Abstract
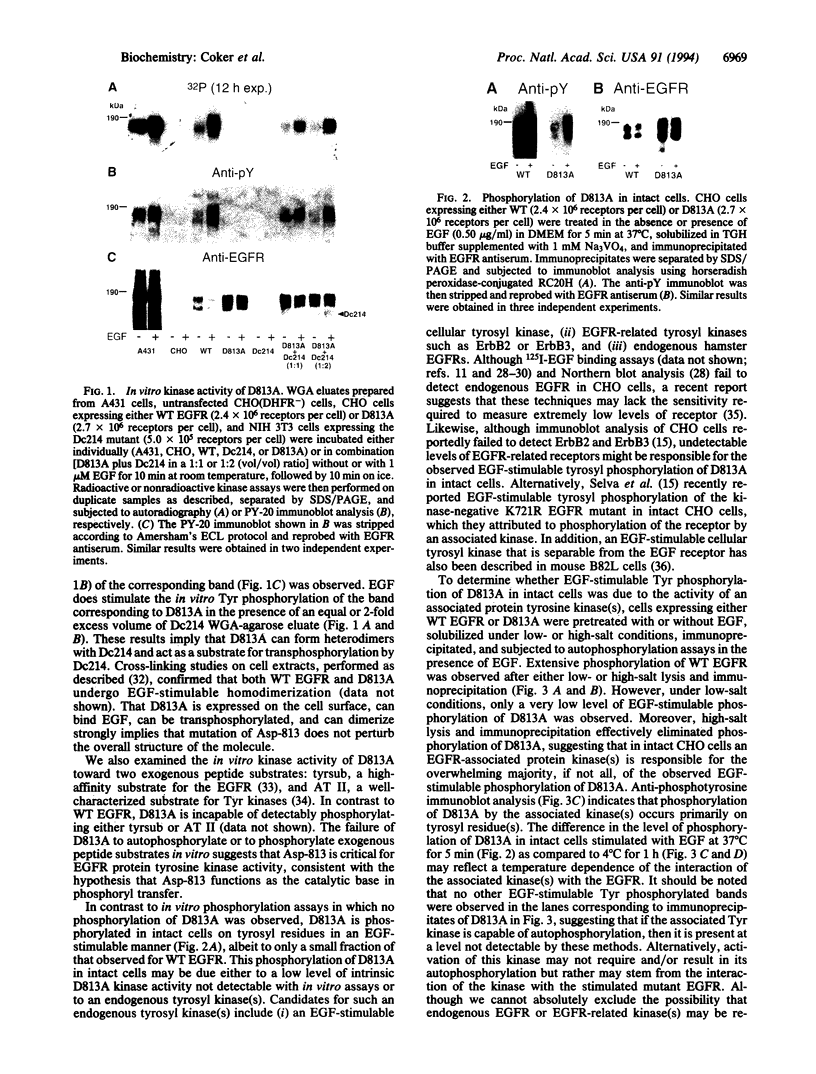
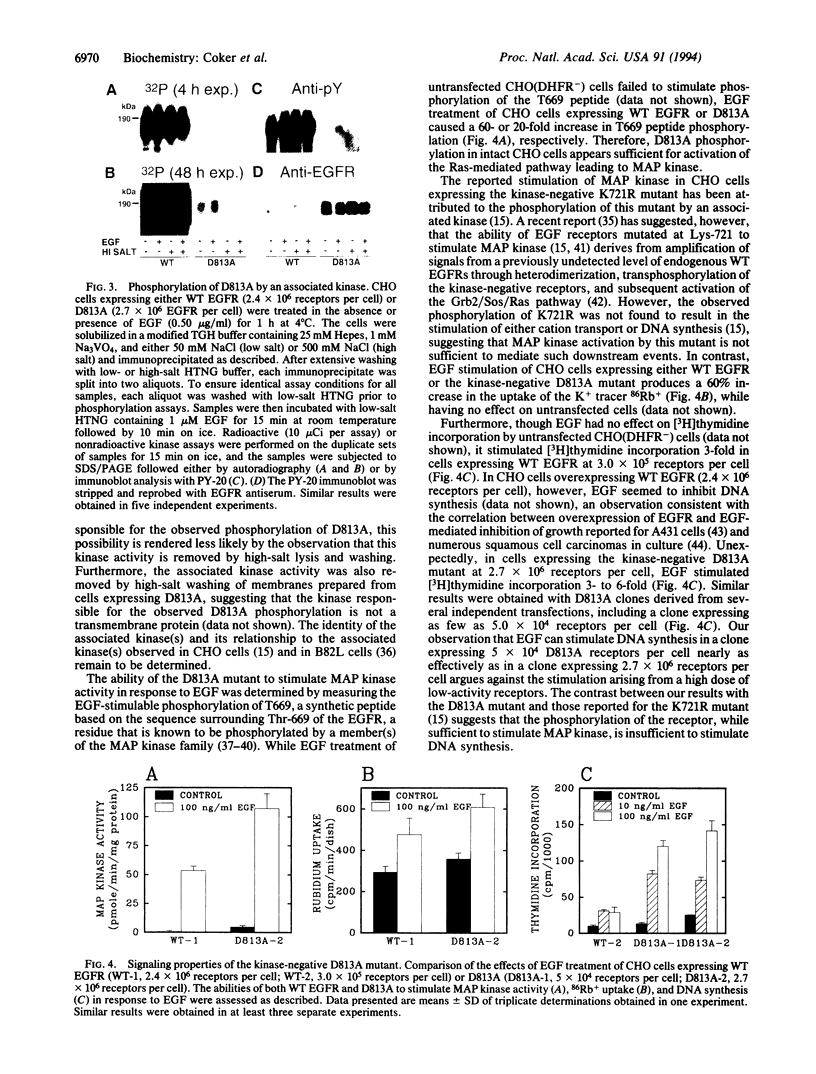
The residue proposed to serve as the catalytic base for phosphoryl transfer, Asp-813, of the human epidermal growth factor receptor (EGFR) was mutated to Ala, and the mutant receptor (D813A) was expressed in Chinese hamster ovary (CHO) cells. Partially purified D813A exhibited no detectable kinase activity in the absence or presence of EGF. A low level of EGF-stimulable phosphorylation of D813A was detectable in intact cells, apparently due to the activity of an associated Tyr kinase(s). As previously observed for kinase-inactive Lys-721 mutants, EGF binding to D813A stimulates mitogen-activated protein kinase activity. Surprisingly, and unlike results reported for Lys-721 mutants, D813A is capable of stimulating both 86Rb+ uptake and DNA synthesis in response to EGF. These data suggest not only that Asp-813 is critical to the catalytic activity of the EGFR but also that differences may exist in the signaling properties of kinase-negative Lys-721 and kinase-negative Asp-813 EGFR mutants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. A., Compton L. A., Clinton G. M. Antibodies against highly conserved sites in the epidermal growth factor receptor tyrosine kinase domain as probes for structure and function. Biochemistry. 1993 May 4;32(17):4659–4664. doi: 10.1021/bi00068a025. [DOI] [PubMed] [Google Scholar]
- Buechler J. A., Taylor S. S. Dicyclohexylcarbodiimide cross-links two conserved residues, Asp-184 and Lys-72, at the active site of the catalytic subunit of cAMP-dependent protein kinase. Biochemistry. 1989 Mar 7;28(5):2065–2070. doi: 10.1021/bi00431a015. [DOI] [PubMed] [Google Scholar]
- Buechler J. A., Taylor S. S. Identification of aspartate-184 as an essential residue in the catalytic subunit of cAMP-dependent protein kinase. Biochemistry. 1988 Sep 20;27(19):7356–7361. doi: 10.1021/bi00419a027. [DOI] [PubMed] [Google Scholar]
- Buhrow S. A., Cohen S., Garbers D. L., Staros J. V. Characterization of the interaction of 5'-p-fluorosulfonylbenzoyl adenosine with the epidermal growth factor receptor/protein kinase in A431 cell membranes. J Biol Chem. 1983 Jun 25;258(12):7824–7827. [PubMed] [Google Scholar]
- Buhrow S. A., Cohen S., Staros J. V. Affinity labeling of the protein kinase associated with the epidermal growth factor receptor in membrane vesicles from A431 cells. J Biol Chem. 1982 Apr 25;257(8):4019–4022. [PubMed] [Google Scholar]
- Campos-González R., Glenney J. R., Jr Tyrosine phosphorylation of mitogen-activated protein kinase in cells with tyrosine kinase-negative epidermal growth factor receptors. J Biol Chem. 1992 Jul 25;267(21):14535–14538. [PubMed] [Google Scholar]
- Carpenter G. Binding assays for epidermal growth factor. Methods Enzymol. 1985;109:101–110. doi: 10.1016/0076-6879(85)09079-6. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Countaway J. L., Northwood I. C., Davis R. J. Mechanism of phosphorylation of the epidermal growth factor receptor at threonine 669. J Biol Chem. 1989 Jun 25;264(18):10828–10835. [PubMed] [Google Scholar]
- Davis R. J. Independent mechanisms account for the regulation by protein kinase C of the epidermal growth factor receptor affinity and tyrosine-protein kinase activity. J Biol Chem. 1988 Jul 5;263(19):9462–9469. [PubMed] [Google Scholar]
- Fanger B. O., Stephens J. E., Staros J. V. High-yield trapping of EGF-induced receptor dimers by chemical cross-linking. FASEB J. 1989 Jan;3(1):71–75. doi: 10.1096/fasebj.3.1.2783412. [DOI] [PubMed] [Google Scholar]
- Filhol O., Chambaz E. M., Gill G. N., Cochet C. Epidermal growth factor stimulates a protein tyrosine kinase which is separable from the epidermal growth factor receptor. J Biol Chem. 1993 Dec 25;268(36):26978–26982. [PubMed] [Google Scholar]
- Fry M. J., Panayotou G., Booker G. W., Waterfield M. D. New insights into protein-tyrosine kinase receptor signaling complexes. Protein Sci. 1993 Nov;2(11):1785–1797. doi: 10.1002/pro.5560021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. S., Zoller M. J. Identification of electrostatic interactions that determine the phosphorylation site specificity of the cAMP-dependent protein kinase. Biochemistry. 1991 Jun 4;30(22):5329–5334. doi: 10.1021/bi00236a001. [DOI] [PubMed] [Google Scholar]
- Gibbs C. S., Zoller M. J. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991 May 15;266(14):8923–8931. [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Raden D. L., Davis R. J. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem. 1991 Nov 25;266(33):22159–22163. [PubMed] [Google Scholar]
- Hack N., Sue-A-Quan A., Mills G. B., Skorecki K. L. Expression of human tyrosine kinase-negative epidermal growth factor receptor amplifies signaling through endogenous murine epidermal growth factor receptor. J Biol Chem. 1993 Dec 15;268(35):26441–26446. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Herberg F. W., Taylor S. S. Physiological inhibitors of the catalytic subunit of cAMP-dependent protein kinase: effect of MgATP on protein-protein interactions. Biochemistry. 1993 Dec 21;32(50):14015–14022. doi: 10.1021/bi00213a035. [DOI] [PubMed] [Google Scholar]
- Herbst R., Shearman M. S., Obermeier A., Schlessinger J., Ullrich A. Differential effects of W mutations on p145c-kit tyrosine kinase activity and substrate interaction. J Biol Chem. 1992 Jul 5;267(19):13210–13216. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Szapary D., Schmidt A., Lyall R., Van Obberghen E., Dull T. J., Ullrich A., Schlessinger J. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol. 1987 Dec;7(12):4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata N., Chida K., Rikimaru K., Horikoshi M., Enomoto S., Kuroki T. Growth-inhibitory effects of epidermal growth factor and overexpression of its receptors on human squamous cell carcinomas in culture. Cancer Res. 1986 Apr;46(4 Pt 1):1648–1653. [PubMed] [Google Scholar]
- Knighton D. R., Cadena D. L., Zheng J., Ten Eyck L. F., Taylor S. S., Sowadski J. M., Gill G. N. Structural features that specify tyrosine kinase activity deduced from homology modeling of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5001–5005. doi: 10.1073/pnas.90.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Lazar C. S., Carpenter C. D., Gill G. N., Evans R. M., Rosenfeld M. G. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986 Mar 28;44(6):839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Livneh E., Prywes R., Kashles O., Reiss N., Sasson I., Mory Y., Ullrich A., Schlessinger J. Reconstitution of human epidermal growth factor receptors and its deletion mutants in cultured hamster cells. J Biol Chem. 1986 Sep 25;261(27):12490–12497. [PubMed] [Google Scholar]
- Magni M., Meldolesi J., Pandiella A. Ionic events induced by epidermal growth factor. Evidence that hyperpolarization and stimulated cation influx play a role in the stimulation of cell growth. J Biol Chem. 1991 Apr 5;266(10):6329–6335. [PubMed] [Google Scholar]
- Moolenaar W. H., Bierman A. J., Tilly B. C., Verlaan I., Defize L. H., Honegger A. M., Ullrich A., Schlessinger J. A point mutation at the ATP-binding site of the EGF-receptor abolishes signal transduction. EMBO J. 1988 Mar;7(3):707–710. doi: 10.1002/j.1460-2075.1988.tb02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Sadowski I., Pawson T. Mutational analysis of a phosphotransfer motif essential for v-fps tyrosine kinase activity. Oncogene. 1988 Dec;3(6):665–672. [PubMed] [Google Scholar]
- Northwood I. C., Gonzalez F. A., Wartmann M., Raden D. L., Davis R. J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991 Aug 15;266(23):15266–15276. [PubMed] [Google Scholar]
- Rolband G. C., Williams J. F., Webster N. J., Hsu D., Olefsky J. M. Deletion of exon 21 of the insulin receptor eliminates tyrosine kinase activity but preserves mitogenic signaling. Biochemistry. 1993 Dec 14;32(49):13545–13550. doi: 10.1021/bi00212a021. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M. W., Lukas T. J., Cohen S., Staros J. V. Identification of residues in the nucleotide binding site of the epidermal growth factor receptor/kinase. J Biol Chem. 1985 May 10;260(9):5205–5208. [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Selva E., Raden D. L., Davis R. J. Mitogen-activated protein kinase stimulation by a tyrosine kinase-negative epidermal growth factor receptor. J Biol Chem. 1993 Jan 25;268(3):2250–2254. [PubMed] [Google Scholar]
- Sorkin A., Helin K., Waters C. M., Carpenter G., Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. Contrasting significance of tyrosine 992 in the native and truncated receptors. J Biol Chem. 1992 Apr 25;267(12):8672–8678. [PubMed] [Google Scholar]
- Takishima K., Griswold-Prenner I., Ingebritsen T., Rosner M. R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated "MAP" kinase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. C., Nocka K., Ray P., Traktman P., Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990 Jan 12;247(4939):209–212. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- Zheng J., Knighton D. R., ten Eyck L. F., Karlsson R., Xuong N., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry. 1993 Mar 9;32(9):2154–2161. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]