. The cost-effectiveness threshold commonly adopted in studies for the US varies between USD 50,000 to 100,000 per QALY [60]. The World Health Organization (WHO) suggests cost-effectiveness thresholds between 2–3 times the gross domestic product (GDP) per person [61]. The Swiss GDP per person was CHF 74,010 in 2012 [62]. According to the threshold suggested by WHO, all results including subgroup analyses would be cost-effective.
An additional point of discussion relates to the fact that thresholds representing the societal willingness to pay for any intervention leading to health gains may vary depending on the context. This was pointed out in a recent article that emphasized how the cost-effectiveness of sofosbuvir may dramatically clash with its affordability if the treatment is to be implemented on a large scale [63]. Not surprisingly, economic studies consider ICERs in distinct subgroups of patients. This appears palatable in those patients in whom the benefit is clear cut, i.e. patients with advanced liver fibrosis at high risk of complications, while it becomes increasingly difficult to accept by payers for patients in whom the life-long risk of complications is relatively small, i.e. patients with no or minimal fibrosis. Those patients are significantly less likely to die of HCV-related consequences and treatment-induced viral clearance may lead to less clear health gains.
Our study has several strengths and limitations relating to the decision-analytic model’s structural assumptions and available input data. Patient characteristics were obtained from the SCCS [19]. Swiss patient characteristics are thus reflected and the local applicability of the results is improved. The validity of the model was assessed by scrutinizing outputs for internal consistency. Model elements and formulae were thoroughly checked for correctness. We also compared the structure with that of other models in the field, which supported the approaches taken.
Clinical input parameters such as transition probabilities and utilities were derived from international literature. Transition probabilities reflect the biological course of CHC and mainly depend on disease characteristics and treatment. European countries with similar treatment options available should have comparable transition probabilities. The utilities used in our model were mainly based on UK studies [21,22,29]. Although the EQ-5D is a generic instrument to measure quality of life, the value sets applied to obtain utilities differ between countries [64]. The values available to us were based on the UK value set. There is no Swiss-specific value set, but a recent study reported that the UK value set generates lower utilities than the German or French value set [65]. Because of the small difference, we have no reason to assume that this had a substantial impact on our results, at least not in the sense of favouring sofosbuvir. For sofosbuvir and alternative treatment regimens we used the same utilities according to health state because treatment-specific utilities were not available. Given the lifelong time horizon of the model, this should not substantially impact the findings. Treatment characteristics and medical resource use based on clinical trial data, international literature and European guidelines were checked by Swiss clinical experts and generally found to be applicable to the Swiss situation. A small number of assumptions were, however, modified. For example, all HCV patients in Switzerland are tested for hepatitis B virus infection while there is no cryoglobulin test performed.
To the extent feasible, the model was populated with Swiss input parameters for patient characteristics, health state costs and other unit costs. This implied some uncertainties. For the patient characteristics implemented in the model, we used data from the SCCS, which reflects local patient characteristics and medical practice. However, patients in the clinical trials of sofosbuvir were younger than patients in the SCCS. If relevant SVR effects, adverse event rates, etc. were age-dependent, this age difference could distort the study results. In addition, we received health state costs from one big university hospital in Switzerland, which were based on a data collection of one year. These costs could differ from those accrued in other settings in Switzerland, which might lead to different results. Despite these aspects and because the model structure, underlying assumptions and model parameters were reviewed for appropriateness by Swiss clinical experts, we believe to have achieved reasonable estimates of the cost effectiveness of CHC treatment with sofosbuvir in Switzerland.
In the base-case analysis, we only included drug costs for the treatment of AEs due to a lack of Swiss specific data. In this respect, this modelling study may provide a conservative estimate of the cost-effectiveness of sofosbuvir-based HCV treatment regimens. In a scenario analysis, total costs of severe AE management were obtained from a US study [43] and adjusted to Swiss costs to the extent possible. ICERs in this scenario analysis changed by less than 5% in comparison with the base case, except for telaprevir-containing and boceprevir-containing regimens. Given differences in regimen-specific AE profiles, data allowing for the inclusion of total AE management costs (i.e. not only drug costs to treat AEs) might improve ICERs of sofosbuvir treatment.
Given the model structure and underlying model input parameters (mild and moderate HCV states (fibrosis stage F0-F3) were combined to a non-cirrhotic state to reflect available data from clinical trials), it was not possible to calculate ICERs for sofosbuvir-based regimens compared to standard treatment according to fibrosis stage. Therefore, the model is not able to reflect the current Swiss treatment limitations. In some cases, SVR rates were obtained from phase II trials due to missing or inapplicable data from phase III trials. Nevertheless, SVR rates from phase II and III trials were consistent. The cost-effectiveness for genotype 1 treatment-experienced could not be assessed due to lack of clinical data. As there are no data available regarding the reversibility of complications after treatment and any assumption made would add uncertainty to the model, we did not consider this and assumed a similar post-treatment clinical course independent from the type of treatment used. Clinical efficacy data were derived from registration trials and may not fully reflect effectiveness in routine clinical practice due to compliance and adherence issues, which limits the generalisability of the results. Finally, the analysis was conducted from the health care system perspective. A broader societal perspective including indirect costs (e.g. due to loss of productivity, absence from work) would provide additional insights into the implications of sofosbuvir-based therapy.
Several authors have criticised the high price of sofosbuvir [66–68]. Given a relevant pool of prevalent, previously untreated HCV cases, budget impact remains an issue among payers and policy makers, especially due to the high up-front costs. Swiss regulators restricted the reimbursement of sofosbuvir-containing treatment to patients with fibrosis stage 3 or 4, or symptomatic patients with extra-hepatic manifestations [17], which reduces initial budget impact. At the same time, our findings for Switzerland generally indicate acceptable ICERs for sofosbuvir-containing regimens, which may initiate further discussion on reimbursement issues. Our results further provide a benchmark for future treatment regimens in patients with CHC that are expected to become available in the near future.
Supporting Information
BOC, boceprevir; GT, genotype; IE, interferon eligible; II, interferon ineligible; NA, not available; PegIFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; TE, treatment-experienced; TEL, telaprevir; TN, treatment-naïve; # 1% erythropoietin and 0.7% blood transfusions; § equally distributed between erythropoietin and blood transfusions.
(PDF)
BOC, boceprevir; C, cirrhotic; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; NC, non-cirrhotic; PegIFN, pegylated interferon; RBV, ribavirin; RNA, ribonucleic acid; SOF, sofosbuvir; SVR, sustained virological response; TEL, telaprevir; *resource use was checked by Swiss clinical experts; 1 stands for 100% of the patients.
(PDF)
AE, adverse event; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon 2a+ribavirin; PbR, pegylated interferon 2b+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TN, treatment-naïve; * values are rounded to one decimal place.
(PDF)
AE, adverse event; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon 2a+ribavirin; PbR, pegylated interferon 2b+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TE, treatment-experienced; TN, treatment-naïve; * values are rounded to one decimal place.
(PDF)
AE, adverse event; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon 2a+ribavirin; PbR, pegylated interferon 2b+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TE, treatment-experienced; TN, treatment-naïve; * values are rounded to one decimal place.
(PDF)
Acknowledgments
We thank Dr. David Semela, Dr. Philip Bruggmann and Dr. Marcel Stöckle for reviewing the model input parameters.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by Gilead Switzerland. The sponsor participated, but did not make final decisions regarding the design, conduct or reporting of the study and had no role in data collection and analysis, decision to publish, or preparation of the manuscript. Helsana Group and OPTUMInsight provided support in the form of salaries for the authors OR, IMG, and SC, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the "author contributions" section.
References
- 1. Kim CW, Chang KM (2013) Hepatitis C virus: virology and life cycle. Clin Mol Hepatol 19: 17–25. 10.3350/cmh.2013.19.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, et al. (2011) A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int 31 Suppl 2: 30–60. 10.1111/j.1478-3231.2011.02539.x [DOI] [PubMed] [Google Scholar]
- 3. Pol S, Vallet-Pichard A, Corouge M, Mallet VO (2012) Hepatitis C: epidemiology, diagnosis, natural history and therapy. Contrib Nephrol 176: 1–9. 10.1159/000332374 [DOI] [PubMed] [Google Scholar]
- 4. Tran TT (2012) Overview of epidemiology, diagnosis, and disease progression associated with hepatitis C. Am J Manag Care 18: S335–339. [PubMed] [Google Scholar]
- 5. Papatheodoridis G, Hatzakis A (2012) Public health issues of hepatitis C virus infection. Best Pract Res Clin Gastroenterol 26: 371–380. 10.1016/j.bpg.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 6. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. (2014) Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370: 1483–1493. 10.1056/NEJMoa1316366 [DOI] [PubMed] [Google Scholar]
- 7. Martinot-Peignoux M, Stern C, Maylin S, Ripault MP, Boyer N, Leclere L, et al. (2010) Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology 51: 1122–1126. 10.1002/hep.23444 [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver (2014) EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 60: 392–420. 10.1016/j.jhep.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 9. Casey LC, Lee WM (2013) Hepatitis C virus therapy update 2013. Curr Opin Gastroenterol 29: 243–249. 10.1097/MOG.0b013e32835ff972 [DOI] [PubMed] [Google Scholar]
- 10.Feld JJ (2014) The beginning of the end: What is the future of interferon therapy for chronic hepatitis C? Antiviral Res. [DOI] [PubMed]
- 11. Umar M, Khan AG, Abbas Z, Arora S, Asifabbas N, Elewaut A, et al. (2014) World gastroenterology organisation global guidelines: diagnosis, management and prevention of hepatitis C april 2013. J Clin Gastroenterol 48: 204–217. 10.1097/MCG.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 12. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. (2013) Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 368: 1867–1877. 10.1056/NEJMoa1214854 [DOI] [PubMed] [Google Scholar]
- 13. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. (2013) Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368: 1878–1887. 10.1056/NEJMoa1214853 [DOI] [PubMed] [Google Scholar]
- 14. Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. (2013) Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA 310: 804–811. 10.1001/jama.2013.109309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. (2013) Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 13: 401–408. 10.1016/S1473-3099(13)70033-1 [DOI] [PubMed] [Google Scholar]
- 16.Lawitz E, Poordad F, Brainard D. Sofosbuvir in combination with PegIFN and ribavirin for 12 weeks provides high SVR rates in HCV-infected genotype 2 or 3 treatment experienced patients with and without compensated cirrhosis: results from the LONESTAR-2 study.; 2013 1–5 November 2013; Washington, DC.
- 17.Swissmedic (2014) Sovaldi.
- 18. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. (2013) Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 346: f1049 10.1136/bmj.f1049 [DOI] [PubMed] [Google Scholar]
- 19. Prasad L, Spicher VM, Zwahlen M, Rickenbach M, Helbling B, Negro F (2007) Cohort Profile: the Swiss Hepatitis C Cohort Study (SCCS). Int J Epidemiol 36: 731–737. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver (2014) EASL Recommendations on Treatment of Hepatitis C 2014. [DOI] [PubMed]
- 21. Hartwell D, Jones J, Baxter L, Shepherd J (2011) Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess 15: i–xii, 1–210 10.3310/hta15440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N (2007) Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 11: 1–205, iii. [DOI] [PubMed] [Google Scholar]
- 23. Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24: 289–293. [DOI] [PubMed] [Google Scholar]
- 24. Swiss Federal Statistical Office (2005) Sterbetafeln für die Schweiz 1998/2003 BAG. [Google Scholar]
- 25. Schad M, John J (2012) Towards a social discount rate for the economic evaluation of health technologies in Germany: an exploratory analysis. Eur J Health Econ 13: 127–144. 10.1007/s10198-010-0292-9 [DOI] [PubMed] [Google Scholar]
- 26. Dedes KJ, Matter-Walstra K, Schwenkglenks M, Pestalozzi BC, Fink D, Brauchli P, et al. (2009) Bevacizumab in combination with paclitaxel for HER-2 negative metastatic breast cancer: an economic evaluation. Eur J Cancer 45: 1397–1406. 10.1016/j.ejca.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 27. Matter-Walstra K, Joerger M, Kuhnel U, Szucs T, Pestalozzi B, Schwenkglenks M (2012) Cost-effectiveness of maintenance pemetrexed in patients with advanced nonsquamous-cell lung cancer from the perspective of the Swiss health care system. Value Health 15: 65–71. 10.1016/j.jval.2011.08.1737 [DOI] [PubMed] [Google Scholar]
- 28. Pfeil AM, Kressig RW, Szucs TD (2012) Alzheimer's dementia: budget impact and cost-utility analysis of a combination treatment of a cholinesterase inhibitor and memantine in Switzerland. Swiss Med Wkly 142: w13676 10.4414/smw.2012.13676 [DOI] [PubMed] [Google Scholar]
- 29. Wright M, Grieve R, Roberts J, Main J, Thomas HC (2006) Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 10: 1–113, iii. [DOI] [PubMed] [Google Scholar]
- 30. Thomson BJ, Kwong G, Ratib S, Sweeting M, Ryder SD, De Angelis D, et al. (2008) Response rates to combination therapy for chronic HCV infection in a clinical setting and derivation of probability tables for individual patient management. J Viral Hepat 15: 271–278. [DOI] [PubMed] [Google Scholar]
- 31. Grishchenko M, Grieve RD, Sweeting MJ, De Angelis D, Thomson BJ, Ryder SD, et al. (2009) Cost-effectiveness of pegylated interferon and ribavirin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care 25: 171–180. 10.1017/S0266462309090229 [DOI] [PubMed] [Google Scholar]
- 32. McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. (2009) Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 360: 1827–1838. 10.1056/NEJMoa0806104 [DOI] [PubMed] [Google Scholar]
- 33.Lawitz E, Zeuzem S, Nyberg LM, Nelson DR, Rossaro L, Balart LA, et al. Boceprevir (BOC) Combined with Peginterferon alfa-2b/Ribavirin (P/RBV) in Treatment-Naïve Chronic HCV Genotype 1 Patients with Compensated Cirrhosis: Sustained Virologic Response (SVR) and Safety Subanalyses from the Anemia Management Study 2012 Nov 9–12; Boston, MA.
- 34.Lalezari JP, Nelson DR, Hyland RH, Lin M, Rossi SJ, Symonds WT, et al. Once-Daily Sofosbuvir Plus Ribavirin Given for 12 or 24 Weeks in Treatment-Naïve Patients with HCV Infection: the QUANTUM Study; 2013 April 24–28, 2013; Amsterdam, The Netherlands.
- 35.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. (2014) Sofosbuvir and Ribavirin in HCV Genotypes 2 and 3. N Engl J Med. [DOI] [PubMed]
- 36. Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. (2013) Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 368: 34–44. 10.1056/NEJMoa1208953 [DOI] [PubMed] [Google Scholar]
- 37. Foster GR, Hezode C, Bronowicki JP, Carosi G, Weiland O, Verlinden L, et al. (2011) Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 141: 881–889 e881. 10.1053/j.gastro.2011.05.046 [DOI] [PubMed] [Google Scholar]
- 38. Lagging M, Rembeck K, Rauning BM, Christensen P, Dalgard O, Farkkila M, et al. (2013) Retreatment with peg-interferon and ribavirin in patients with chronic hepatitis C virus genotype 2 or 3 infection with prior relapse. Scand J Gastroenterol 48: 839–847. 10.3109/00365521.2013.793389 [DOI] [PubMed] [Google Scholar]
- 39. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. (2001) Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358: 958–965. [DOI] [PubMed] [Google Scholar]
- 40. Federal Office of Public Health (FOPH) Spezialitätenliste (SL). Federal Office of Public Health (BAG). [Google Scholar]
- 41. TARMED Suisse TARMED Tarif-Browser. TARMED Suisse. [Google Scholar]
- 42. Federal Office of Public Health (FOPH) (2014) Analysenliste (AL), Gesamtliste. Federal Office of Public Health. [Google Scholar]
- 43. Bichoupan K, Martel-Laferriere V, Sachs D, Ng M, Schonfeld EA, Pappas A, et al. (2014) Costs of telaprevir-based triple therapy for hepatitis C: $189,000 per sustained virological response. Hepatology 60: 1187–1195. 10.1002/hep.27340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swiss Federal Statistical Office Landesindex der Konsumentenpreise. SFSO. [Google Scholar]
- 45. Organisation for Economic Co-Operation and Development Purchasing Power Parities for GDP and related indicators: OECD. [Google Scholar]
- 46. Organisation for Economic Co-Operation and Development Health expenditure since 2000—Main indicators: OECD. [Google Scholar]
- 47.AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C.
- 48. Food and Drug Administration F (2014) FDA approves first combination pill to treat hepatitis C FDA News Release: FDA. [Google Scholar]
- 49.Gilead (2014) European Commission Grants Marketing Authorization for Gilead’s Harvoni▼ (Ledipasvir/Sofosbuvir), the First Single Tablet Regimen to Treat the Majority of Chronic Hepatitis C Patients With Genotype 1 and 4—Gilead Sciences Inc.
- 50. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (2014) Sofosbuvir—Nutzenbewertung gemäs § 35a SGB V. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen,. 119 119. [Google Scholar]
- 51. Kim H, Gurrin L, Ademi Z, Liew D (2014) Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. Br J Clin Pharmacol 77: 116–121. 10.1111/bcp.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leleu H, Blachier M, Rosa I (2014) Cost-effectiveness of sofosbuvir in the treatment of patients with hepatitis C. J Viral Hepat. [DOI] [PMC free article] [PubMed]
- 53. Deuffic-Burban S, Schwarzinger M, Obach D, Mallet V, Pol S, Pageaux G-P, et al. (2014) Should we await IFN-free regimens to treat HCV genotype 1 treatment-naive patients? A cost-effectiveness analysis (ANRS 95141). J Hepatol 61: 7–14. 10.1016/j.jhep.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 54. Petta S, Cabibbo G, Enea M, Macaluso FS, Plaia A, Bruno R, et al. (2014) Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology 59: 1692–1705. 10.1002/hep.27010 [DOI] [PubMed] [Google Scholar]
- 55.Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z (2014) Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. [DOI] [PubMed]
- 56.San Miguel R, Gimeno-Ballester V, Blazquez A, Mar J (2014) Cost-effectiveness analysis of sofosbuvir-based regimens for chronic hepatitis C. Gut. [DOI] [PubMed]
- 57. Briggs A (2010) Transportability of comparative effectiveness and cost-effectiveness between countries. Value Health 13 Suppl 1: S22–25. 10.1111/j.1524-4733.2010.00751.x [DOI] [PubMed] [Google Scholar]
- 58.(2011) Bundesgerichtsentscheid 9C_334/2010—Regeste und Auszüge: Regeste des Entscheids vom 23. November 2010. Bioethica Forum 4. [Google Scholar]
- 59. McCabe C, Claxton K, Culyer AJ (2008) The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 26: 733–744. [DOI] [PubMed] [Google Scholar]
- 60. Chambers JD, Neumann PJ, Buxton MJ (2010) Does Medicare have an implicit cost-effectiveness threshold? Med Decis Making 30: E14–27. 10.1177/0272989X10371134 [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization Cost-effectiveness thresholds.
- 62.Bundesamt für Statistik (2014) Bruttoinlandprodukt pro Einwohner.
- 63. Neumann PJ, Cohen JT, Weinstein MC (2014) Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371: 796–797. 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 64. Knies S, Evers SM, Candel MJ, Severens JL, Ament AJ (2009) Utilities of the EQ-5D: transferable or not? Pharmacoeconomics 27: 767–779. 10.2165/11314120-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 65. Matter-Walstra K, Klingbiel D, Szucs T, Pestalozzi BC, Schwenkglenks M (2014) Using the EuroQol EQ-5D in Swiss cancer patients, which value set should be applied? Pharmacoeconomics 32: 591–599. 10.1007/s40273-014-0151-0 [DOI] [PubMed] [Google Scholar]
- 66. Hill A, Cooke G (2014) Medicine. Hepatitis C can be cured globally, but at what cost? Science 345: 141–142. 10.1126/science.1257737 [DOI] [PubMed] [Google Scholar]
- 67. Carroll J (2014) Payers consider waiting out budget-busting hepatitis C drug. Manag Care 23: 7, 9. [PubMed] [Google Scholar]
- 68. Brennan T, Shrank W (2014) New expensive treatments for hepatitis C infection. JAMA 312: 593–594. 10.1001/jama.2014.8897 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BOC, boceprevir; GT, genotype; IE, interferon eligible; II, interferon ineligible; NA, not available; PegIFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; TE, treatment-experienced; TEL, telaprevir; TN, treatment-naïve; # 1% erythropoietin and 0.7% blood transfusions; § equally distributed between erythropoietin and blood transfusions.
(PDF)
BOC, boceprevir; C, cirrhotic; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; NC, non-cirrhotic; PegIFN, pegylated interferon; RBV, ribavirin; RNA, ribonucleic acid; SOF, sofosbuvir; SVR, sustained virological response; TEL, telaprevir; *resource use was checked by Swiss clinical experts; 1 stands for 100% of the patients.
(PDF)
AE, adverse event; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon 2a+ribavirin; PbR, pegylated interferon 2b+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TN, treatment-naïve; * values are rounded to one decimal place.
(PDF)
AE, adverse event; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon 2a+ribavirin; PbR, pegylated interferon 2b+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TE, treatment-experienced; TN, treatment-naïve; * values are rounded to one decimal place.
(PDF)
AE, adverse event; IE, interferon eligible; II, interferon ineligible; NT, no treatment; PaR, pegylated interferon 2a+ribavirin; PbR, pegylated interferon 2b+ribavirin; QALY, quality-adjusted life year; RBV, ribavirin; SOF, sofosbuvir; TE, treatment-experienced; TN, treatment-naïve; * values are rounded to one decimal place.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


