Abstract
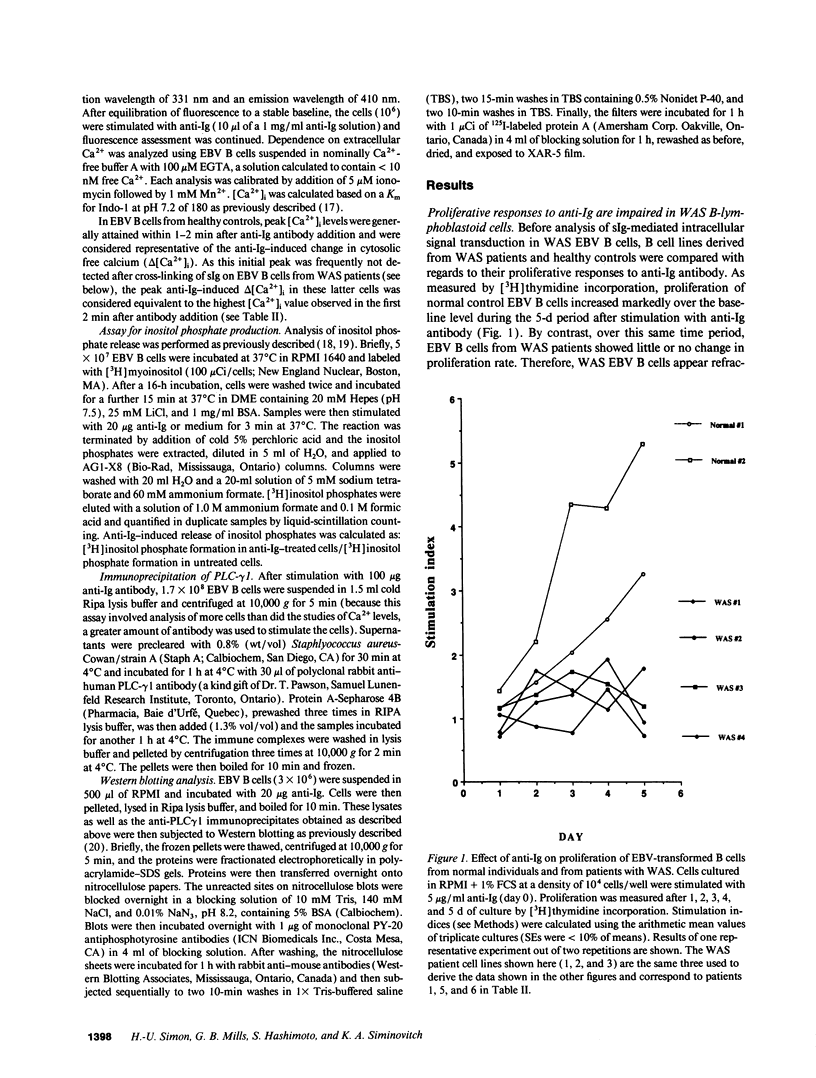
B lymphocytes from patients expressing the X chromosome-linked immune deficiency disorder, Wiskott-Aldrich syndrome (WAS), fail to produce antibodies in response to stimulation with polysaccharides and other type-2 T cell-independent antigens. To investigate whether this abnormality reflects a defect in the signal transduction cascade normally triggered by ligation of surface immunoglobulin (sIg) on B cells, we have examined early signaling events induced by anti-Ig antibody stimulation of EBV B lymphoblastoid cell lines from WAS patients and healthy controls. Despite the expression of comparable levels of sIg and sIgM on WAS and control EBV B cells, WAS cells failed to manifest the increased proliferation in response to anti-Ig treatment observed in the control cell lines. WAS and control EBV B cells also differed in the magnitude of the change in cytosolic free calcium ([Ca2+]i) induced by sIg ligation; WAS cells showed either markedly diminished or no changes in [Ca2+]i levels whereas control EBV B cells consistently showed increases in [Ca2+]i. Anti-Ig-induced changes in inositol phosphate release were also markedly reduced in WAS compared with control cells. As protein tyrosine phosphorylation is thought to represent a proximal event in the activation of B cells, inducing increases in [Ca2+]i by virtue of tyrosine phosphorylation of phospholipase C (PLC)-gamma, profiles of protein tyrosine phosphorylation and expression of tyrosine-phosphorylated PLC-gamma 1 were compared between WAS and normal EBV B cells before and after sIg cross-linking. These studies revealed that in addition to defective mobilization of Ca2+, the WAS cells manifested little or no increase in tyrosine phosphorylation of PLC-gamma 1 or other intracellular proteins after sIg ligation. Together these results indicate the association of WAS with a defect in the coupling of sIg to signal transduction pathways considered prerequisite for B cell activation, likely at the level of tyrosine phosphorylation. The abnormalities observed in these early transmembrane signaling events in WAS EBV B cells may play a role not only in the nonresponsiveness of WAS patient B cells to certain T independent antigens, but also in the genesis of some of the other cellular deficits exhibited by these patients.
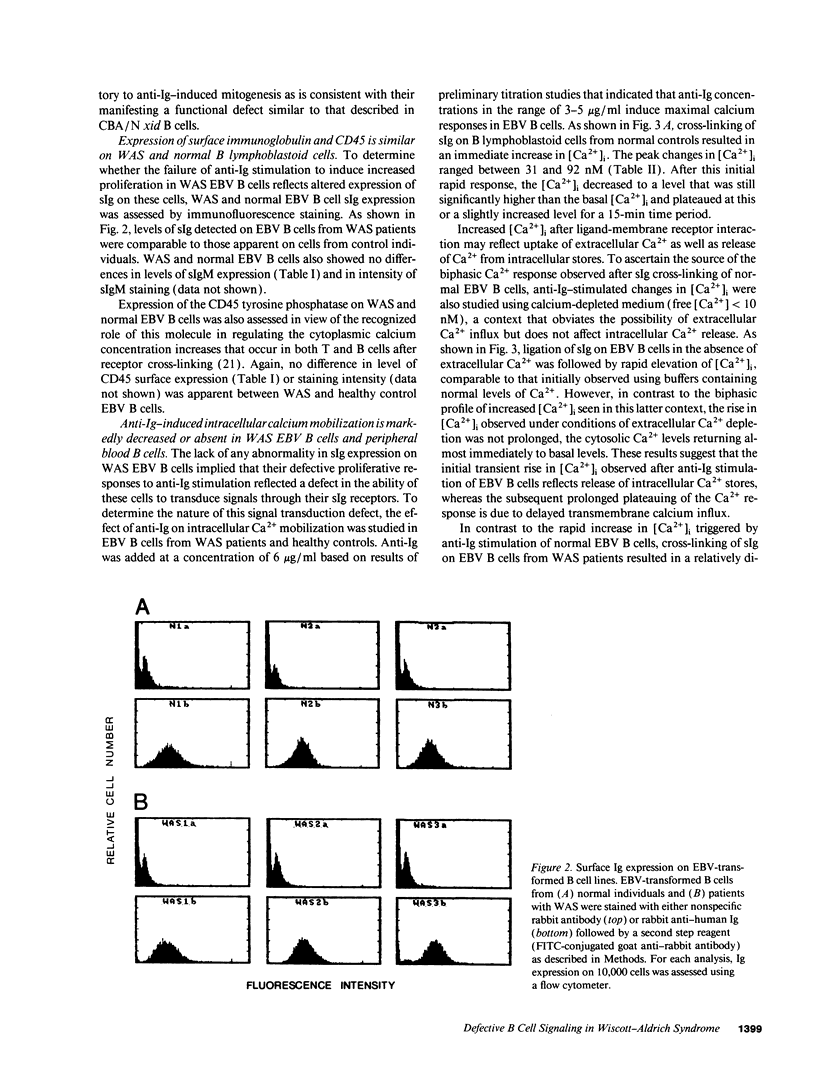
Full text
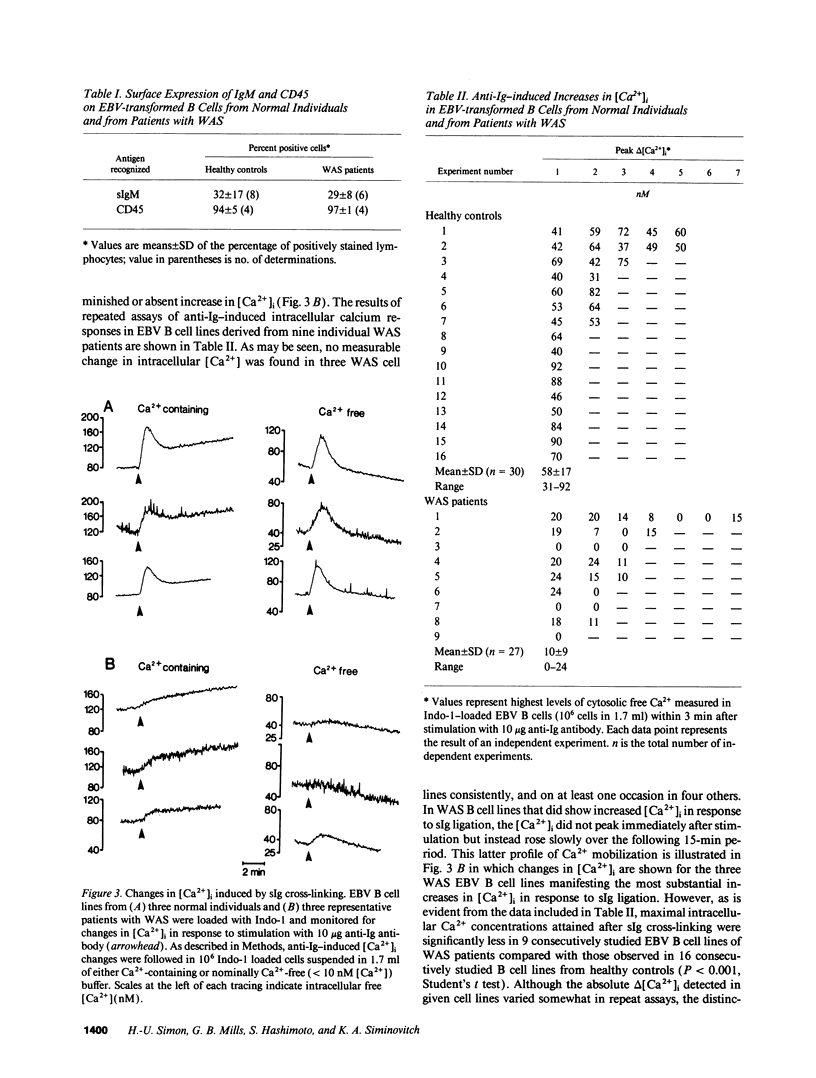
PDF

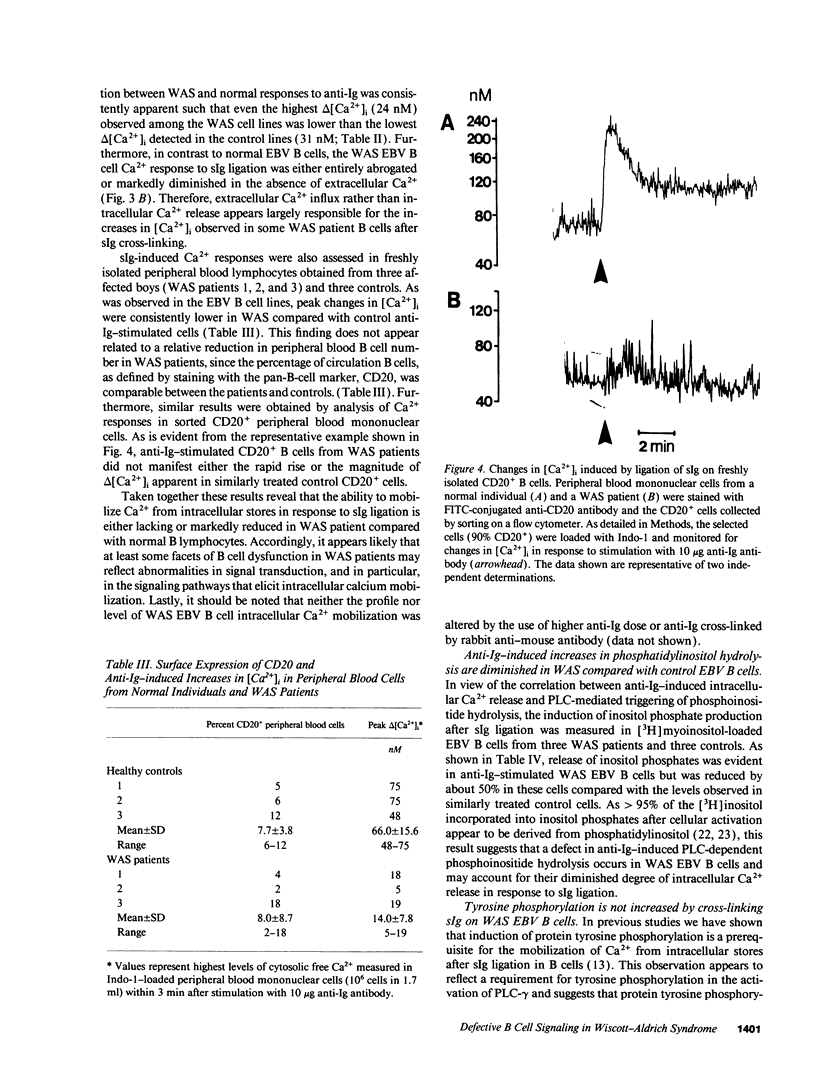



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRICH R. A., STEINBERG A. G., CAMPBELL D. C. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954 Feb;13(2):133–139. [PubMed] [Google Scholar]
- Alés-Martínez J. E., Cuende E., Martínez C., Parkhouse R. M., Pezzi L., Scott D. W. Signalling in B cells. Immunol Today. 1991 Jun;12(6):201–205. doi: 10.1016/0167-5699(91)90054-w. [DOI] [PubMed] [Google Scholar]
- Brunswick M., Finkelman F. D., Highet P. F., Inman J. K., Dintzis H. M., Mond J. J. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J Immunol. 1988 May 15;140(10):3364–3372. [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Friedrich R. J., Cambier J. C. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. H., Park D. J., Rhee S. G., Fearon D. T. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2745–2749. doi: 10.1073/pnas.88.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Z., Stall A. M., Herzenberg L. A., Herzenberg L. A. Differences in glycoprotein complexes associated with IgM and IgD on normal murine B cells potentially enable transduction of different signals. EMBO J. 1990 Jul;9(7):2117–2124. doi: 10.1002/j.1460-2075.1990.tb07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien M. M., Ashman R. F. Phospholipid synthesis by activated human B lymphocytes. J Immunol. 1983 Jun;130(6):2568–2573. [PubMed] [Google Scholar]
- Cooper M. D., Chae H. P., Lowman J. T., Krivit W., Good R. A. Wiskott-Aldrich syndrome. An immunologic deficiency disease involving the afferent limb of immunity. Am J Med. 1968 Apr;44(4):499–513. doi: 10.1016/0002-9343(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Golding B., Muchmore A. V., Blaese R. M. Newborn and Wiskott-Aldrich patient B cells can be activated by TNP-Brucella abortus: evidence that TNP-Brucella abortus behaves as a T-independent type 1 antigen in humans. J Immunol. 1984 Dec;133(6):2966–2971. [PubMed] [Google Scholar]
- Greer W. L., Kwong P. C., Peacocke M., Ip P., Rubin L. A., Siminovitch K. A. X-chromosome inactivation in the Wiskott-Aldrich syndrome: a marker for detection of the carrier state and identification of cell lineages expressing the gene defect. Genomics. 1989 Jan;4(1):60–67. doi: 10.1016/0888-7543(89)90315-7. [DOI] [PubMed] [Google Scholar]
- Grupp S. A., Harmony J. A. Increased phosphatidylinositol metabolism is an important but not an obligatory early event in B lymphocyte activation. J Immunol. 1985 Jun;134(6):4087–4094. [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein regulation of receptor signalling. Immunol Today. 1988 Oct;9(10):315–320. doi: 10.1016/0167-5699(88)91325-4. [DOI] [PubMed] [Google Scholar]
- Higgins E. A., Siminovitch K. A., Zhuang D. L., Brockhausen I., Dennis J. W. Aberrant O-linked oligosaccharide biosynthesis in lymphocytes and platelets from patients with the Wiskott-Aldrich syndrome. J Biol Chem. 1991 Apr 5;266(10):6280–6290. [PubMed] [Google Scholar]
- Holub B. J. The Mn2+-activated incorporation of inositol into molecular species of phosphatidylinositol in rat liver microsomes. Biochim Biophys Acta. 1974 Oct 16;369(1):111–122. doi: 10.1016/0005-2760(74)90197-0. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Justement L. B., Wienands J., Hombach J., Reth M., Cambier J. C. Membrane IgM and IgD molecules fail to transduce Ca2+ mobilizing signals when expressed on differentiated B lineage cells. J Immunol. 1990 May 1;144(9):3272–3280. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Zilberstein A., Franks C., Felder S., Kremer S., Ullrich A., Rhee S. G., Skorecki K., Schlessinger J. Effect of phospholipase C-gamma overexpression on PDGF-induced second messengers and mitogenesis. Science. 1990 May 4;248(4955):607–610. doi: 10.1126/science.2333512. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Interleukin 2-induced lymphocyte proliferation is independent of increases in cytosolic-free calcium concentrations. J Immunol. 1985 Apr;134(4):2431–2435. [PubMed] [Google Scholar]
- Mills G. B., Stewart D. J., Mellors A., Gelfand E. W. Interleukin 2 does not induce phosphatidylinositol hydrolysis in activated T cells. J Immunol. 1986 Apr 15;136(8):3019–3024. [PubMed] [Google Scholar]
- Morio T., Takase K., Okawa H., Oguchi M., Kanbara M., Hiruma F., Yoshino K., Kaneko T., Asamura S., Inoue T. The increase of non-MHC-restricted cytotoxic cells (gamma/delta-TCR-bearing T cells or NK cells) and the abnormal differentiation of B cells in Wiskott-Aldrich syndrome. Clin Immunol Immunopathol. 1989 Aug;52(2):279–290. doi: 10.1016/0090-1229(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Blaese R. M., Waldmann T. A. Defective lymphocyte transformation and delayed hypersensitivity in Wiskott-Aldrich syndrome. J Immunol. 1970 Apr;104(4):835–844. [PubMed] [Google Scholar]
- Padeh S., Levitzki A., Gazit A., Mills G. B., Roifman C. M. Activation of phospholipase C in human B cells is dependent on tyrosine phosphorylation. J Clin Invest. 1991 Mar;87(3):1114–1118. doi: 10.1172/JCI115074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal J. T., Carroll A. J., Prchal J. F., Crist W. M., Skalka H. W., Gealy W. J., Harley J., Malluh A. Wiskott-Aldrich syndrome: cellular impairments and their implication for carrier detection. Blood. 1980 Dec;56(6):1048–1054. [PubMed] [Google Scholar]
- Reth M., Hombach J., Wienands J., Campbell K. S., Chien N., Justement L. B., Cambier J. C. The B-cell antigen receptor complex. Immunol Today. 1991 Jun;12(6):196–201. doi: 10.1016/0167-5699(91)90053-V. [DOI] [PubMed] [Google Scholar]
- Rigley K. P., Harnett M. M., Phillips R. J., Klaus G. G. Analysis of signaling via surface immunoglobulin receptors on B cells from CBA/N mice. Eur J Immunol. 1989 Nov;19(11):2081–2086. doi: 10.1002/eji.1830191117. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Sekar M. C., Roufogalis B. D. Muscarinic-receptor stimulation enhances polyphosphoinositide breakdown in guinea-pig ileum smooth muscle. Biochem J. 1984 Oct 15;223(2):527–531. doi: 10.1042/bj2230527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitler L. E., Levin A. S., Stites D. P., Fudenberg H. H., Huber H. The Wiskott-Aldrich syndrome. Immunologic studies in nine patients and selected family members. Cell Immunol. 1975 Oct;19(2):201–218. doi: 10.1016/0008-8749(75)90204-x. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- van Noesel C. J., van Lier R. A., Cordell J. L., Tse A. G., van Schijndel G. M., de Vries E. F., Mason D. Y., Borst J. The membrane IgM-associated heterodimer on human B cells is a newly defined B cell antigen that contains the protein product of the mb-1 gene. J Immunol. 1991 Jun 1;146(11):3881–3888. [PubMed] [Google Scholar]





