Abstract
Resiniferatoxin (RTX) is the most potent amongst all known endogenous and synthetic agonists for the transient receptor potential vanilloid 1 (TRPV1) receptor, which is a calcium permeable non-selective cation channel, expressed on the peripheral and central terminals of small diameter sensory neurons. [11,32] Prolonged calcium influx induced by RTX causes cytotoxicity and death of only those sensory neurons that express the TRPV1 ion channel leading to selective targeting and permanent deletion of the TRPV1-expressing C-fiber neuronal cell bodies in the dorsal root ganglia. [10,17] The goal of this project was to provide pre-clinical efficacy data, that intrathecal RTX could provide effective pain relief and improve function in dogs with bone cancer without significant long-term side effects. In a single blind, controlled study, 72 companion dogs with bone cancer pain were randomized to standard of care analgesic therapy alone (control, n=36) or 1.2 mcg/kg intrathecal RTX in addition to standard of care analgesic therapy (treated, n=36). Significantly more dogs in the control group (78%) required unblinding and adjustment in analgesic protocol or euthanasia within 6 weeks of randomization, than dogs that were treated with RTX (50%; p<0.03); and overall, dogs in the control group required unblinding significantly sooner than dogs that had been treated with RTX (p<0.02). The analgesic effect was documented in these dogs without any evidence of development of deafferentation pain syndrome that can be seen with neurolytic therapies.
Introduction
Patients with advanced cancer commonly experience, life-altering, pain and bone pain is the most common pain syndrome encountered in cancer patients. [12,22] The frequency and intensity of the pain increases during the advanced stages of disease and the efficacy of potent opioids with or without NSAIDs can be variable with significant side effects. [12,22] In the last days of life, some patients undergo nonselective chemical or surgical neuroablative interventions and palliative sedation. Novel analgesics and innovative procedures with greater efficacy and fewer side effects are clearly needed.
There is much discussion around why development of optimal analgesics for chronic pain states including cancer pain has been hampered. Of particular interest is the development and utilization of a full set of clinically appropriate animal models for both basic science translational research and clinical trials of promising agents. [2,3,3,9,16,21,22,26–29,31,31,31,33,35,37,38] [24] Because bone cancer pain is a unique pain state that changes with progression of the disease, utilizing animal models that are specific to bone cancer is important to identifying novel treatments. [25,34] Rodent models have been instrumental in informing the pathophysiology of bone cancer pain, but very rapid disease progression occurs in these models making clinically relevant efficacy evaluations of novel compounds challenging.. [25,34] The canine model of naturally-occurring bone cancer closely mirrors the progression of clinical disease in humans and has been useful in evaluations of efficacy of novel interventions for bone cancer pain. [4,5,8,17]. In addition to similarities in histopathology, physiology, and response to treatment; the issues associated with managing pain in human cancer patients are precisely mirrored in canine patients, where pain severity becomes refractory to conventional pain therapeutics as disease progresses. [4,5,8,17] These parallels make companion dogs with bone cancer an attractive model for efficacy evaluations of novel interventions for bone cancer pain.
In the present work, we explore the intrathecal administration of resiniferatoxin (RTX) as an approach to control bone cancer pain using the companion dog model. RTX, derived from Euphorbia resinifera, is the most potent amongst all known endogenous and synthetic agonists for the transient receptor potential vanilloid 1 (TRPV1) receptor. TRPV1 is a calcium permeable non-selective cation channel expressed on the peripheral and central terminals of small diameter sensory neurons. [11,13,20,32] When applied to the sensory neuron perikarya, the prolonged calcium influx induced by RTX causes cytotoxicity and death of only those sensory neurons that express the TRPV1 ion channel. [17] Thus, intrathecal RTX administration leads to selective targeting and permanent deletion of the TRPV1-expressing C-fiber neuronal cell bodies in the dorsal root ganglia. [10,17] Loss of these C-fiber neurons interrupts the transmission of pain information from the body to second-order spinal cord neurons, which in turn convey the information to the brain. Mechanosensation, proprioception, and locomotor capability are retained. The goal of this project was to provide pre-clinical efficacy data, that intrathecal RTX could provide effective pain relief and improve function in dogs with bone cancer without long-term side effects. We hypothesized that control dogs would require additional analgesics significantly sooner, have high pain scores and worsening lameness compared to RTX treated dogs.
Methods
Overall Study design
The protocol was reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee and the consent form was reviewed and approved by the Privately Owned Animal Protocol Review Committee. This was a single-blind (owner), randomized, controlled study. Seventy two companion dogs with appendicular bone cancer were randomized to receive standard of care analgesics alone (controls) or standard of care analgesics plus a single intrathecal injection of RTX (treated). Sequentially numbered opaque sealed envelopes were used to allocate dogs to treated and control groups. [14] To maintain owner blinding, all dogs were admitted to the hospital for randomization and the fur was clipped over the intravenous catheter and intrathecal injection sites. Only the dogs randomized to the treated group were anesthetized and underwent intrathecal injection. All dogs were hospitalized overnight to allow treated dogs to fully recover and the following day were discharged from the hospital to owners who were unaware as to which group their dog was randomized. Dogs were evaluated two weeks following the randomization visit and then at monthly intervals until death. Following death, dogs underwent full necropsy.
Population
Inclusion criteria included dogs with appendicular bone cancer confirmed via history, physical, and radiographic examination (Figure 1). Exclusion criteria included clinically significant abnormalities on screening complete blood count and serum biochemistry profile; clinically significant neurologic disease; or history of unexplained coagulopathy. All dogs were on a consistent standard of care (non-steroidal anti-inflammatory drugs, tramadol, and gabapentin) analgesic regimen for at least two weeks prior to enrollment. In addition, The Canine Brief Pain Inventory (BPI) was administered to ensure that the owners were documenting pain behaviors at the screening visit and that they were still present at the randomization visit.
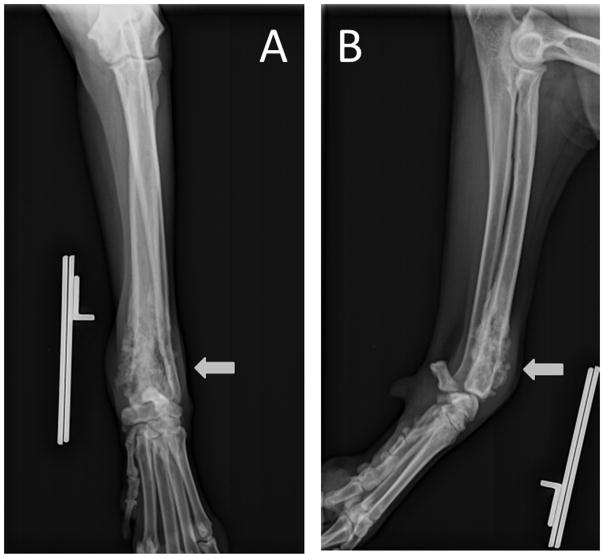
Figure 1.
Anterior-posterior (A) and lateral (B) radiographic views of the left antebrachium of an 11 year old, female, Golden Retriever that was diagnosed with osteosarcoma. There is a moth-eaten lytic lesion in the distal radial metaphyseal region with sunburst periosteal reactions associated with it.
RTX Injection
Just prior to induction of general anesthesia, dogs were pilled with a VitalSense ingestible capsule thermometer to collect core body temperature (VitalSense Integrated Physiological Monitoring System, Mini Mitter Company, Inc. Bend, OR). General anesthesia was induced according to standard of care considering dog breed, age, and anesthesiologist preferences. Anesthesia was maintained with isoflurane in oxygen at 1.5 mean alveolar concentration. Chlorhexidine solution was used for sterile preparation of the intrathecal injection site. A 20 gauge 1.5 inch spinal needle (B–D Quincke Type Point Spinal Needle, Becton Dickinson and Company, Franklin Lakes, NJ) was placed percutaneously into the intrathecal space at the level of the cisterna magna for dogs with forelimb tumors. Flow of clear cerebrospinal fluid confirmed proper needle placement. 1.2 mcg/kg RTX (100mcg/ml solution) was injected into the cisternal space through the spinal needle, followed by 0.1 ml/kg sterile saline. A sterile 22G 3-inch needle was introduced percutaneously to the subarachnoid space between L5 and L6 for dogs with hind limb tumors. Proper needle placement was confirmed by cerebrospinal fluid flow or, in one case, 1 ml injection of a non-ionic, iodinated contrast medium (Omnipaque; Iohexol 240 mg/ml, Amersham Health, PA) into the subarachnoid space under fluoroscopy. 1.2 mcg/kg RTX (100mcg/ml solution) RTX was injected into the lumbar space through the spinal needle, followed by 0.5 ml sterile saline. The dose of RTX and volume of injection for a 50kg dog was used as a maximum for all dogs weighing more than 50 kg. Following RTX injection, the head and neck were elevated approximately 30 degrees above the posterior half of the body to inhibit craniad flow of RTX. General anesthesia was maintained throughout the initial excitation phase of TRPV1 activation. At 60 minutes post injection dogs were maintained on increasingly lower concentrations of isofluorane based on changes in heart rate and blood pressure until they could be safely extubated.
Primary Outcomes
Unblinding occurred when an owner believed that their dog had an unacceptable level of discomfort and required an intervention. At unblinding, dogs in the treated group had their standard of care pain management regimen adjusted and dogs in the control group were offered RTX injection. Unblinding also occurred at the time of spontaneous death or euthanasia of the dog. The primary outcomes were 1) the time to unblinding and 2) the number of dogs that required unblinding within six weeks of randomization.
Secondary outcomes compared the change in 3 measurements from prerandomization to two weeks following randomization. These included 1) pain severity score and pain interference score on the owner-completed Canine Brief Pain Inventory (Canine BPI) [5], and 2) veterinarian assessed lameness based on blinded video analysis.
Statistical Analysis
Primary Outcomes
1) Time to unblinding was determined by the use of the Kaplan-Meier product limit method and log rank analysis was used to compare the failure curves between the two groups (control vs. treated). 2) The Fisher’s exact test was used to compare the number of control versus treated dogs unblinded within 6 weeks of randomization.
Secondary Outcomes
1) The Canine BPI contains 4 questions pertaining to the severity of pain (the averaged responses for these questions result in a pain severity score) and 6 questions pertaining to how the pain interferes with the dog’s routine activities (the averaged responses for these question result in a pain interference score). As long as the same owner provides all assessments for their dog throughout the study, the Canine BPI provides a valid and reliable owner assessment of chronic pain in their dog.[5,6] The Mann Whitney test was used to compare the percent change in pain severity and pain interference scores from pre randomization to 2 weeks post randomization between control and treated dogs. 2) A board certified orthopedist, blinded to treatment group and visit, reviewed videos and rated lameness using an 11 point numerical rating scale with 0=‘sound’ and 10=‘could not be more lame’. Dogs with a decrease in lameness score ≥ 1 from the randomization visit to the 2 week post randomization visit were considered responders. Fisher’s exact test was used compare the number of responders between the treated and control groups.
All analyses were performed using STATA statistical software (Version 11, Statacorp LC, College Station, TX).
Results
Demographics
Thirty-six dogs were randomized to the control group; 21 males and 15 females; median age 8 years, range 2–13 years; median weight 42kg, range 21–75 kg; 28 pure breeds and 8 mixed breeds. Thirty-six dogs were randomized to the treated group; 17 males and 19 females; median age 8 years, range 1–18 years; median weight 41kg, range 21–72 kg; 30 pure breeds and 6 mixed breeds.
Pathology
In the control group 13 dogs had a tumor of the radius; 10 in the humerus; 5 each in the femur and tibia; 1 each in the ilium, ischium, and ulna. Histopathology was available in 31 of these dogs. 30 had osteosarcoma and 1 had a histiocytic sarcoma. In the treated group group 14 dogs had a tumor of the humerus; 8 in the radius; 7 in the femur; 6 in the tibia; 1in the radius and ulna. Histopathology was available in 35 of these dogs. 32 had osteosarcoma, 2 chondrosarcoma, and 1hemangiosarcoma. Time from diagnosis to randomization was not significantly different between groups with median 53 days (range 1 to 393 days) for control dogs and median 41 days (range 12 to 285 days) for treated dogs. Median baseline pain severity score for control dogs was 4.6 (range 1.5 to 8.3) and for treated dogs was 4.5 (range 1.0 to 7.0). Median baseline pain interference score for control dogs was 5.0 (range 1.0 to 9.2) and for treated dogs was 5.8 (range 1.0 to 8.2).
Peri-injection effects of RTX
As described in prior work [8], significant increases in blood pressure and heart rate occur following intrathecal RTX injection in dogs. These effects typically peak within 5 minutes of injection and then gradually return to baseline over the hour that the dog remains anesthetized through the excitation phase. Immediately after extubation, many dogs begin panting heavily and may continue to do so for several hours, during which time they tend to become hypothermic (Figure 2). The hypothermia can persist for many hours while the animals make an otherwise uneventful recovery. [8]
Figure 2.
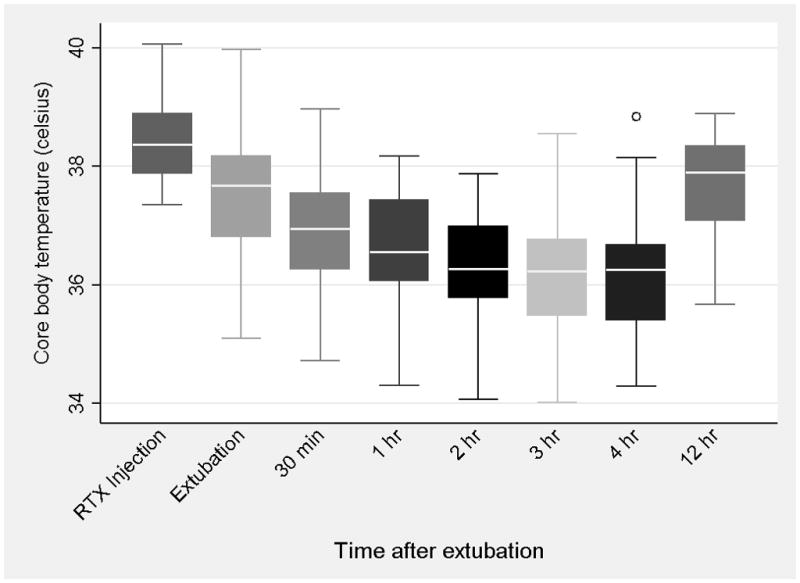
Core body temperature of dogs undergoing general anesthesia and intrathecal injection of resiniferatoxin shows the development of hypothermia that plateaus three to four hours following extubation.
Primary Outcomes
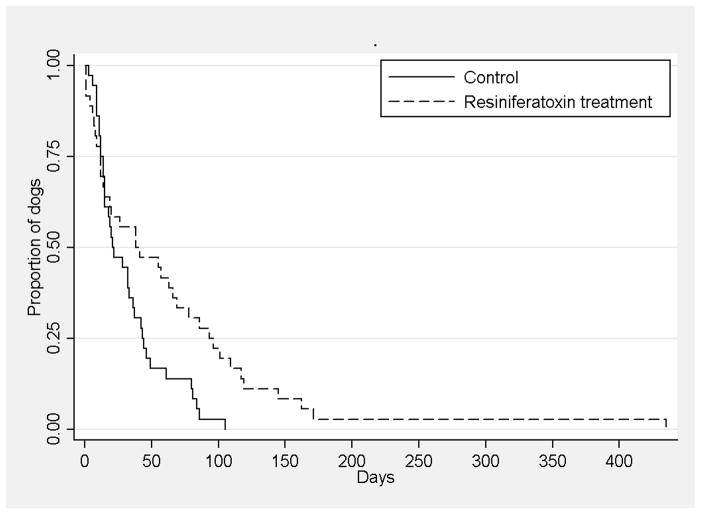
Both primary outcomes suggested a positive effect of IT RTX. 1) Kaplan-Meier product limit method and log rank analysis revealed that dogs receiving standard of care therapy alone were unblinded significantly (p<0.02) sooner than dogs that were treated with RTX in addition to standard of care therapy (Figure 3). 2) Twenty eight dogs (78%) in the control group required unblinding within 6 weeks of randomization and 18 RTX treated dogs (50%) required unblinding (Figure 4). This was a statistically significant difference between groups (p<0.03).
Figure 3.
Kaplan-Meier product-limit method and log rank analysis demonstrated that owners of dogs that received standard of care therapy analgesics alone (controls) sought additional intervention including additional analgesics or euthanasia for their dogs significantly (p=0.01) sooner than owners of dogs that received resiniferatoxin in addition to standard of care analgesics.
Figure 4.
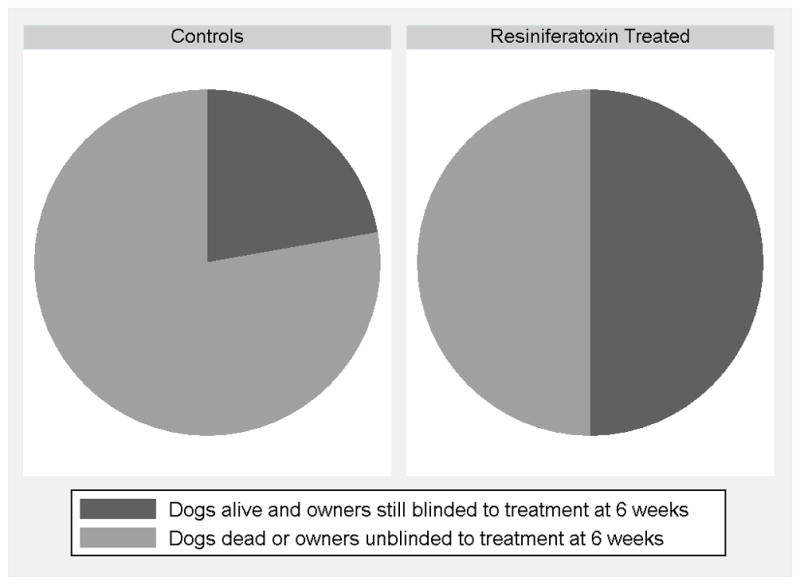
Seventy eight percent of dogs in the control group required unblinding and additional intervention within 6 weeks of randomization, while only 50% of dogs treated with intrathecal resiniferatoxin required unblinding and additional intervention during that same time frame. This was a statistically significant difference between groups (p<0.03).
Secondary Outcomes at Two-week Follow-up
All 36 dogs in the control group were evaluated two weeks following randomization. Seven dogs in the treated group were euthanized prior to the two week time point. Three dogs had previously undiagnosed disease that complicated their recovery from anesthesia (1 with pheochromocytoma, 2 with intervertebral disc herniation) Two dogs developed central neurologic deficits while under anesthesia; one due to multiple brain infarcts and one due a fibrocartilagneous embolism affecting C6-T4. One dog had explosive tumor growth with severe drainage reaction and one dog developed severe lymphedema. One of the three outcomes measured at this 2-week time point suggested a positive effect of RTX. On the Canine BPI (Figure 5), 1) dogs in the control group had a median 5% increase in pain severity score (i.e. more pain) from baseline to 2 weeks post randomization while treated dogs had a median 0% change in pain severity score from baseline to 2 weeks post randomization. The difference between the two groups was not statistically significant on Mann Whitney test (p=0.93). 2) Dogs in the control group had a median 9% increase (i.e. more pain) in pain interference score from baseline to 2 weeks post randomization while treated dogs had a median 10% increase in pain interference score from baseline to 2 weeks post randomization. The difference between the two groups was not statistically significant on Mann Whitney test (p=0.71). 3) For the lameness outcome (Figure 6), 30 sets of randomization and 2-week postrandomization videos were available for review from dogs in the control group and 24 sets of randomization and 2-week postrandomization videos were available for review from dogs in the treated group. Two dogs (7%) in the control group were responders for improved lameness while 8 dogs (33%) in the treated group were responders for improved lameness at 2 weeks post randomization (A lameness responder in the RTX treated group is presented in the ‘Baseline’ video and ‘Week 2’ follow-up video). This difference between groups was statistically significant (p=0.02).
Figure 5.
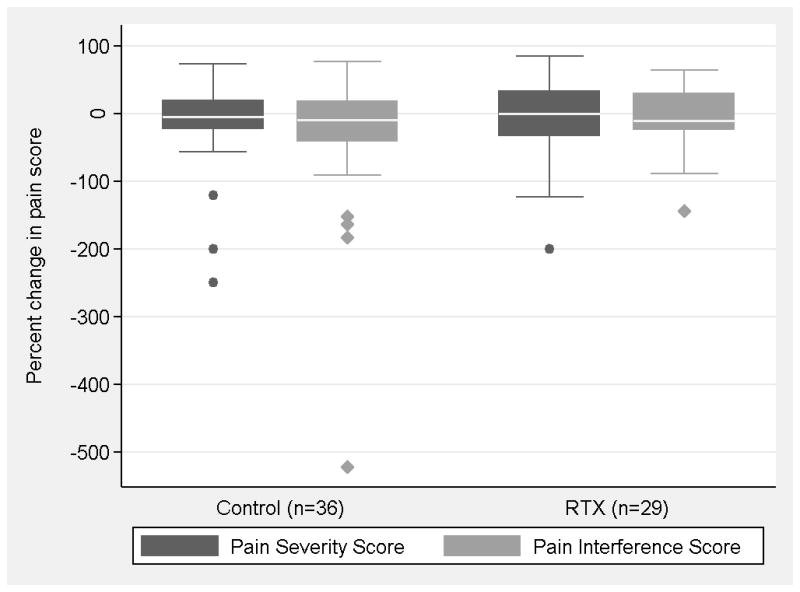
On the owner completed Canine Brief Pain Inventory, there was no significant difference between treated and control groups in the pain severity and pain interference scores two weeks following intrathecal resiniferatoxin administration.
Figure 6.
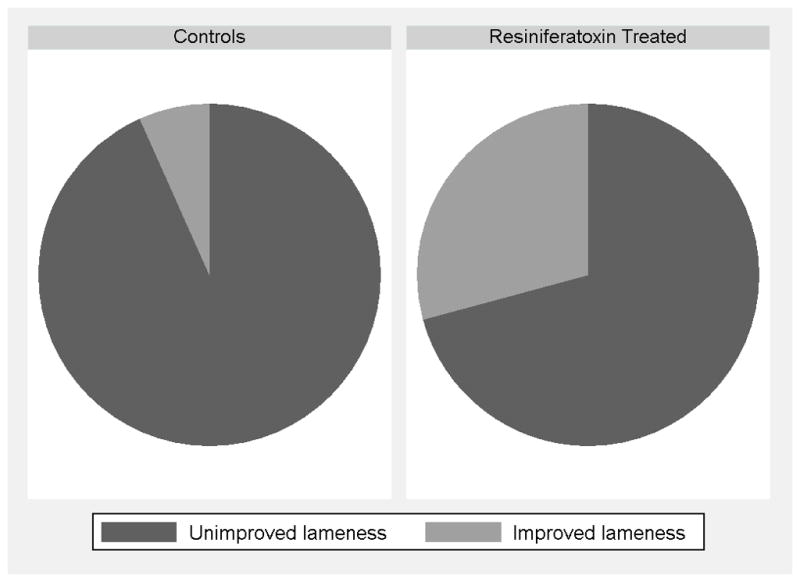
An orthopedist, blinded to treatment group, evaluated lameness through video analysis and determined that seven percent of dogs in the control group had improved lameness while 33% of dogs in the RTX treated group had improved lameness 2 weeks post randomization. This was a statistically significant difference between groups (p<0.03).
Discussion
Because bone cancer pain is a unique pain state that changes with the progression of disease, the identification of effective novel interventions are enhanced by utilizing animal models that are specific to bone cancer and have disease progression that parallels the human condition. [4,5,8,15,17]. In this report, we describe the evaluation of intrathecal RTX in a canine bone cancer model with a complex pain state that parallels the human disease. [4,8,15,23,39] In this randomized, blinded, controlled study we demonstrated analgesic efficacy and some challenges associated with the selective deletion of TRPV1 bearing sensory neurons in complex pain states.
Studies in companion dogs are a novel additional step in evaluating intervention safety and efficacy in human translational therapy. Medical surveillance of dogs is second only to that of people and dogs develop cancer twice as frequently. The presentation, histology, and biology of bone cancer in dogs closely parallel those of humans and the evolution of pain is similar as well. [30,36,39] The frequency and intensity of the pain in dogs increases over weeks or months, allowing time to evaluate effectiveness of antinociceptive agents through evolution of the pain process. The extended course of disease, compared to rodent models, allows for clinically relevant efficacy data collection, while the shorter overall lifespan of dogs, compared to humans, provides a time course of disease within a time-frame reasonable for data collection. This canine model has predominantly been used to evaluate antitumor interventions. Despite the shorter history of use for evaluating analgesic interventions, the canine model has been integral in moving compounds forward to human clinical trials, for example: substance-p saporin (ClinicalTrials.gov Identifier: NCT02036281) [4, 15], and resiniferatoxin (ClinicalTrials.gov Identifier: NCT00804154) [8, 17]. While the design and implementation of studies in this canine model can be challenging due the variable nature of the disease, concurrent medical conditions, and ethical standard of care considerations, these are the same issues present in the design and implementation of human clinical trials. The ability to document a positive result with an intervention in this model with the same hurdles as human studies may alleviate some of the inconsistencies found when translating drugs from more homogeneous, induced rodent models to humans. [4,8,17,23,24] The results of the study described here provide proof of concept data for efficacy of TRPV1 sensory neuron deletion in a complex pain state as well as for informing clinical trial designs by describing issues around choice of efficacy measures, timing of endpoints, and potential adverse events.
Five efficacy outcomes were evaluated in this study. While both of the primary outcomes and the lameness secondary outcome revealed a positive effect of RTX, the owner-completed Canine BPI pain severity and interference scores did not. This could be attributable to several factors. Seven dogs in the treated group were euthanized prior to the 2-week endpoint, so lack of statistical could be due to a loss of power in the study. In addition, there were 18 dogs in the treated group that recovered from general anesthesia with what appeared to be a spinal headache. In hospital, prior to discharge, these dogs were slowly moving, photophobic, reluctant to be pet on the head, and maintained a neck-extended posture. At home, the owners might describe lethargy, lack of interaction with the family, and inappetance. These behaviors could last about a week, and then the owner would describe the dog suddenly ‘snapping out of it’ and returning to normal behaviors that were improved compared to prerandomization. These negative behaviors in the first week post-randomization could influence the owners’ responses on the Canine BPI that encompass an assessment of the behaviors in the week prior to the 2-week post randomization time point.
Spinal headaches are not a well described phenomenon in dogs and would not be expected to occur at the rate described in this report in the average dog undergoing a diagnostic spinal tap. It is also not reported frequently for dogs undergoing injection of contrast agent for myelography, which is a typically a larger volume (0.3–0.5 ml/kg) than the volume of RTX and flush used in this study. As 17 of the 18 dogs with spinal headache behavior had cisternal injections, one might consider whether there was direct effect of craniad flow of RTX to the brain at injection which was responsible for these behaviors. Regardless of the cause, it suggests that the 2-week time point might not be the ideal choice of time-point for outcome assessment.
The two week evaluation point for the secondary outcomes in current study was chosen based on the data suggesting calcium influx, cytotoxicity, and cell death begins to occur immediately upon RTX injection. One would expect that the analgesic effects of RTX could be documented once any effects of hospitalization and general anesthesia have worn off and the dog is back in its home environment to be evaluated for routine behaviors. The 2-week time point would maximize the likelihood that dogs randomized to the control group would remain stable enough to not warrant additional intervention prior to that endpoint. In further canine studies it is advisable to evaluate these secondary outcome measures at a 3– 4 week post injection time point and increase sample size to accommodate the potential for some dogs in the control group necessitating unblinding prior to that time point
Interestingly, the lameness evaluation performed at 2 weeks post-randomization did identify a significant difference between groups. This is likely due to the fact that this assessment evaluates the dog at a single point in time, well beyond any residual effects of the spinal tap, by a veterinarian who is unaware of any behaviors other than the video assessment of the dog walking. Owners are more focused on the ability of their dog to perform its activities of daily living as opposed to how much weight the dog is willing to put on its leg. While a dog may be more weight bearing on its leg following an intervention, if it does not have an overall improved ability to do things like go up and down the stairs or jump up on the bed, they may not consider the dog ‘better’. Force plate gait analysis (FPGA) data shows that lameness is not highly correlated with the owner’s assessment of outcome in the dog. [7] While FPGA is a highly sensitive method of lameness assessment, it was not used in this study because the level of debilitation of dogs with bone cancer can make achieving the required number of valid passes over the force platform impossible and repeated attempts to collect the data throughout the study might increase the risk of pathologic fracture. The level of lameness in these dogs made the less sensitive blinded veterinary assessment a reasonable alternative to FPGA and was indeed sufficient to detect a significant difference between treated and control groups.
Both of the primary outcomes, associated with when the dog required unblinding and an additional intervention (either adjustment of analgesic protocol or euthanasia), showed a significant difference between treated and control dogs. This is notable based on the number of dogs in the treated group that required unblinding very early and did not make it to the 2-week endpoint. Once the dogs make it at least 2 weeks post injection, they tend to do very well and there is great separation in the time-to-event curves (Figure 3). The early loss of cases in the treated group may be avoided with more rigorous screening and a more selective process to study inclusion. For example, the dogs with adrenal tumor and significant intervertebral disc prolapse may not have been selected as good candidates for general anesthesia, spinal column manipulation, and RTX injection if that pathology was apparent at screening. With more strict inclusion criteria, the separation in the time-to-event curves may be even greater than identified in this study.
A major concern in translating neurolytic therapies to clinical application is the development of deafferentation pain syndromes. Patients can experience abnormal sensory phenomena such as allodynia, hyperalgesia, dysesthesias, and hyperpathia when there is complete or partial interruption of afferent nerve impulses. In animals, deafferentation is manifested as self-mutilation of the region in which they might feel pain or paresthetic sensations. [1,18,19] While nociceptive testing to document allodynia or hyperalgesia was not included in this study, self-mutilation did not manifest in any of these dogs, which were all monitored until death.
In summary, the present study demonstrates an analgesic effect of a single intrathecal injection of RTX in bone cancer pain. Significantly more dogs in the control group required unblinding (adjustment in analgesic protocol or euthanasia) within 6 weeks of randomization, than dogs in the RTX treated group; and overall, dogs in the control group required unblinding sooner than treated dogs. In addition, self-mutilation, which can be indicative of deafferentation pain syndromes, did not occur in any of the dogs in the study. The positive analgesic effects of RTX documented in these dogs with bone cancer that closely parallels the human condition provides proof-of-concept data that encourages further investigation into the use of intrathecal RTX for chronic pain control in complex pain states.
Supplementary Material
‘Baseline’. Two year old, male, Mastiff, with osteosarcoma of the right distal tibia, just prior to intrathecal injection of resiniferatoxin.
‘Week 2’. The same Mastiff seen in the ‘Baseline’ video, two weeks following intrathecal injection of resiniferatoxin.
Summary.
Intrathecal resiniferatoxin provides effective pain relief in a naturally occurring canine bone cancer model without occurrence of self-mutilation, which can be indicative of deafferentation pain.
Acknowledgments
For their assistance with this project, the authors would like to thank Michael DiGregorio CVT, Managing Director; and Molly Love RN, Clinical Trials Coordinator; of the Veterinary Clinical Investigations Center, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA USA
This study was funded by a grant from the National Institute of Health (5K08DA017720), Bethesda, MD USA
Footnotes
The authors declare no competing interests at the time of study design, funding, implementation, and analysis. As of 3/22/2014 Dr. Brown acts as a consultant for Sorrento Therapeutics, which is developing RTX for human and veterinary applications, as a key opinion leader with the FDA assisting in study design and data interpretation.
References
- 1.Albe-Fessard D. Neurophysical studies in rats deafferented by dorsal root sections. In: Nashold B, Ovelman-Levitt J, editors. Deafferentation pain syndromes: Pathophysiology and treatments. New York: Raven Press; 1991. pp. 125–139. [Google Scholar]
- 2.Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol. 2006;18:537–547. doi: 10.1097/01.bor.0000240369.39713.af. [DOI] [PubMed] [Google Scholar]
- 3.Bendele A, McComb J, Gould T, McAbee T, Sennello G, Chlipala E, Guy M. Animal models of arthritis: relevance to human disease. Toxicol Pathol. 1999;27:134–142. doi: 10.1177/019262339902700125. [DOI] [PubMed] [Google Scholar]
- 4.Brown DC, Agnello K. Intrathecal substance P-saporin in the dog: efficacy in bone cancer pain. Anesthesiology. 2013;119:1178–1185. doi: 10.1097/ALN.0b013e3182a95188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DC, Boston R, Coyne JC, Farrar JT. A novel approach to the use of animals in studies of pain: validation of the canine brief pain inventory in canine bone cancer. Pain Med. 2009;10:133–142. doi: 10.1111/j.1526-4637.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DC, Boston RC, Coyne JC, Farrar JT. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am J Vet Res. 2007;68:631–637. doi: 10.2460/ajvr.68.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DC, Boston RC, Farrar JT. Comparison of force plate gait analysis and owner assessment of pain using the Canine Brief Pain Inventory in dogs with osteoarthritis. J Vet Intern Med. 2013;27:22–30. doi: 10.1111/jvim.12004. [DOI] [PubMed] [Google Scholar]
- 8.Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Cain DM, Wacnik PW, Simone DA. Animal models of cancer pain may reveal novel approaches to palliative care. Pain. 2001;91:1–4. doi: 10.1016/s0304-3959(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 11.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Dinh QT, Groneberg DA, Peiser C, Mingomataj E, Joachim RA, Witt C, Arck PC, Klapp BF, Fischer A. Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiolo Neurobiol. 2004;144:15–24. doi: 10.1016/j.resp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20:187–191. doi: 10.1016/j.jcrc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida K. Substance P-saporin for bone cancer pain in dogs: can man’s best friend solve the lost in translation problem in analgesic development? Anesthesiology. 2013;119:999–1000. doi: 10.1097/ALN.0b013e3182a951a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter AJ. Have animal models of disease helped or hindered the drug discovery process? Ann N Y Acad Sci. 2011;1245:1–2. doi: 10.1111/j.1749-6632.2011.06375.x. [DOI] [PubMed] [Google Scholar]
- 17.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriz N, Rokyta R. Effect of unilateral deafferentation on extracellular potassium concentration levels in rat thalamic nuclei. Neuroscience. 2000;96:101–108. doi: 10.1016/s0306-4522(99)00540-0. [DOI] [PubMed] [Google Scholar]
- 19.Kriz N, Yamamotova A, Tobias J, Rokyta R. Tail-flick latency and self-mutilation following unilateral deafferentation in rats. Physiol Res. 2006;55:213–220. doi: 10.33549/physiolres.930782. [DOI] [PubMed] [Google Scholar]
- 20.Lazzeri M, Vannucchi MG, Zardo C, Spinelli M, Beneforti P, Turini D, Faussone-Pellegrini MS. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur Urol. 2004;46:792–798. doi: 10.1016/j.eururo.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis”--the need for consensus? Osteoarthritis Cartilage. 2012;20:261–267. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Luger NM, Mach DB, Sevcik MA, Mantyh PW. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage. 2005;29:S32–46. doi: 10.1016/j.jpainsymman.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9:125–136. doi: 10.1007/BF00046339. [DOI] [PubMed] [Google Scholar]
- 24.Mao J. Translational pain research: bridging the gap between basic and clinical research. Pain. 2002;97:183–187. doi: 10.1016/S0304-3959(02)00109-4. [DOI] [PubMed] [Google Scholar]
- 25.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain. 2002;96:129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 26.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 27.Mogil JS. The etiology and symptomatology of spontaneous pain. J Pain. 2012;13:932–933. doi: 10.1016/j.jpain.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Neff MW, Rine J. A fetching model organism. Cell. 2006;124:229–231. doi: 10.1016/j.cell.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Ostrander EA, Galibert F, Patterson DF. Canine genetics comes of age. Trends Genet. 2000;16:117–124. doi: 10.1016/s0168-9525(99)01958-7. [DOI] [PubMed] [Google Scholar]
- 31.Pritzker KP. Animal models for osteoarthritis: processes, problems and prospects. Ann Rheum Dis. 1994;53:406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raisinghani M, Pabbidi RM, Premkumar LS. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J Physiol (Lond) 2005;567:771–786. doi: 10.1113/jphysiol.2005.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Preclinical Pain C, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stambaugh J. Issues for chronic pain models with specific emphasis on the chronic cancer pain model. In: Max M, Portenoy R, Laska E, editors. Advances in pain research. New York: Raven Press; 1991. pp. 287–290. [Google Scholar]
- 36.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 37.Wade GJ. Rethinking the model of osteoarthritis: a clinical viewpoint. J Am Osteopath Assoc. 2011;111:631–637. [PubMed] [Google Scholar]
- 38.Wang LX, Wang ZJ. Animal and cellular models of chronic pain. Adv Drug Deliv Rev. 2003;55:949–965. doi: 10.1016/s0169-409x(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 39.Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop. 1991:159–168. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
‘Baseline’. Two year old, male, Mastiff, with osteosarcoma of the right distal tibia, just prior to intrathecal injection of resiniferatoxin.
‘Week 2’. The same Mastiff seen in the ‘Baseline’ video, two weeks following intrathecal injection of resiniferatoxin.